Material Formation of Recombinant Spider Silks through Aqueous Solvation using Heat and Pressure
Summary
Here, we present a protocol to produce water soluble recombinant spider silk protein solutions and the material forms that can be formed from those solutions.
Abstract
Many spiders produce seven types of silks. Six of the silks are fiber in form when produced by the spiders. These fibers are not water soluble. In order to reproduce the remarkable mechanical properties of spider silks, they must be produced in heterologous hosts as spiders are both territorial and cannibalistic. The synthetic analogs of spider silk also tend to be insoluble in aqueous solutions. Thus, a large percentage of research in recombinant spider silks rely upon organic solvents that are detrimental to large scale production of materials. Our group’s method forces the solvation of these recombinant spider silks into water. Remarkably, when these proteins are prepared using this method of heat and pressure, a wide range of material forms can be prepared from the same solution of recombinant spider silk proteins (rSSp) including: films, fibers, sponge, hydrogel, lyogel, and adhesives. This article demonstrates the production of the solvated rSSp and material forms in a manner that is more easily understood than from written materials and methods alone.
Introduction
Spider silks have garnered the interest of material scientists for their impressive combination of strength, elasticity, and biocompatibility. Recreating fibers has traditionally been the thrust of the research. This effort was hampered by the recombinant spider silk protein (rSSp) insolubility in water as well as the inability of traditional solvation techniques (chaotropic agents and detergents) to achieve aqueous solvation. Further, techniques that have been developed for solvating versions of rSSp do not work on all rSSp variants and also require substantial manipulation and time that often results in protein loss1,2. This has largely resulted in the field utilizing 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) as a solvent from which to form fibers, and other limited material forms. The advantage being that all known rSSp are soluble in HFIP, providing data uniformity between each research group. The disadvantage is that HFIP is a toxic solvent that is expensive and impractical to scale due to health concerns and environmental considerations.
A novel approach to rSSp solvation was developed that bridged the technological gap between the harsh organic solvent HFIP and other techniques that selectively worked for rSSp solvation. The combination of specific heats and pressures was applied to suspensions of rSSp and water. The results were near 100% solvation and recovery of the rSSp, as well as high protein concentrations; a variety of materials forms were determined to be possible from these formulations that were not all achievable using HFIP or other organic solvents3,4,5,6. The objective of this approach is to efficiently and easily solubilize purified and dried recombinant spider proteins in an aqueous solution that can then be utilized for the production of a variety of material forms.
Fibers, films, coatings, adhesives, hydrogels, lyogels, microspheres, and sponge materials are all readily accomplishable from the same aqueous rSSp solution using this method. The continued evolution of this method, not only with additional rSSp but with other proteins, could lead to new material forms and alternative protein purification and solubilization avenues.
Protocol
1. Recombinant spider silk mixture preparation from lyophilized protein stocks
- Determine the necessary formulation and volume required for the intended material formations. Typical formulations range from 3% (w/v) up to 15% (w/v). Using this selection, calculate the appropriate rSSp, concentrations, and ratios.
- Use the following respective formulations to prepare each material described in this protocol: hydrogels/sponges/lyogels, 6% (w/v) 50:50 MaSp1:MaSp2; films/coatings, 5% (w/v) 80:20 MaSp1:MaSp2; adhesives, 12% (w/v) 50:50 MaSp1:MaSp2; fibers, 12.5% (w/v) 80:20 MaSp1:MaSp2.
NOTE: Even though most formulations are better fitted for specific forms and materials, there is a wide range of formulations that can often overlap. Additionally, the final rSSp materials can also be tailored during formation and processing to produce the desired properties. Generally, each protein will require investigation into appropriate or useful parameters.
- Use the following respective formulations to prepare each material described in this protocol: hydrogels/sponges/lyogels, 6% (w/v) 50:50 MaSp1:MaSp2; films/coatings, 5% (w/v) 80:20 MaSp1:MaSp2; adhesives, 12% (w/v) 50:50 MaSp1:MaSp2; fibers, 12.5% (w/v) 80:20 MaSp1:MaSp2.
- Select a clean and new 8 mL autoclavable borosilicate glass culture vial with a rubber lined screw cap.
- Remove the cap and place the empty vial on an analytical balance. Tare the mass of the empty vial so that the balance reads zero mass.
- Add the desired lyophilized rSSp powder to the empty vial for each specific material.
- Use these specific masses of each protein type for each material, when preparing a 2 mL of solution: hydrogels/sponges/lyogels, 60 mg of MaSp1 and 60 mg of MaSp2; films/coatings, 80 mg of MaSp1 and 20 mg of MaSp2; adhesives, 120 mg of MaSp1 and 120 mg of MaSp2; fibers, 200 mg of MaSp1 and 50 mg of MaSp2.
- Add the desired amount of ultrapure water, at least 2 mL, to the vial that already contains the weighted rSSp powders.
NOTE: A minimal volume of 2 mL is recommended for all solvation procedures. - Seal the vial cap and briskly vortex the contents to create a dispersed, and homogenous, rSSp mixture that is now ready for the solvation procedure. Additional homogenization approaches such as sonication or impeller mixing can be employed with, or in addition, the vortex mixing.
2. Recombinant spider silk solvation
CAUTION: High heats and pressures are generated during the solvation procedure. Proper personal protective equipment, especially goggles, long sleeves, and heat resistant gloves are required for this process.
- Perform a final check of the vial, or vessel, cap to ensure that it has been firmly and securely tightened. Then transfer the suspended rSSp mixture to a conventional microwave oven.
NOTE: Microwave units within the power range of 700 to 1,500 Watts, possessing smaller internal chamber capacities, and rotating platforms are recommended to provide better solvation conditions. - Begin operation of the microwave with 5 s bursts at full power. After each burst briefly open the door and carefully mix/swirl the vial to prevent settling and maintain the suspended mixture.
- Repeat this microwave process until the mixture and/or solution has obtained a temperature of at least 130 °C, when measured with an infrared thermometer directly against the solution containing portions of the vial. Repeat this process until all of the solid particulates have been completely dissolved and are no longer visible.
NOTE: It is suggested to allow the vial and solution to cool occasionally, especially if the formulation has a high concentration of rSSp present. Temperatures exceeding 200 °C increase the risk of vial seal failure. Special attention must also be given to prevent the superheated mixture/solution from touching the seal, which will also result in vial containment failure. - After successfully solvating the rSSp mixture into a solution allow the temperature of the solution and the vial cap to cool below 100 °C (boiling point) before opening.
3. Hydrogels
- Prepare a hydrogel from the solution after removing it from the microwave and allowing it to cool and set. Cast the hydrogel into specific geometries prior to allowing it to fully cool.
NOTE: Different rSSps will require varied amounts of times to transition to a hydrogel. For example, MaSp2-like sequences tend to form hydrogels more rapidly in comparison to MaSp1-like sequences. Protein concentrations, salinity, and pH also directly affect the rate of transition to a hydrogel.
4. Sponges
- Prepare a rSSp sponges by first allowing the primary solvated solution to form a hydrogel.
- Place the hydrogel in a water bath, place this bath in the freezer at -20 °C, and wait until the bath is frozen completely.
- Complete the sponge formation process by removing the frozen hydrogel and water bath from the freezer and thawing at 25 °C. The resultant sponge can now be removed from the thawed water.
5. Lyogel
- Prepare a rSSp lyogel by directly freezing a formed hydrogel, either with or without a water bath, and transferring the frozen hydrogel sample to a lyophilizer (freeze dryer).
- Remove the final lyophilized gel material from the vessel that the moisture sublimation occurred in.
6. Films and coatings
- Use one of the three following methods: solution casting, solution spraying, or dip coating to produce films or coatings of rSSp.
- Cast the solubilized silk solution into/onto PDMS forms of the desired shape.
- Pour and spread 200 µL of the film solution and allow this to dry before peeling them off of the PDMS substrate for testing or treatment.
- After allowing these to dry, remove the formed films for mechanical testing or post-treat the films to improve the mechanical properties.
- To prepare a coating, or a film that cannot be removed from the substrate, use either spray or dip coating to produce a thin film layer.
NOTE: To spray coat, this protocol has found success with a master airbrush model paint sprayer.- Form a dip coating by simply submerging the substrate of choice into the solubilized rSSp and repeat after drying to achieve the desired thickness.
- Perform an initial spray coat before applying a dip coat to increase the consistency and effectiveness of the final coating.
7. Adhesives
NOTE: The formation of adhesives is achieved through one of the following methods.
- Directly add the solubilized rSSp onto a substrate and then apply a second substrate over the top of the solution. Firmly clamp the pieces together and then dry the samples in an oven with a minimal temperature of 25 °C for at least 16 h.
- Alternatively, spray the two substrate surfaces with a spray coating and then clamp the substrates together.
- Applying the rSSp through the dip method of coating the substrates and sticking the substrates can also be used to prepare and adhesive.
8. Wet-Spun fibers
- Load the solubilized dope solution into a concentric syringe with Luer-Lok tip through a 19 G glide needle. Eject air bubbles and let the dope sit at the Luer-Lok end of the syringe.
- Insert at least 25 mm of PEEK tubing, internal diameter 0.01 inch, into the PEEK tubing’s one-piece finger tight Fittings for 1/16 inch OD and 10/32 cone. Attach this fitting to a PEEK tubing to Luer-Lok female adapter.
- Replace the 19 gauge needle with this set up on the loaded syringe.
- Fill a tall, clear glass bath with 99% pure isopropanol to use for coagulation bath.
- Fill the stretch baths, located below the stretch godets. These will have 80:20 isopropanol: distilled water in the first stretch bath, and 20:80 isopropanol: distilled water in the second stretch bath.
- Set the godet stretch system such that the first godet after the coagulation bath and the first godet in the first stretch bath are rotating at the same speed.
- Initiate the first stretch by adjusting the speeds of the final godet in stretch bath 1, the middle upper godet, and the first godet in stretch bath 2 to the same speed. This speed will be 2x as fast as the initial fiber removal speed.
- Initiate the second stretch by adjusting the speeds of the final godet in stretch bath 2, the last upper godet, and the winder to the same speed. This speed will be 2x as fast as the speed used for the first stretch or 4x the initial fibers removal speed.
- Place nitrile gloves on the outside of the intermediate godets to keep the fiber from slipping.
- Start to slowly extrude the solution into the coagulation bath. In an automated system set the extrusion rate to match a removal speed of 10 mm/s.
- Allow fiber extrusion to become uniform before pulling the fibers out of the bath using with a thin metal hook or forceps. Verify removing the fiber from the bath created a loop between the PEEK tubing tip and path the fiber leaving the bath the bath.
- Guide the retrieved fiber through the series of godets such that the fiber is submerged in the stretch baths but drying in the air between the stretch baths and before going onto a spool. This drying is achieved by the higher placed intermediate godets.
NOTE: The fiber removal rate and/or extrusion rate will need to be adjusted based upon the protein concentration, additives, and protein type to allow ample coagulation time without pooling fibers on the bottom of the coagulation bath. - Attach the fully stretched fiber to the spool on the winder mechanism using tape.
Representative Results
From the described method of solubilization of rSSp, a variety of material forms can be achieved as seen in Figure 1. The method of solubilization is to apply heat and pressure, generated by a conventional microwave, to a suspension of rSSp and water. When critical temperatures and pressures are achieved, the protein will solubilize. From this solubilized rSSp solution, the required conditions are presented for seven material forms: hydrogels, lyogels, sponge, adhesives, coatings, films, and fibers. Hydrogels are prepared by allowing the solubilized rSSp to cool and naturally self-associate. A lyogel is prepared by lyophilizing the hydrogel. Sponge material is formed by freezing the hydrogel while it is immersed in water. Films can be prepared by casting the solubilized rSSp onto PDMS surfaces (and other amenable surfaces) and dried. The PDMS allows the film to be easily removed for post treatments or analysis. Coatings and adhesives are generated using either spray or dip methods or combinations of spray and dip. Fibers require the most extensive processing by extruding into a coagulation bath and then serially stretching the raw fiber in post-spin stretch baths. Fibers can be generated by extruding into a coagulation bath alone. However, the best mechanical ability in the fibers requires stretching in post-spin stretch baths3,7,8,9.
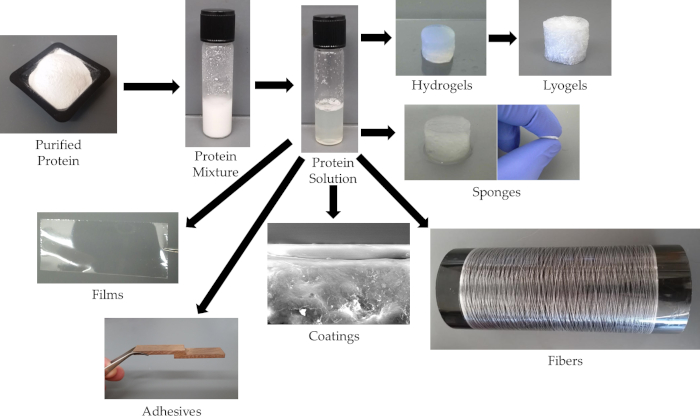
Figure 1: Aqueous solvation and rSSp materials. Representative pictures of the materials that have been formulated using this solubilization method of heat and pressure with rSSp solvated in water. Please click here to view a larger version of this figure.
Discussion
After recombinant spider silk proteins are purified they have to then be prepared in a solution that can be used for material formation. By mixing lyophilized spider silk protein with water and exposing this mixture to microwave irradiation, to generate heat and pressure, it possible to prepare a rSSp solution. A wide variety of material forms can be produced from this simple and efficient method of rSSp solubilization. Each material has to be uniquely prepared and processed to achieve the desired outcome and properties. With minor alterations to the initial formulations, formation conditions, and/or processing parameters, each material can be easily adjusted using this method. There are more forms than are presented here and through further investigations by others in the field, these materials will continue to evolve to explore new material forms using this technique.
Provided that the solution is comprised of primarily water and protein (additives can be utilized to delay gelation and improve stability of the solutions) the possibility for functionalization with biologically active components is greatly improved in comparison to HFIP based rSSp solutions. A variety of components, but not exhaustive samplings have been included in the dopes and thus material forms including: antibiotics, antimycotics, heparin, silver nanoparticles, and integrins for cell adhesion. In addition to additives, multiple recombinant spider silk proteins of various sizes, sequences, natures, and sources have been successfully solvated with this method and used in the formation of materials described in this protocol.
Further expanding the usefulness of this method of solubilization for not just rSSp but all proteins solvated in this method, is that the solutions are sterile provided the temperature and pressures inside the vial or chamber are sufficiently high. These solutions can be and have been taken directly to cell culture without contaminating the cultures.
If these materials are to be taken directly into in vivo systems, endotoxin levels must be addressed. A triple autoclave method that destroys endotoxins so that their levels are at, or below, the recommended 0.25 EU/mL has recently been reported10. While the autoclave is useful for destroying the endotoxin, its pressures and temperatures usually fail to reach the critical temperature or pressure required to completely solvate all of the rSSp samples attempted to date6. This necessitates microwaving or a temperature/pressure reactor necessary to complete the solvation.
Uniquely, removal of endotoxin and solvation of the material using heat and pressure does not degrade the protein or the mechanical ability of the resultant material form4,5,6,7. It is appreciated that there likely is a tipping point of obtaining too high of pressure and/or temperature and too many cycles of heat and pressure that results in degraded mechanical ability and/or destruction of the protein. This tipping point will likely vary for the type of rSSp solvated and, to some degree, the length of the rSSp utilized. However, with this basic method of solvation, several scouting solvation experiments can be performed in short order to delineate appropriate solvation temperature and pressures required for specific proteins.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to gratefully acknowledge funding from the Utah Science and Technology Research (USTAR) initiative.
Materials
| 3 mL Syringe with Luer-Lok Tip | BD | 309657 | Other size syringes can be used but to keep the tips on, it is advised to use luer-lok tips |
| 4 mL culture vial, clear with rubber lined cap | Wheaton | 225142 | Minimum dope volume is 1mL, max is 2mL |
| 8 mL culture vial, clear with rubber lined cap | Wheaton | 225144 | Minimum dope volume is 2mL, max is 4mL |
| 99% Isopropyl Alcohol, Reagent ACS/USP Grade | Pharmco-Aaper | 231000099 | |
| Freezone 4.5 Plus | Labconco | 7386030 | Freeze Dryer |
| Luer Adapter Female Luer x 10-32 Female, Tefzel (ETFE) | IDEX | P-629 | |
| Microwave | Magic Chef | HMD1110B | 120V, 60Hz AC; 1000 watts; 1.1 cu. ft. capacity; with glass turn table |
| One-Piece Fingertight 10-32 Coned, for 1/16" OD | IDEX | F-120X | |
| PEEK Tubing 1/16" OD x 0.010" ID | IDEX | 1531B | |
| Sprayer: Master Airbrush | Master Airbrush | TC-60 |
References
- Huemmerich, D., et al. Primary Structure Elements of Spider Dragline Silks and Their Contribution to Protein Solubility. Biochimie. 43 (42), 13604-13612 (2004).
- Schacht, K., Scheibel, T. Controlled Hydrogel Formation of a Recombinant Spider Silk Protein. Biomacromolecules. 12 (7), 2488-2495 (2011).
- Jones, J. A., et al. More Than Just Fibers: An Aqueous Method for the Production of Innovative Recombinant Spider Silk Protein Materials. Biomacromolecules. 16 (4), 1418-1425 (2015).
- Tucker, C. L., et al. Mechanical and Physical Properties of Recombinant Spider Silk Films Using Organic and Aqueous Solvents. Biomacromolecules. 15 (8), 3158-3170 (2014).
- Harris, T. I., et al. A Sticky Situation: An Investigation of Robust Aqueous-Based Recombinant Spider Silk Protein Coatings and Adhesives. Biomacromolecules. 17 (11), 3761-3772 (2016).
- Jones, J. A., et al. Importance of Heat and Pressure for Solubilization of Recombinant Spider Silk Proteins in Aqueous Solution. International Journal of Molecular Sciences. 17 (11), 1955 (2016).
- Copeland, C. G., Bell, B. E., Christensen, C. D., Lewis, R. V. Development of a Process for the Spinning of Synthetic Spider Silk. ACS Biomaterials Science and Engineering. 1 (7), 557-584 (2015).
- Arcidiacono, S., et al. Aqueous Processing and Fiber Spinning of Recombinant Spider Silks. Macromolecules. 35 (4), 1262-1266 (2002).
- Work, R. W. Mechanisms of Major Ampullate Silk Fiber Formation by Orb-Web-Spinning Spiders. Transactions of the American Microscopical Society. 96 (2), 170-189 (1977).
- Decker, R. E., et al. Method for the Destruction of Endotoxin in Synthetic Spider Silk Proteins. Scientific Reports. 8 (12166), 1-6 (2018).

