Studying Oxidative Stress Caused by the Mitis Group Streptococci in Caenorhabditis elegans
Summary
The nematode Caenorhabditis elegans is an excellent model to dissect host-pathogen interactions. Described here is a protocol to infect the worm with members of the mitis group streptococci and determine activation of the oxidative stress response against H2O2 produced by this group of organisms.
Abstract
Caenorhabditis elegans (C. elegans), a free-living nematode, has emerged as an attractive model to study host-pathogen interactions. The presented protocol uses this model to determine the pathogenicity caused by the mitis group streptococci via the production of H2O2. The mitis group streptococci are an emerging threat that cause many human diseases such as bacteremia, endocarditis, and orbital cellulitis. Described here is a protocol to determine the survival of these worms in response to H2O2 produced by this group of pathogens. Using the gene skn-1 encoding for an oxidative stress response transcription factor, it is shown that this model is important for identifying host genes that are essential against streptococcal infection. Furthermore, it is shown that activation of the oxidative stress response can be monitored in the presence of these pathogens using a transgenic reporter worm strain, in which SKN-1 is fused to green fluorescent protein (GFP). These assays provide the opportunity to study the oxidative stress response to H2O2 derived by a biological source as opposed to exogenously added reactive oxygen species (ROS) sources.
Introduction
Mitis group streptococci are human commensals of the oropharyngeal cavity1. However, these organisms can escape this niche and cause a variety of invasive diseases2. The infections caused by these microorganisms include bacteremia, endocarditis, and orbital cellulitis2,3,4,5,6. Furthermore, they are emerging as causative agents of bloodstream infections in immunocompromised, neutropenic, and cancer patients that have undergone chemotherapy5,7,8,9.
The mechanisms underlying mitis group pathogenesis is obscure, because few virulence factors have been identified. The mitis group is known to produce H2O2, which has shown to play an important role in oral microbial communities10. More recently, several studies have highlighted a role for H2O2 as a cytotoxin that induces epithelial cell death11,12. S. pneumonia, which belongs to this group, has been shown to produce high levels of H2O2 that induces DNA damage and apoptosis in alveolar cells13. Using an acute pneumonia animal model, the same researchers demonstrated that production of H2O2 by the bacteria confers a virulence advantage. Studies on pneumococcal meningitis have also shown that pathogen-derived H2O2 acts synergistically with pneumolysin to trigger neuronal cell death14. These observations clearly establish that H2O2 produced by this group of bacteria is important for their pathogenicity.
Interestingly, it has also been shown that members of the mitis group S. mitis and S. oralis cause death of the nematode C. elegans via the production of H2O215,16. This free-living nematode has been used as a simple, genetically tractable model to study many biological processes. More recently, the worm has emerged as a model to study host-pathogen interactions17,18. In addition, several studies have highlighted the importance of studying oxidative stress using this organism19,20,21. Its short life cycle, ability to knockdown genes of interest by RNAi, and use of green fluorescent protein (GFP)-fused reporters to monitor gene expression are some of the attributes that make it an attractive model system. More importantly, the pathways that regulate oxidative stress and innate immunity in the worm are highly conserved with mammals20,22.
In this protocol, it is demonstrated how to use C. elegans to elucidate the pathogenicity caused by streptococcal-derived H2O2. A modified survival assay is shown, and members of the mitis group are able to kill the worms rapidly via the production of H2O2. Using members of the mitis group, a sustained biological source of reactive oxygen species (ROS) is provided, as opposed to chemical sources that induce oxidative stress in the worms. Furthermore, the bacteria are able to colonize the worms rapidly, which allows for H2O2 to be directly targeted to the intestinal cells (compared to other sources that have to cross several barriers). The assay is validated either 1) by determining the survival of the skn-1 mutant strain or 2) by knocking down skn-1 using RNAi in worms relative to the N2 wild-type and vector control treated worms. SKN-1 is an important transcription factor that regulates the oxidative stress response in C. elegans23,24,25. In addition to survival assays, a worm strain expressing a SKN-1B/C::GFP transgenic reporter is used to monitor activation of the oxidative stress response via the production of H2O2 by the mitis group.
Protocol
1. Preparation of THY (Todd-Hewitt Yeast Extract) Agar Plates
- For 1 L of media, add 30 g of Todd-Hewitt powder, 2 g of yeast extract and 20 g of agar to a 2 L Erlenmeyer flask. Add 970 mL of deionized water to the contents of the flask and include a stir bar. Autoclave the media at a temperature of 121 °C and pressure of 15 lb/inch2 for 30 min. Thereafter, set the media on a stir plate and allow for cooling with gentle stirring.
- Pour the media into appropriately sized sterile Petri dishes (100 mm x 15 mm dishes for growth and maintenance of bacteria, 35 mm x 10 mm dishes for killing assays) under a laminar flow. Allow the media to dry for 2 h under the laminar hood. Thereafter, the plates can be stored at 4 °C for 1 month.
2. Preparation of Nematode Growth Medium (NGM) and RNAi Feeding Plates (NGM RNAi)
- Using a stir bar, dissolve 2.5 g of peptone and 3 g of NaCl in 970 mL of deionized water in a 2 L Erlenmeyer flask. Add 20 g of agar to the media. Autoclave the media at a temperature of 121 °C and pressure of 15 lb/inch2 for 30 min. Set the media on a stir plate and allow for cooling with gentle stirring.
- Add the following solutions to the media for preparation of NGM plates: 25 mL of 1 M potassium phosphate buffer (pH = 6.0), 1 mL of 1 M MgSO4, 1 mL of 1 M CaCl2, 1 mL of (5 mg/mL in 95% ethanol) cholesterol, 1 mL of (10% v/w in ethanol) nystatin, and 1 mL of 25 mg/mL streptomycin.
- Add the following solutions to the media for preparation of NGM RNAi feeding plates: 25 mL of 1 M potassium phosphate buffer (pH = 6.0), 1 mL of 1 M MgSO4, 1 mL of 1 M CaCl2, 1 mL of (5 mg/mL in 95% ethanol) cholesterol, 1 mL of (10% v/w in ethanol) nystatin, 1 mL of 50 mg/mL carbenicillin, and 1 mL of IM IPTG.
- Pour the media into 60 mm x 15 mm sterile Petri dishes under laminar flow. Allow the media to dry for 2 h under the laminar hood. Subsequently, plates can be stored at 4 °C for 1 month.
3. Maintenance of C. elegans
- Seed the NGM plates by spotting 50 µL of overnight grown E. coli OP50 in the center of the plates. The E. coli culture is prepared previously in Luria-Bertani (LB) media and stored at 4 °C for several months. Cover the plates and allow them to dry for 24 h under a laminar hood and thereafter store the plates in polystyrene container.
- Under a dissecting microscope, pick up 10 to 12 gravid adults using a sterile worm pick and transfer the worms to an E. coli OP50 seeded NGM plate. Incubate the plates at 20 °C overnight.
- The next day, remove the adults using a sterile worm pick and allow the embryos to develop to L4 larvae at 20 °C (~2.5 days).
4. Preparation of Age Synchronous Population of Worms
- Wash gravid adults from two to four NGM plates using M9W and collect them in a 15 mL conical tube.
- To prepare M9W: combine 3 g of NaCl, 6 g of Na2HPO4, and 3 g of KH2PO4 and dissolve in a final volume of 1 L of deionized water. Autoclave the solution and add 1 mL of 1 M MgSO4.
- Spin the tube at 450 x g for 1 min, then decant the supernatant while ensuring that the worm pellet stays intact.
- Add 400 µL of 8.25% sodium hypochlorite (household bleach) and 100 µL of 5 N NaOH to prepare the worm lysis solution. Add the lysis solution to the worm pellet and mix the contents by flicking the tube until 70% of the adult worms are lysed. Periodically observe the contents of the tube under a dissecting microscope to ensure there is no overbleaching of the eggs.
- Dilute the bleach mix by adding 10 mL of M9W to the contents of the conical tube.
- Spin the tube at 450 x g for 1 min. Decant supernatant, then add 10 mL of M9W.
- Repeat step 4.5 two more times.
- Resuspend the resulting egg pellet in 3-5 mL of M9W. Place the tube on a tube rotator. Allow the tubes to rotate at a speed of 18 rpm at room temperature (RT) overnight.
- The next day, spin the tube at 450 x g for 1 min to pellet the L1 larvae. Remove most of M9W by aspiration, leaving behind ~250 µL of liquid in the tube. Resuspend the L1 larvae and place three 5 µL drops of the worm suspension onto a Petri dish lid, then estimate the number of worms per µL using a dissecting microscope.
5. Induction of RNAi in Worms
- Using a sterile loop or pick, streak out the desired strains of RNAi-containing E. coli from frozen stocks (Ahringer and Vidal libraries) onto 100 mm x 15 mm LB agar plates containing 50 µg/mL carbenicillin and 15 µg/mL of tetracycline. Incubate the plates at 37 °C for 24 h.
- Pick and inoculate an isolated colony from the desired RNAi strain into sterile 15 mL conical tubes containing 2 mL of LB supplemented with 50 µg/mL carbenicillin. Incubate the tubes at 37 °C for 16 h in an orbital shaker at 150 rpm.
- The next day, spread 150 µL of overnight grown culture onto 65 mm x 15 mm NGM RNAi feeding plates using a sterile spreader. Incubate the plates at 37 °C for 24 h.
- Allow the plates to cool to RT after incubation at 37 °C. Add an appropriate volume of M9W containing ~200 L1 larvae (obtained from the age synchronous population of worms steps) to the E. coli seeded NGM RNAi feeding plates. Incubate the plates at 20 °C until the larvae reach the L4 stage (~2.5 days).
6. Preparation of Mitis Group Streptococci for Infection
- Streak out desired strains of streptococci on the 100 mm x 15 mm THY agar (if plates were stored at 4 °C, pre-warm the plates to 37 °C before streaking the respective strains), then incubate the plates at 37 °C overnight (~18 h) in a candle jar providing a microaerophilic environment for the growth of the streptococci (the streaked plates can be stored for a week at 4 °C).
- To propagate clinical isolates of streptococci, use tryptic soy blood agar. Incubate the plates at 37 °C in a candle jar overnight (~18 h).
- The next day, remove the plates from the candle jar and pick isolated colonies using a sterile loop. Inoculate 15 mL sterile conical tubes containing 2 mL of THY broth. To propagate clinical isolates of streptococci, supplement the THY broth with 5% v/v of sheep blood. Close the caps tight and incubate the tubes at 37 °C under static conditions.
7. Survival Assays
NOTE: The steps involved in this assay are depicted in Figure 1. To demonstrate that the H2O2 derived by the mitis group is responsible for the killing of the worm, supplement the media with catalase, or the mutant strain ΔspxB and complement strain ΔspxB;spxB+ of S. gordonii can be used. SpxB encodes for a pyruvate oxidase, which is responsible for the production of H2O2 in the mitis group.
- Pre-warm 35 mm x 10 mm THY plates to 37 °C. Add 80 µL of overnight grown cultures of the desired strains of streptococci and spread the bacteria completely across the agar surface using a sterile spreader. Incubate the plates at 37 °C in a candle jar overnight (~18 h). As a control, seed two 35 mm x 10 mm NGM plates with 80 µL of overnight grown cultures of E. coli OP50. Incubate the plates at 37 °C overnight.
- To confirm that the H2O2 produced by the mitis group is responsible for killing of the worms, add 50 µL containing 1,000 units of catalase c onto the THY plate. Spread the catalase solution using a sterile spreader and allow the plates to dry in the laminar flow for 30 min. Seed the plates thereafter with the respective streptococcus strains as described in step 7.1.
- The next day, remove the plates from the candle jar and allow the plates to cool to RT for 10-15 min. Using a sterile worm pick, transfer 30 L4 larvae from the NGM or NGM RNAi feeding plates to the streptococcus seeded THY plates. Use two seeded THY plates with a total of 60 worms per strain of streptococcus. Incubate the plates at 25 °C.
- Using a dissecting microscope, count the number of live and dead L4 larvae on each plate at several timepoints. Initially, score the worms as dead or live every 30 min. Thereafter, when worms rapidly start to die, score them at 15 min intervals. Use the sterile worm pick to gently prod the worms and determine if they are dead or alive. A worm is considered dead if there is no movement in response to the prodding.
- The assay will take 5-6 h to complete. Repeat the experiment two more times. After completion of the assay, pool the data from the two plates. Input the data of each group, compare the survival curves, and perform Kaplan-Meier survival analysis using statistical software.
8. Preparation of Agarose Pads for Microscopy
- Dissolve 2% w/v of agarose in deionized water by heating the solution in a microwave. A volume of 5 mL of solution is adequate to prepare 20 slides.
- Stick lab tape lengthwise along two glass slides. This will determine the thickness of the agarose pads. Place a clean glass slide between the two taped slides.
- Place 100 µL of molten agarose on the center of the clean slide. Immediately place another clean glass on top of the molten agarose and gently press down to make a pad. Allow the agarose to solidify and subsequently remove the top slide. The agarose pad is ready for use.
9. Observation of SKN-1 Localization in Response to Streptococcus Infection
NOTE: The steps involved in this assay are depicted in Figure 2. Localization of SKN-1 was determined using the SKN-1B/C::GFP transgenic worm strain. To demonstrate localization of SKN-1 due to the production of H2O2 by the mitis group, wild-type (WT), ΔspxB, and the complement strain ΔspxB;spxB+ of S. gordonii were used. Furthermore, the transgenic reporter strain SKN-1B/C::GFP and RNAi interference technique were used to demonstrate that components of the p38 MAPK pathway regulate the localization of SKN-1.
- Pre-warm 35 mm x 10 mm THY plates to 37 °C. Add 80 µL of overnight grown cultures of the desired strains of streptococcus and spread the bacteria completely across the agar surface using a sterile spreader. Incubate the plates at 37 °C in a candle jar overnight (~18 h). As a control, seed three 35 mm x 10 mm NGM plates with 80 µL of overnight grown cultures of E. coli OP50. Incubate these plates at 37 °C for ~18 h.
- The next day, remove plates from the candle jar and allow the plates to cool to RT for 10-15 min. Wash L4 larvae using M9W from NGM and NGM RNAi feeding plates. Collect the worms in 15 mL conical tubes.
- Spin the tubes at 450 x g for 1 min. Decant the supernatant and add 10 mL of M9W.
- Repeat step 9.3 three more times.
- Resuspend the worms in ~250 µL of M9W and place three 5 µL drops of the worm suspension onto a clean Petri dish lid and estimate the number of worms per µL using a dissecting microscope.
- Add ~100 L4 larvae to each THY streptococcus seeded and NGM E. coli seeded plates. Use three plates per strain of bacteria. Incubate the plates for 2 to 3 h at 25 °C.
- Thereafter, remove the plates from the incubator, wash them with M9W, and collect the worms in 15 mL conical tubes.
- Wash the worms 3x as described in steps 9.3.
- Remove most of the M9W by aspiration and add 500 µL of M9W containing 2 mM sodium azide or 2 mM tetramisole hydrochloride to the worm pellet. This will anesthetize the worms, ensuring that no movement occurs when imaged under the microscope.
CAUTION: Use personal protective equipment (PPE) when handling sodium azide. Prepare the azide solution under a chemical hood. - Incubate the worm pellets at RT for 15 min. Then, spot 15 µL of the worm suspension onto a prepared agarose pad. Gently place a no. 1.5 coverslip over the agarose pad containing the anesthetized worms.
- Using a fluorescent microscope, visualize the localization of SKN-1 utilizing FITC and DAPI filters.Image worms at 10x and 20x magnifications.
- Score the worms based on the level of localization of SKN-1. No nuclear localization, localization of SKN-1B/C::GFP in the anterior or posterior of the worm, and nuclear localization of SKN-1B/C::GFP in all intestinal cells are categorized as low, medium, and high levels of localization, respectively.
- After scoring the fluorescent micrographs, determine the statistical differences by chi- squared and Fisher's exact tests using statistical software.
Representative Results
Members of the mitis group S. mitis, S. oralis, and S. gordonii rapidly killed the worms, as opposed to S. mutans, S. salivarius, and non-pathogenic E. coli OP50 (Figure 3A). The median survival for S. mitis, S. oralis, and S. gordonii was 300 min, 300 min, and 345 min, respectively. To determine if the killing was mediated by H2O2, catalase was supplemented to THY agar. The killing of the worms was abolished in the presence of catalase (Figure 3B). To further confirm whether streptococcal derived H2O2 mediated killing of the worms, survival on the ΔspxB mutant strain, WT strain, and complement strain ΔspxB;spxB+ of S. gordonii was analyzed. Death of the worms was not observed on the ΔspxB mutant strain compared to the wild-type and complement strains (Figure 3C). These data suggest that the H2O2 produced by the mitis group mediates killing of the worms. We also observed similar killing kinetics when the worms were exposed to clinical isolates of the mitis group streptococci obtained from the blood of cancer patients (Figure 3D). Based on the data, the pathogenicity caused by H2O2 produced by the mitis group streptococci was assessed.
To identify host genes that are essential against streptococcal infections, skn-1 was knocked down, which encodes for the oxidative stress response transcription factor in C. elegans. Then, survival relative to the vector control treated worms was compared. A significant decrease in the survival of the skn-1 knockdown worms was observed compared to the vector control treated worms (Figure 4A). This data was further validated using a skn-1 mutant strain, and its survival was compared to that of the N2 wild-type worms. We observed a similar killing phenotype as the skn-1 mutant, as seen with the skn-1 knockdown, demonstrating that SKN-1 influenced the survival of the worms on the mitis group (Figure 4B).
Next, it was determined whether the H2O2 produced by the mitis group caused localization of SKN-1B/C::GFP in the worms. Localization of SKN-1B/C::GFP was observed in worms exposed to the wild-type and complement stains and not in response to the ΔspxB mutant strain of S. gordonii (Figure 5A,B). Furthermore, to determine the activation of SKN-1, components of the p38 MAPK pathway were knocked down. Reduced localization of SKN-1B/C::GFP in nsy-1, sek-1, pmk-1, and skn-1 knockdown worms relative to the vector control treated worms was observed. The data suggests the p38 MAPK is required for the activation of SKN-1 in response to H2O2 produced by the mitis group (Figure 5C,D).
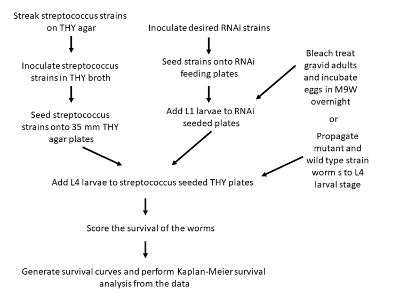
Figure 1: Flowchart depicting the steps involved in preparation of the survival assays. Please click here to view a larger version of this figure.
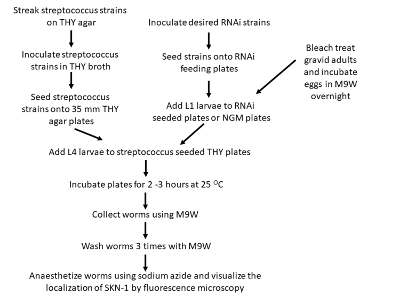
Figure 2: Flowchart depicting the steps involved in localization of SKN-1 during infection. Please click here to view a larger version of this figure.
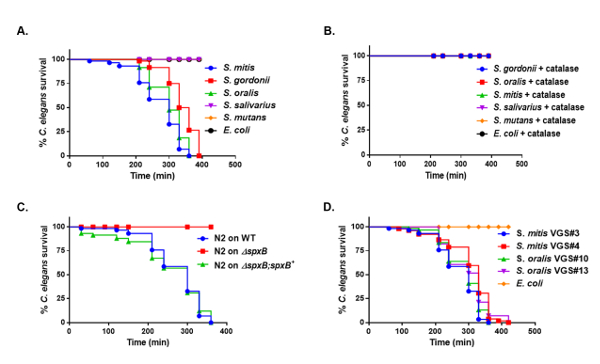
Figure 3: H2O2-mediated killing of C. elegans by mitis group streptococci. Kaplan-Meier survival curves of L4 larvae exposed to (A) S. gordonii, S. oralis, S. mitis, S. salivarius, S. mutans, and E. coli OP50. (B) S. gordonii, S. oralis, S. mitis, S. salivarius, S. mutans, and E. coli OP50 on THY plates in the presence of 1,000 U of catalase. (C) S. gordonii WT, ΔspxB mutant, and ΔspxB;spxB+ complement strains on N2 L4 larvae. (D) S. oralis (VGS#3), S. oralis (VGS#4), S. mitis (VGS#10), S. mitis (VGS#13), and E. coli OP50. The data are representative of experiments repeated two or more times, with n = 60 worms for each condition. Kaplan-Meier log rank analysis was used to compare survival curves and calculate the median survival. P values < 0.05 were considered to be statistically significant. This figure has been modified and adapted with permission15. Please click here to view a larger version of this figure.
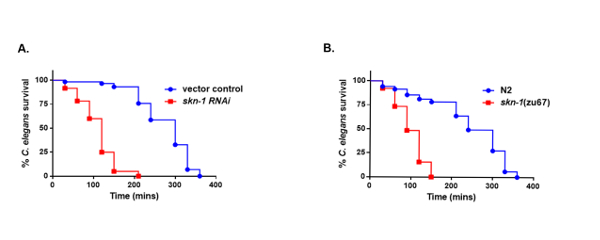
Figure 4: SKN-1 is required for survival of the worms on S. gordonii. (A) Survival of vector control treated and skn-1 knockdown worms exposed to S. gordonii. (B) Survival of N2 and skn-1(zu67) mutant worms fed on S. gordonii. The data are representative of experiments repeated two or more times, with n = 60 worms for each condition. Kaplan-Meier log rank analysis was used to compare survival curves and calculate the median survival. P values < 0.05 were considered to be statistically significant. This figure has been modified and adapted with permission15. Please click here to view a larger version of this figure.
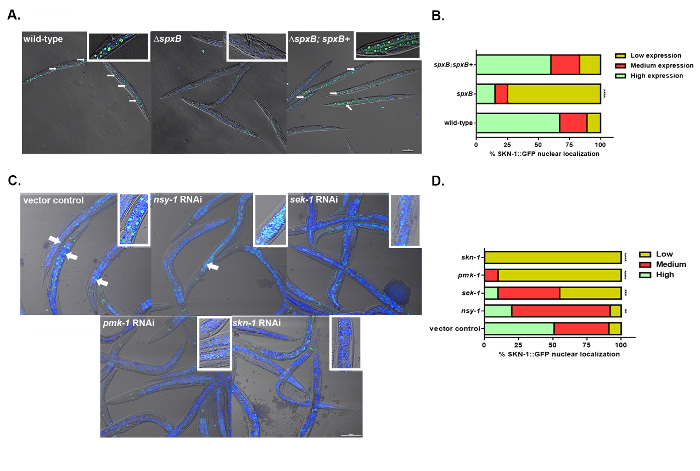
Figure 5: Streptococcal H2O2 mediated activation of SKN-1 is dependent on the p38 MAPK pathway. (A) Representative images of the localization of SKN-1B/C::GFP in worms exposed to the WT, ΔspxB mutant, and ΔspxB;spxB+ complement strains of S. gordonii. Closeups are shown in the upper righthand corners of each image. Scale bar = 100 µm. (B) The degree of nuclear localization of SKN-1B/C::GFP and percentage of worms in each category fed on WT, ΔspxB mutant, and ΔspxB;spxB+ complement strains of S. gordonii. Significantly low levels of nuclear localization of SKN-1B/C::GFP were observed in the ΔspxB mutant (p < 0.0001) compared to the WT and ΔspxB;spxB+ complement strains of S. gordonii. (C) Representative images of the localization of SKN-1B/C::GFP in nsy-1, sek-1, pmk-1, skn-1 knockdown, and vector control treated worms on S. gordonii. Closeups are shown in the upper righthand corners of each image. Scale bar = 100 µm. (D) The degree of SKN-1B/C::GFP nuclear localization and percentage of worms in each category fed on nsy-1, sek-1, pmk-1, skn-1 knockdown, and vector control treated worms on S. gordonii. Significantly low levels of nuclear localization of SKN-1B/C::GFP were observed in the nsy-1 (p < 0.01), sek-1 (p < 0.001), pmk-1 (p < 0.0001),and skn-1 knockdown (p < 0.0001) compared to the vector control treated worms on S. gordonii. Greater than 100 worms exposed to each strain were imaged, and the experiment was repeated three times. This figure has been modified and adapted with permission15. Please click here to view a larger version of this figure.
Discussion
The methods described can be used for other pathogenic bacteria such as Enterococcus faecium, which also produces H2O2 grown under anaerobic or microaerophilic conditions26. Typically, for most pathogenic organisms, it takes several days to weeks to complete the survival assays. However, due to the robust production of H2O2 by members of the mitis group, these assays could be completed within 5-6 h under the conditions described. This ensures the capability to screen several gene candidates involved in host immunity and the oxidative stress response over a short time period.
In this protocol, H2O2 produced by the bacteria is in direct contact with the intestinal cells of the worm, as opposed to other exogenous ROS sources that must cross several barriers. This ensures that the H2O2 is delivered to the intestinal cells; hence, a more robust killing response is observed in the worm. Using fluorescently labeled bacteria, it was determined that the worms must be exposed to pathogens for 30 min for complete colonization of the intestinal tract15. It is advised to use less than 1 week old streak plates of streptococcal strains to ensure their viability and ability to produce H2O2. In addition, the streptococcal strains must be grown under microaerophilic conditions for optimal production of H2O2. L4 larvae or 1 day old adults can be used for this assay. It is critical that all worms used in an experiment are the same age and sex. Younger worms tend to die more slowly compared to older hermaphrodites. L4 animals are more easily distinguished because their developing vulva is visible at mid-body as a clear patch that contrasts with the rest of the body. It is also important that no E. coli OP50 are transferred to the streptococcus seeded plates. Contamination of killing plates with E. coli can cause the attenuation or abrogation of killing of the worms. To avoid contamination, it is necessary to pick worms away from the E. coli lawn. When scoring the survival assays, it is advised to observe the pharyngeal pumping, foraging behavior of the head, and body movement. To ensure that the worm is dead, it is recommended to gently prod the nose, side of the body, or tail and observe any movement.
RNAi feeding and the survival of the worms on the mitis group was combined to identify candidates that are involved in the defense against H2O2. Using the gene skn-1 that encodes for an oxidative stress response transcription factor, its requirement for survival of the worm in response to H2O2 is demonstrated here. Hence, this assay can be adapted to screen for several genes and identify potential candidates required for oxidative stress response and immunity. RNAi feeding of the worms is achieved by adding age synchronized L1 larvae to the RNAi expressing E. coli lawns. During the worm synchronization protocol, it is essential to monitor lysis of the worm cuticles in the presence of bleach and sodium hydroxide. The cuticle of adults and larvae will continually dissolve, while the embryos are partially protected by the thick eggshell. However, prolonged incubation in the presence of the blead sodium hydroxide mix may cause the embryos to die. Therefore, it is important to observe the tube containing the worms under a dissecting microscope periodically during bleaching. Another step to consider in the synchronization protocol is the maintenance of arrested L1 larvae. The arrested larvae can be maintained on the tube rotator for 5 days at room temperature. It is recommended to use the larvae for RNAi feeding within 1 to 2 days after hatching. Prolonged maintenance in M9W can result in the formation of the dauer stage.
Lastly, a transgenic worm expressing SKN-1 fused to GFP was used to monitor activation of the oxidative stress response in the presence of the mitis group. It is shown by RNAi that the components of the p38 MAPK are required for localization of SKN-1B/C::GFP to the nuclei of the intestinal cells. It is important to use the L3 or L4 stages of this strain to observe localization of SKN-1B/C::GFP, as localization of SKN-1 tends to diminish in the adult stage. To better observe the localization of SKN-1B/C::GFP, it is advised to overlap the obtained images using the FITC and DAPI filter settings. Autofluorescence generated by the lipofuscins help provide contrast for observation of SKN-1 localization in the worm. However, it has also been shown that the signal from weakly expressed GFP reporters is masked by autofluorescence emitted by various sources in the gut of the worm. Autofluorescence has been shown to increase with age and is highest in the intestine and uterus of the C. elegans27. To overcome this problem, a recent study utilized a triple band GFP filter setup to monitor the localization of SKN-1B/C::GFP in C. elegans28. This setup separates the GFP signal from autofluoresence, displaying the GFP and autofluoresence in the green and yellow channels, respectively.
C. elegans is used in this protocol to study host-pathogen interactions and ascertain how H2O2 produced by the mitis group causes pathogenicity. More importantly, by using this model, the effects of H2O2 on endoplasmic reticular stress, mitochondrial damage, mitophagy, autophagy, and oxidative stress can be studied. Furthermore, mechanisms by which H2O2 acts as a virulence factor to elicit immune responses by disrupting core processes of the cell can be identified. Hence, this worm is recognized as a powerful model system for discovering new insights into host-pathogen interactions.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Bing-Yan Wang, Dr. Gena Tribble (The University of Texas, School of Dentistry), Dr. Richard Lamont (University of Louisville, School of Dentistry), and Dr. Samuel Shelburne (MD Anderson Cancer Center) for providing laboratory and clinical strains of the mitis group streptococci. We also thank Dr. Keith Blackwell (Department of Genetics, Harvard Medical School) for the C. elegans strains. Finally, we thank Dr. Danielle Garsin and her lab (The University of Texas, McGovern Medical School) for providing reagents and worm strains to conduct the study. Some worm strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Materials
| Media and chemicals | |||
| Agarose | Sigma Aldrich | A9539-50G | |
| Bacto peptone | Fisher Scientific | DF0118-17-0 | |
| BD Bacto Todd Hewitt Broth | Fisher Scientific | DF0492-17-6 | |
| BD BBL Sheep Blood, Defibrinated | Fisher Scientific | B11947 | |
| BD Difco Agar | Fisher Scientific | DF0145-17-0 | |
| BD Difco LB Broth | Fisher Scientific | DF0446-17-3 | |
| Blood agar (TSA with Sheep Blood) | Fisher Scientific | R01200 | |
| Calcium Chloride | Fisher Scientific | BP510-500 | |
| Carbenicillin | Fisher Scientific | BP26481 | |
| Catalase | Sigma Aldrich | C1345-1G | |
| Cholesterol | Fisher Scientific | ICN10138201 | |
| IPTG | Fisher Scientific | MP21021012 | |
| Magnesium sulfate | Fisher Scientific | BP213-1 | |
| Nystatin | Acros organics | AC455500050 | |
| Potassium Phosphate Dibasic | Fisher Scientific | BP363-500 | |
| Potassium phosphate monobasic | Fisher Scientific | BP362-500 | |
| Sodium Azide | Sigma Aldrich | S2002-25G | |
| Sodium chloride | Fisher Scientific | BP358-1 | |
| Sodium Hydroxide | Fisher Scientific | SS266-1 | |
| 8.25% Sodium Hypochlorite | |||
| Sodium Phosphate Dibasic | Fisher Scientific | BP332-500 | |
| Streptomycin Sulfate | Fisher Scientific | BP910-50 | |
| Tetracyclin | Sigma Aldrich | 87128-25G | |
| (−)-Tetramisole hydrochloride | Sigma Aldrich | L9756 | |
| Yeast extract | Fisher Scientific | BP1422-500 | |
| Consumables | |||
| 15mL Conical Sterile Polypropylene Centrifuge Tubes | Fisher Scientific | 12-565-269 | |
| Disposable Polystyrene Serological Pipettes 10mL | Fisher Scientific | 07-200-574 | |
| Disposable Polystyrene Serological Pipettes 25mL | Fisher Scientific | 07-200-575 | |
| Falcon Bacteriological Petri Dishes with Lid (35 x 10 mm) | Fisher Scientific | 08-757-100A | |
| No. 1.5 18 mm X 18 mm Cover Slips | Fisher Scientific | 12-541A | |
| Petri Dish with Clear Lid (60 x 15 mm) | Fisher Scientific | FB0875713A | |
| Petri Dishes with Clear Lid (100X15mm) | Fisher Scientific | FB0875712 | |
| Plain Glass Microscope Slides (75 x 25 mm) | Fisher Scientific | 12-544-4 | |
| Software | |||
| Prism | Graphpad | ||
| Bacterial Strains | |||
| S. oralis ATCC 35037 | |||
| S. mitis ATCC 49456 | |||
| S. gordonii DL1 Challis | |||
| E. coli OP50 | |||
| E. coli HT115 | |||
| Worm Strains | |||
| Strain | Genotype | Transgene | Source |
| N2 | C. elegans wild isolate | CGC | |
| EU1 | skn-1(zu67) IV/nT1 [unc-?(n754) let-?] (IV;V) | CGC | |
| LD002 | IdIs1 | SKN-1B/C::GFP + rol-6(su1006) | Keith Blackwell |
References
- Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature. 486 (7402), 207-214 (2012).
- Mitchell, J. Streptococcus mitis: walking the line between commensalism and pathogenesis. Molecular Oral Microbiology. 26 (2), 89-98 (2011).
- Dyson, C., Barnes, R. A., Harrison, G. A. Infective endocarditis: an epidemiological review of 128 episodes. Journal of Infection. 38 (2), 87-93 (1999).
- Sahasrabhojane, P., et al. Species-level assessment of the molecular basis of fluoroquinolone resistance among viridans group streptococci causing bacteraemia in cancer patients. International Journal of Antimicrobial Agents. 43 (6), 558-562 (2014).
- Shelburne, S. A., et al. Streptococcus mitis strains causing severe clinical disease in cancer patients. Emerging Infectious Diseases. 20 (5), 762-771 (2014).
- van der Meer, J. T., et al. Distribution, antibiotic susceptibility and tolerance of bacterial isolates in culture-positive cases of endocarditis in The Netherlands. European Journal of Clinical Microbiology & Infectious Diseases. 10 (9), 728-734 (1991).
- Han, X. Y., Kamana, M., Rolston, K. V. Viridans streptococci isolated by culture from blood of cancer patients: clinical and microbiologic analysis of 50 cases. Journal of Clinical Microbiology. 44 (1), 160-165 (2006).
- Hoshino, T., Fujiwara, T., Kilian, M. Use of phylogenetic and phenotypic analyses to identify nonhemolytic streptococci isolated from bacteremic patients. Journal of Clinical Microbiology. 43 (12), 6073-6085 (2005).
- Kohno, K., et al. Infectious complications in patients receiving autologous CD34-selected hematopoietic stem cell transplantation for severe autoimmune diseases. Transplant Infectious Disease. 11 (4), 318-323 (2009).
- Zhu, L., Kreth, J. The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxidative Medicine and Cellular Longevity. , 717843 (2012).
- Okahashi, N., et al. Hydrogen peroxide contributes to the epithelial cell death induced by the oral mitis group of streptococci. PLoS One. 9 (1), 88136 (2014).
- Stinson, M. W., Alder, S., Kumar, S. Invasion and killing of human endothelial cells by viridans group streptococci. Infection and Immunity. 71 (5), 2365-2372 (2003).
- Rai, P., et al. Streptococcus pneumoniae secretes hydrogen peroxide leading to DNA damage and apoptosis in lung cells. Proceedings of the National Academy of Sciences of the United States of America. 112 (26), 3421-3430 (2015).
- Braun, J. S., et al. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. Journal of Clinical Investigation. 109 (2), 19-27 (2002).
- Naji, A., et al. The activation of the oxidative stress response transcription factor SKN-1 in Caenorhabditis elegans by mitis group streptococci. PLoS One. 13 (8), 0202233 (2018).
- Bolm, M., Jansen, W. T., Schnabel, R., Chhatwal, G. S. Hydrogen peroxide-mediated killing of Caenorhabditis elegans: a common feature of different streptococcal species. Infection and Immunity. 72 (2), 1192-1194 (2004).
- Sifri, C. D., Begun, J., Ausubel, F. M. The worm has turned–microbial virulence modeled in Caenorhabditis elegans. Trends in Microbiology. 13 (3), 119-127 (2005).
- Irazoqui, J. E., Ausubel, F. M. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Caenorhabditis elegans as a model to study tissues involved in host immunity and microbial pathogenesis. Clinical & Experimental Immunology. 160 (1), 48-57 (2010).
- Van Raamsdonk, J. M., Hekimi, S. Reactive Oxygen Species and Aging in Caenorhabditis elegans: Causal or Casual Relationship. Antioxidants & Redox Signaling. 13 (12), 1911-1953 (2010).
- Tissenbaum, H. A. Using C. elegans for aging research. Invertebrate Reproduction & Development. 59, 59-63 (2015).
- Blackwell, T. K., Steinbaugh, M. J., Hourihan, J. M., Ewald, C. Y., Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radical Biology & Medicine. 88, 290-301 (2015).
- Irazoqui, J. E., Urbach, J. M., Ausubel, F. M. Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nature Reviews Immunology. 10 (1), 47-58 (2010).
- Park, S. K., Tedesco, P. M., Johnson, T. E. Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell. 8 (3), 258-269 (2009).
- An, J. H., et al. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proceedings of the National Academy of Sciences of the United States of America. 102 (45), 16275-16280 (2005).
- An, J. H., Blackwell, T. K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes & Development. 17 (15), 1882-1893 (2003).
- Moy, T. I., Mylonakis, E., Calderwood, S. B., Ausubel, F. M. Cytotoxicity of hydrogen peroxide produced by Enterococcus faecium. Infection and Immunity. 72 (8), 4512-4520 (2004).
- Pincus, Z., Mazer, T. C., Slack, F. J. Autofluorescence as a measure of senescence in C. elegans: look to red, not blue or green. Aging (Albany NY). 8 (5), 889-898 (2016).
- Teuscher, A. C., Ewald, C. Y. Overcoming Autofluorescence to Assess GFP Expression During Normal Physiology and Aging in Caenorhabditis elegans. Bio-protocol. 8 (14), (2018).

