Daily Phototherapy with Red Light to Regulate Candida albicans Biofilm Growth
Summary
Here, we present a protocol to assess the outcome of red light application on the growth of Candida albicans biofilm. A non-coherent red light device with the wavelength of 635 nm and energy density of 87.6 J·cm-2 was applied throughout the growth of Candida albicans biofilms for 48 h.
Abstract
Here, we present a protocol to assess the outcomes of per diem red light treatment on the growth of Candida albicans biofilm. To increase the planktonic growth of C. albicans SN425, the inoculums grew on Yeast Nitrogen Base media. For biofilm formation, RPMI 1640 media, which have high concentrations of amino acids, were applied to help biofilm growth. Biofilms of 48 h were treated twice a day for a period of 1 min with a non-coherent light device (red light; wavelength = 635 nm; energy density = 87.6 J·cm-2). As a positive control (PC), 0.12% chlorhexidine (CHX) was applied, and as a negative control (NC), 0.89% NaCl was applied to the biofilms. Colony forming units (CFU), dry-weight, soluble and insoluble exopolysaccharides were quantified after treatments. Briefly, the protocol presented here is simple, reproducible and provides answers regarding viability, dry-weight and extracellular polysaccharide amounts after red light treatment.
Introduction
The increased incidence of diabetes, immunosuppressive therapy applications, HIV infection, AIDS epidemic, invasive clinical procedures and broad-spectrum antibiotic consumption in the past years have increased the incidence of Candida albicans related diseases1,2. C. albicans infections are commonly related to biofilm development and may cause clinical manifestations, such as candidiasis, or systemic manifestations, such as candidemia1,2. One of the most noteworthy virulence factors of biofilm growth is the extracellular polysaccharide matrix establishment. Biofilm formation cooperates to increase the resistance to existing antifungal drugs, environmental stress, and host immune mechanisms3.
The biofilm growth of C. albicans begins with the early adherence of planktonic cells to a substrate, followed by the propagation of yeast cells through the substrate surface, and hyphal growth. The last phase of biofilm growth is the maturation phase, wherein yeast-like development is suppressed, the hyphal development expands, and the extracellular matrix encloses the biofilm4. C. albicans exopolysaccharides (EPS) in the matrix interact to form the mannan-glucan complex5,6. The interaction of exopolysaccharides is critical for the defense of the biofilms against drugs7. Hence, the reduction of EPS from the C. albicans extracellular matrix could support the development of new antibiofilm protocols for oral candidiasis control.
Light regulates the growth, development, and behavior of several organisms8 and it has been applied as an antimicrobial in photodynamic antimicrobial chemotherapy (PACT). PACT applies a visible light of a specific wavelength and a light-absorbing photosensitizer9. However, the photosensitizers have difficulties in penetrating the biofilm, causing lower efficacy10. The failure of therapeutic agents to fully infiltrate biofilms is a reason that biofilms occasionally resist traditional antimicrobial therapy3,5. To deactivate the enclosed microbial cells, antimicrobials need to permeate through the extracellular matrix; nevertheless, the EPS characterizes a diffusional obstacle for such molecules by prompting their level of carriage into the biofilm or by influencing the response of the antimicrobial with the matrix itself11.
Considering the disadvantages of PACT, the use of light by itself emerges as a valuable improvement. Preliminary data revealed that the treatment with blue light twice a day significantly inhibited the production of EPS-insoluble in Streptococcus mutans biofilm. By the decrease of EPS-insoluble, blue light diminished biofilm growth. Nevertheless, the outcomes of phototherapy using red light in C. albicans biofilms are scarce. Hence, the objective of this investigation was to evaluate in what manner phototherapy using red light influences the growth and arrangement of C. albicans biofilm. For the twice-daily treatment, we adapted our laboratory's previous protocols9,12 to provide an easy and reproducible biofilm model that delivers answers regarding viability, dry-weight and extracellular polysaccharides amounts after red light treatment. The same protocol can be used for testing other therapies.
Protocol
1. Preparation of culture media
- Prepare sabouraud dextrose agar (SDA). Suspend 65 g of SDA supplemented with chloramphenicol (50 mg/L) in 1,000 mL of distilled water. Boil to dissolve the medium completely. Sterilize by autoclaving at 15 PSI (121°C) for 30 min. Cool to 45-50 °C. Mix well and pour 20 mL of SDA into sterile Petri plates (size: 100 mm x 15 mm).
- Prepare yeast nitrogen base (YNB) medium supplemented with 100 mM glucose by mixing 6.7 g of YNB and 18 g of dextrose to 1,000 mL of ultrapure water. Mix using a stirrer and sterilize the medium using a 0.22 µm vacuum filter system.
- Prepare RPMI by mixing 10.4 g of RPMI 1640 and 34.32 g of 3-(N-morpholino)propanesulfonic acid (MOPS) to 1,000 mL of ultrapure water. Mix in a stirrer without heat and adjust the pH to 7 by adding 1 N NaOH (Add 2 g of NaOH to 50 mL of ultrapure water). Sterilize the medium using a 0.22 µm vacuum filter system.
2. Pre-inoculum and inoculum
- Remove the microorganism C. albicans SN425 from the −80 °C freezer and let it thaw at ambient temperature. Seed 10 µL of the stock culture on Petri dishes containing SDA supplemented with chloramphenicol (50 mg/L). Incubate the Petri dishes aerobically at 37 °C for 48 h.
- After 48 h of incubation, add 10 colonies of C. albicans to 10 mL of yeast nitrogen base (YNB) medium supplemented with 100 mM glucose, and incubate aerobically at 37 °C for 16 h for the pre-inoculum.
- After incubating for 16 h, prepare the inoculums by diluting the starter cultures into fresh YNB medium supplemented with 100 mM glucose in a 1:10 proportion (1 mL of the starter culture diluted in 9 mL of YNB). Measure the initial optical density (OD) at 540 nm.
- Incubate the inoculums aerobically at 37 °C for 8 h until the strains reach the mid-log growth phase and measure the final OD at 540 nm. Subtract the final OD from the initial OD to check if the OD of the inoculums are on the mid-log growth phase (8 h, based on the growth curves in Figure 1).
- To reach an inoculum with 107 cells mL-1, centrifuge the inoculum for 5 min at 5,500 x g, discard the supernatant and concentrate the inoculum to half of the volume of the tubes.
3. Biofilm formation and phototherapy
- Add 1 mL of the inoculum to the wells of a 24-well polystyrene plate. Incubate aerobically at 37 °C for 90 min to allow cell adhesion to the bottom of the wells.
- Use a non-coherent red light (see Table of Materials) as the light source with wavelength of 635 ± 10 nm and a fixed output power of 1,460 mW cm−2.
- Use a power meter to measure the power density of the light. The radiant exposure applied is 87.6 J cm-2, equivalent to about 1 min of exposure. Use the formula Energy density (J/cm2)= power density (W/cm2) x irradiation time (s). Apply a distance of 5 mm between the light source and the biofilm to avoid warming the sample.
- Treat the positive control biofilms with 0.12% chlorhexidine for 1 min and negative control biofilms with 0.89% NaCl for 1 min.
- After treatments, wash all the biofilms twice with 0.89% NaCl solution.
- Add 1 mL of RPMI 1640 buffered with 3-(N-morpholino)propanesulfonic acid (MOPS) at pH 7 to the biofilms and incubate the plates at 37 °C overnight.
- In the morning, apply the same treatments as steps 3.2 to 3.6, wash the biofilms twice with 0.89% NaCl, add new RPMI buffered with MOPS (1 mL, pH 7) to the wells and incubate aerobically at 37 °C.
- At the same day, after 6 h, expose the biofilms to red light. Treat the positive control biofilms with 0.12% chlorhexidine for 1 min and negative control biofilms with 0.89% NaCl for 1 min. After treatments, wash the biofilms twice with 0.89% NaCl solution.
- Repeat the steps 3.2-3.8 until achieving 48 h of biofilm development.
NOTE: Basically, one treatment will happen in the morning and the other one will happen in the afternoon on the same day (6 h apart).
4. Processing
- Add 1 mL of sterile 0.89% NaCl to the well to remove the biofilm by scratching it from the well using a pipette tip. Add the removed biofilm to a sterile tube.
- Add another 1 mL of sterile 0.89% NaCl to the well. Scratch it again and add the suspension to the same tube for a total volume of 2 mL.
- Colony-forming units (CFU)
- Vigorously vortex the biofilm suspension for 1 min. From the biofilm suspension, use an aliquot of 0.1 mL for the serial dilution.
- Dilute the biofilm suspension from 10-1 to 10-4 in 0.89% NaCl solution.
- Seed 50 μL of each dilution to SDA plates and incubate the plates at 37 °C for 48 h.
- Count the colonies and apply the formula CFU/mL = number of colonies x 10n / q (n= dilution; q= seeded volume).
- Dry weight
- Weigh and label microcentrifuge tubes.
- From the biofilm suspension, use 0.1 mL for dry weight (biomass) determination.
- Add 1 mL of absolute ethanol to an aliquot of 0.1 mL of the biofilms and store it at -20 °C for 18 h. After 18 h, centrifuge at 10,000 x g for 10 min.
- Discard the supernatant, place the tubes on a desiccator to dry the samples for one week and weigh the microcentrifuge tubes again.
- Water-soluble polysaccharides (WSPs) and alkali-soluble polysaccharides (ASPs)
- From the biofilm suspension, vigorously vortex the remainder of the volume (1.8 mL) for 1 min and centrifuge at 5,500 × g for 10 min at 4 °C. Store the supernatant in a new tube and wash the precipitate twice with the cells, which contain the insoluble constituents of the extracellular matrix with sterile ultrapure water (5,500 × g, 10 min at 4 °C).
- Re-suspend the insoluble content at 1.8 mL of sterile ultrapure water.
- Water-soluble polysaccharides (WSPs)
NOTE: Perform this experiment at a fume hood.- From the stored supernatant, reserve 1 mL for the quantification of water-soluble polysaccharides (WSPs).
- Homogenize the stored supernatant for 1 min. Then, transfer to sterile tubes and add 2.5 volumes of 95% ethanol. Store the tubes for 18 h at -20 °C for WSPs precipitation.
- After 18 h, centrifuge the tubes at 9,500 x g for 20 min at 4 °C. After centrifugation, discard the supernatants.
- Wash the samples three times with 75% ethanol and air-dry the pellets. Re-suspend the pellet with 1 mL of sterile ultrapure water, and quantify the WSP using the phenol-sulfuric acid method.
- Use 0.1% glucose (0.01 g of glucose to 10 mL of ultrapure water) for the standard curve (0, 2.5, 5, 10, 15, 20, and 25 µL of glucose per tube).
- Add 200 µL of 5% phenol (2.7 mL of 90% phenol to 47.3 mL of ultrapure water) to a glass tube comprising 200 µL of the sample or standard curve point (in triplicate per sample) and mix cautiously.
- Add 1 mL of sulfuric acid to the tube and mix. Wait 20 min for the reaction and measure the samples using a spectrophotometer (490 nm).
- Alkali-soluble polysaccharides (ASPs)
NOTE: Perform this experiment in a fume hood.- From the insoluble re-suspended amount, separate 1 mL for the determination of ASPs. Centrifuge the aliquots of each biofilm suspension (13,000 x g, for 10 min at 4 °C).
- Cautiously remove the supernatant of each tube. Then place the samples on a vacuum concentrator to dry the pellets.
- Weigh the pellets and add 300 µL of 1 N NaOH (2 g of NaOH to 50 mL of ultrapure water) per 1 mg of the dry weight. Incubate them for 2 h at 37 °C and then centrifuge at 13,000 × g for 10 min.
- Cautiously collect the supernatants with a pipette and transfer to microcentrifuge tubes, maintaining the pellet.
- Once again, add an equal volume of 1 N NaOH to the tubes comprising the pellets, and repeat the same steps as above for the extraction of ASP.
- After incubation for 2 h, centrifuge the samples (13,000 x g for 10 min), carefully collect the supernatants and add to the previously collected supernatants.
- For the third extraction, repeat the same steps as above, but this time, do not incubate the samples for 2 h before centrifugation.
- Afterwards, add three volumes of 95% ethanol to each sample. Then, stock the samples at −20 °C for 18 h for precipitation of ASP.
- Centrifuge the tubes (9,500 × g for 20 min at 4 °C) and discard the supernatants.
- Wash each pellet three times with 75% ethanol and air-dry (same procedures did for WSP). Re-suspend the pellets in equal total volume of the first extraction with 1 N NaOH.
- Read the samples for quantification of ASP using the phenol-sulfuric (same as for WSP analysis).
Representative Results
Figure 2 displays the outcomes of Log10 CFU/mL of C. albicans after per diem treatments with red light for 1 min. Red light significantly reduced the Log10 CFU/mL compared to the NC (p = 0.004). Figure 3 presents the outcomes of the biomass (mg) of C. albicans biofilms after daily treatments. All treated groups showed reduction of the biomass compared to the NC (p = 0.000) and the red light treated groups presented similar reduction of biomass to that observed in the PC. Figure 4 and Figure 5 display inferior amounts of C. albicans EPS-soluble and EPS-insoluble in PC compared to NC (p = 0.000). Even though not statistically significant, per diem application of red light for 1 min to C. albicans biofilms numerically diminished the amounts of EPS-soluble and EPS- insoluble.
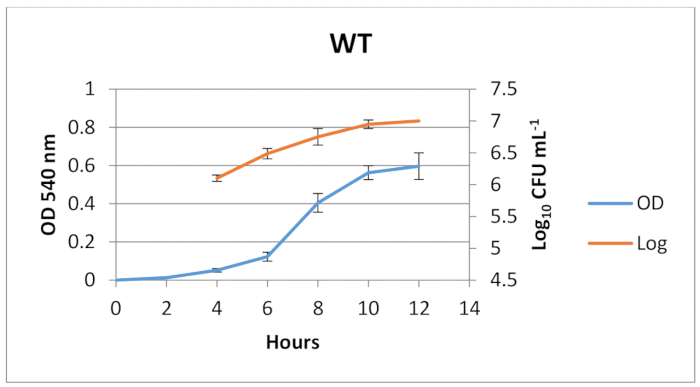
Figure 1. Growth curve of C. albicans strain SN 425. Planktonic culture was made in YNB medium supplemented with 100 mM of glucose and incubated at 37 °C. The optical density (OD at 540 nm) and Log10 CFU/mL were determined over time. Standard deviation is shown. Please click here to view a larger version of this figure.
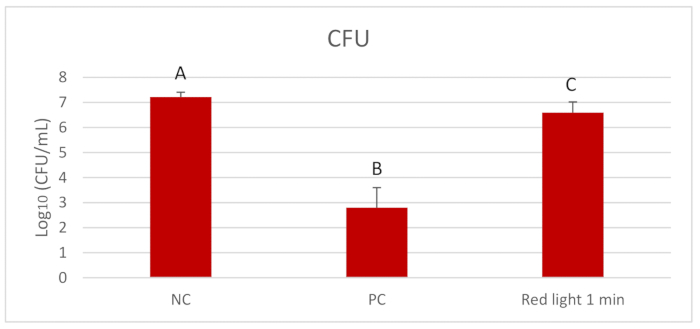
Figure 2. Mean and standard deviations of Log10 CFU/mL of C. albicans. Assessments were made between the treatment with red light twice a day and the controls-0.12% CHX (PC) and 0.89% NaCl (NC). Equal letters represent statistical similarity between groups (p > 0.05). Please click here to view a larger version of this figure.
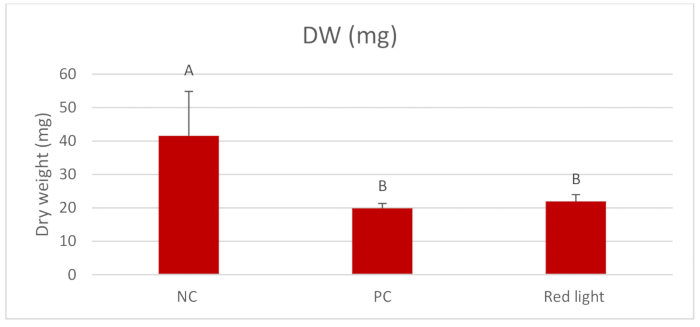
Figure 3. Mean and standard deviations of dry-weight (mg) of C. albicans. Assessments were made between the treatment with red light twice a day and the controls-0.12% CHX (PC) and 0.89% NaCl (NC). Equal letters represent statistical similarity between groups (p > 0.05). Please click here to view a larger version of this figure.
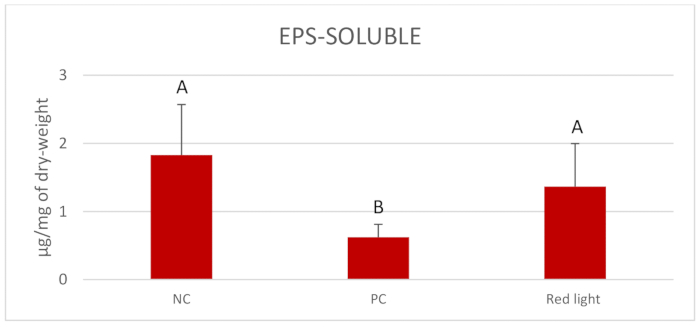
Figure 4. Mean and standard deviations of EPS-soluble amount in C. albicans biofilm (µg/mg of dry-weight). Comparisons were made between the treatment with red light twice a day and the controls-0.12% CHX (PC) and 0.89% NaCl (NC). Equal letters represent statistical similarity between groups (p < 0.001). Please click here to view a larger version of this figure.
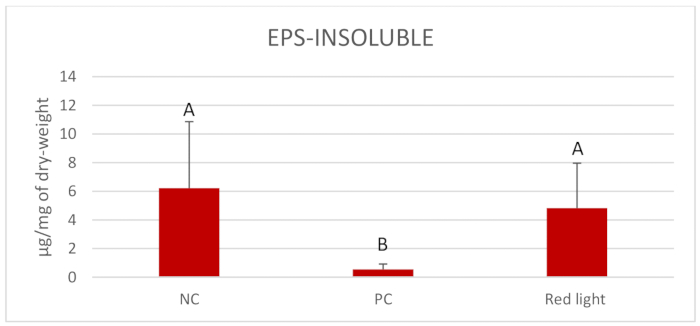
Figure 5. Mean and standard deviations of EPS-insoluble content in C. albicans biofilm (µg/mg of dry-weight). Assessments were made between the treatment with red light twice a day and the controls-0.12% CHX (PC) and 0.89% NaCl (NC). Equal letters represent statistical similarity between groups (p < 0.001). Please click here to view a larger version of this figure.
Discussion
The most critical steps for successful culturing of C. albicans biofilm are: 1) to do the pre-inoculum and the inoculum in YNB medium complemented with 100 mM glucose; 2) to wait 90 min for the adhesion phase and carefully wash twice the wells with 0.89% NaCl to remove non-adhered cells; and 3) to add RPMI medium to the adhered cells to start biofilm formation, since RPMI will stimulate hyphae growth. Aneuploidies can occur when culturing C. albicans. Consequently, it is important not to use colonies that are more than seven days old, not to store plates at 4°C, and not to re-streak cells from existing plates. Likewise, strains should be used prior to 18 hours of overnight growth13.
A study limitation is that it was performed in vitro. Whilst in vitro studies have greatly increased the understanding of the biology of biofilm, they do not precisely represent in vivo conditions14. However, in vitro tests provide high-throughput screening in addition to being a cheap and simple methodology14 to answer questions regarding new antibiofilm therapies. Choosing the correct culture media for each phase of biofilm development is an important step for the success of the method. RPMI 1640 is a nutrient-rich medium that has amino acids and simulates the composition of human fluids15. RPMI 1640 contains L-glutamine, L-arginine, L- asparagine in addition to vitamins and inorganic salts. Nevertheless, YNB media has an elevated amount of glucose (18 g/L) compared to RPMI 1640 medium (2 g/L glucose). The high glucose content has been described to increase the planktonic growth of Candida species16. In contrast, the existence of higher concentrations of amino acids will help biofilm growth in RPMI medium compared to YNB medium16. Qualitative data applying SEM micrographs showed that the structural design of C. albicans biofilms in RPMI presents a complex organization with a solid growth of yeasts with ramifying hyphae, budding elements and bud scars with an abundant extracellular matrix16. Such outcomes are in accordance with a previous study that reported that RPMI 1640 augmented the hyphal formation in C. albicans biofilms15. These results demonstrate the differences in substrate utilization by Candida throughout different biofilm growth phases and show the importance of changing media during planktonic growth and biofilm formation.
The selection of the correct temperature and pH to cultivate C. albicans biofilms is also important to accomplish the method with the adequate formation of hyphae. C. albicans undertakes the conversion from blastospores to filaments in reaction to conditions that mimic the milieu of mammalian host tissues17. These conditions involve growing at body temperature (37 °C) and at neutral pH17, and this is the reason why the pH of the RPMI medium is adjusted to 7.
The methods applied in this study are significant since biofilms of C. albicans SN 425 were characterized before12, showing to have great amounts of EPS-soluble and -insoluble, which makes it a reliable method to analyze the outcome of the lights on the extracellular polysaccharides. Moreover, the same non-coherent light device applied in the experiments was successfully applied to bacterial planktonic suspensions18 and biofilms9, and the utmost benefit of this device is the reduction in treatment time, what makes the device more feasible for clinical applications18. The analysis of daily phototherapy applied to C. albicans biofilms is significant since the methodology applied in the present study simulates a treatment that is fast and can be easily done by the patient at home.
Red light daily treatment meaningfully reduced C. albicans viable colony count and biofilm biomasses. More studies might try a combination of treatments, starting with phototherapy and later applying topical antifungal. This strategy might assist in disorganizing the extracellular matrix shielding C. albicans biofilm, permitting better drug infiltration through the biofilm to reach and finally eradicate C. albicans cells. Considering the limitations of this in vitro experiment, the use of red light for 1 min may assist as an adjuvant to topic antifungals on the treatment of oral candidiasis.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Paula da Silveira, Dr. Cecília Atem Gonçalves de Araújo Costa, Shawn M. Maule, Shane M. Maule, Dr. Malvin N. Janal and Dr. Iriana Zanin for the development of this study. We also acknowledge Dr. Alexander D. Johnson (UCSF) for donating the strain analyzed in this study.
Materials
| Clorhexidine 20% | Sigma-Aldrich | C9394 | |
| Dextrose (D-Glucose) Anhydroous | Fisher Chemical | D16-500 | |
| Ethanol 200 proof | Decon Laboratories | DSP-MD.43 | |
| LumaCare LC-122 A | LumaCare Medical Group, Newport Beach, CA, USA | ||
| NaCl | Fisher Chemical | S641-500 | |
| NaOH | Fisher Bioreagents | BP 359-500 | |
| Phenol 5% | Milipore Sigma | 843984 | |
| RPMI 1640 buffered with 3-(N-morpholino) | Sigma | R7755 | |
| Sabouraud dextrose agar supplemented with chloramphenicol | Acumedia | 7306A | |
| Sulfuric acid | Fisher Chemical | SA200-1 | |
| Yeast nitrogen base | Difco | DF0392-15-9 | |
| 3-(N-morpholino)propanesulfonic acid MOPS | Sigma-Aldrich | M1254 | |
| 24-well polystyrene plate | Falcon | 353935 |
References
- Sardi, J. C. O., Scorzoni, L., Bernardi, T., Fusco-Almeida, A. M., Mendes Giannini, J. M. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. Journal of Medical Microbiology. 62 (Pt 1), 10-24 (2013).
- Harriott, M. M., Noverr, M. C. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrobial Agents and Chemotherapy. 53 (9), 3914-3922 (2009).
- Srinivasan, A., Lopez-Ribot, J. L., Ramasubramanian, A. K. Overcoming antifungal resistance. Drug Discovery Today Technologies. 11, 65-71 (2014).
- Finkel, J. S., Mitchell, A. P. Genetic control of Candida albicans biofilm development. Nature Reviews Microbiology. 9 (2), 109-118 (2011).
- Zarnowski, R., et al. Novel entries in a fungal biofilm matrix encyclopedia. MBio. 5, e013333 (2014).
- Mitchell, K. F., et al. Community participation in biofilm matrix assembly and function. Proceedings of the National Academy of Sciences of the United States of America. 112 (13), 4092-4097 (2015).
- Mitchell, K. F., Zarnowski, R., Andes, D. R. Fungal super glue: the biofilm matrix and its composition, assembly, and functions. PLoS Pathogens. 12, e1005828 (2016).
- Dai, T., et al. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrobial Agents and Chemotherapy. 57 (3), 1238-1245 (2013).
- de Sousa, D. L., Lima, R. A., Zanin, I. C., Klein, M. I., Janal, M. N., Duarte, S. Effect of twice-daily blue light treatment on matrix-rich biofilm development. PLoS One. 10 (7), e0131941 (2015).
- Fontana, C. R., et al. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. Journal of Periodontal Research. 44 (6), 751-759 (2009).
- Donlan, R. M., Costerton, J. W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews. 15 (2), 167-193 (2002).
- Panariello, B. H. D., Klein, M. I., Pavarina, A. C., Duarte, S. Inactivation of genes TEC1 and EFG1 in Candida albicans influences extracellular matrix composition and biofilm morphology. Journal of Oral Microbiology. 9 (1), 1385372 (2017).
- Gulati, M., Lohse, M. B., Ennis, C. L., Gonzalez, R. E., Perry, A. M., Bapat, P., Valle Arevalo, A., Rodriguez, D. L., L, D., Nobile, C. J. In vitro culturing and screening of Candida albicans biofilms. Current Protocols in Microbiology. 50 (1), e60 (2018).
- Roberts, A. E., Kragh, K. N., Bjarnsholt, T., Diggle, S. P. The limitations of in vitro experimentation in understanding biofilms and chronic infection. Journal of Molecular Biology. 427 (23), 3646-3661 (2015).
- Kucharíková, S., Tournu, H., Lagrou, K., Van Dijck, P., Bujdáková, H. Detailed comparison of Candida albicans and Candida glabrata biofilms under different conditions and their susceptibility to caspofungin and anidulafungin. Journal of Medical Microbiology. 60 (Pt 9), 1261-1269 (2011).
- Weerasekera, M. M., Wijesinghe, G. K., Jayarathna, T. A., et al. Culture media profoundly affect Candida albicans and Candida tropicalis growth, adhesion and biofilm development. Memórias Do Instituto Oswaldo Cruz. 111 (11), 697-702 (2016).
- Kadosh, D., Johnson, A. D. Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: a genome-wide analysis. Molecular biology of the cell. 16 (6), 2903-2912 (2005).
- Paschoal, M. A., Lin, M., Santos-Pinto, L., Duarte, S. Photodynamic antimicrobial chemotherapy on Streptococcus mutans using curcumin and toluidine blue activated by a novel LED device. Lasers in Medical Science. 30 (2), 885-890 (2015).

