Isolation and Staining of Mouse Skin Keratinocytes for Cell Cycle Specific Analysis of Cellular Protein Expression by Mass Cytometry
Summary
This protocol describes how to isolate skin keratinocytes from mouse models, to stain with metal-tagged antibodies, and to analyze stained cells by mass cytometry in order to profile the expression pattern of proteins of interest in the different cell cycle phases.
Abstract
The goal of this protocol is to detect and quantify protein expression changes in a cell cycle-dependent manner using single cells isolated from the epidermis of mouse skin. There are seven important steps: separation of the epidermis from the dermis, digestion of the epidermis, staining of the epidermal cell populations with cisplatin, sample barcoding, staining with metal tagged antibodies for cell cycle markers and proteins of interest, detection of metal-tagged antibodies by mass cytometry, and the analysis of expression in the various cell cycle phases. The advantage of this approach over histological methods is the potential to assay the expression pattern of >40 different markers in a single cell at different phases of the cell cycle. This approach also allows for the multivariate correlation analysis of protein expression that is more quantifiable than histological/imaging methods. The disadvantage of this protocol is that a suspension of single cells is needed, which results in the loss of location information provided by the staining of tissue sections. This approach may also require the inclusion of additional markers to identify different cell types in crude cell suspensions. The application of this protocol is evident in the analysis of hyperplastic skin disease models. Moreover, this protocol can be adapted for the analysis of specific sub-type of cells (e.g., stem cells) by the addition of lineage-specific antibodies. This protocol can also be adapted for the analysis of skin cells in other experimental species.
Introduction
Correlation of gene expression with cell cycle stages remains a challenge in the analysis of animal models of hyperplastic diseases like cancer. Part of this challenge is the co-detection of proteins of interest (POI) with markers of proliferation. Proliferative cells can be found in various cell cycle phases including G1, S, G2, and M. Ki67 is one of most commonly used markers of proliferation and is expressed in all phases of the cell cycle. It has been extensively used in the analysis of both human and mouse tissues1,2,3. However, like other general proliferation markers, Ki67 does not discern individual cell cycle phases. A more precise approach uses the incorporation of thymidine nucleotide analogs like Bromodeoxyuridine (BrdU) into cells that are actively replicating their genome (i.e., S-phase)4,5. One drawback to the use of nucleotide analogs is the need to administer them to live animals hours before analysis. Ki67 and BrdU are commonly detected on fixed tissue sections by the use of antibodies. One advantage of this approach is that the location of POIs can be ascertained within the tissue architecture (e.g., the basal layer of skin epidermis). This approach also does not require tissue dissociation that may lead to changes in gene expression. One disadvantage is that the tissue fixation or the processing of the tissue for OCT frozen or paraffin sectioning may occlude antibody targets (i.e., antigens). Retrieval of antigens typically requires heat or tissue digestion. Quantification of staining intensities can also be challenging. This is due to variations in staining, section thickness, signal detection, and experimenter bias. Moreover, a limited number of markers can be detected simultaneously in most typical laboratory setups. Yet, newer multiplex staining approaches promise to overcome these limitations; examples are imaging mass cytometry and Tyramide signal amplification6,7.
Flow cytometry is another powerful technology to detect proliferating cells. It allows for multiplex detection of markers in the same cells but requires tissue dissociation for most non-hematopoietic cell types. Analysis of proliferating cells is routinely done by the use of dyes that bind DNA (e.g., Propidium Iodide (PI))8. Flow cytometry also permits a more precise determination of cell cycle phases when coupled with the detection of BrdU incorporation9. Although a powerful approach, BrdU/PI flow cytometry does have its disadvantages. It is unable to resolve the G2/M and G0/G1 phases without the inclusion of phase-specific antibodies. However, the number of antibodies that can be used is limited by cellular autofluorescence, spectral spillover of fluorophore emissions, and the use of compensation controls. This limitation markes it more challenging and laborious to co-detect the expression of cell cycle markers with POIs. A more facile approach is to use mass cytometry10,11. This technology uses metal conjugated antibodies that have a narrower detection spectrum. Once cells are stained with metal-tagged antibodies, they are vaporized, and the metals detected by cytometry time-of-flight (CyTOF) mass spectrometry. Due to these properties, mass cytometry enables the multiplex detection of >40 different markers using existing platforms10,11. In addition, it is possible to barcode samples with metals that result in the savings of precious antibodies while reducing sample-to-sample staining variability. On the other hand, mass cytometry does have several disadvantages. There are a limited number of commercially available metal-tagged antibodies for non-blood derived cells. Quantification of DNA content is less sensitive compared to the use of fluorescent DNA dyes and mass cytometry has a reduced dynamic range of signal detection compared to fluorescence flow cytometry.
The protocol described here was designed to analyze cell cycle dynamics from newly isolated keratinocytes (KCs) from mouse skin and characterize cell cycle specific protein expression in these cells using mass cytometry. This protocol can also be used with cultured cells or adapted to other cell types.
Protocol
The University of Colorado Anschutz Medical Campus' Institutional Animal Care and Use Committee approved the animal experiments described in this protocol.
1. Preparations
- Design a metal-tagged antibody panel. Use the free online panel design software12 and include 127IododeoxyUridine (IdU), 164Dy (Dysprosium) labeled anti-CCNB1 (CYCLIN B1), 175Lu (Lutetium) phospho (p)-HISTONEH3Ser28 (pHH3), and 150Nd (Neodymium)-pRETINOBLASTOMA proteinSer807/811 (pRB)13. Add additional metal-tagged antibodies that do not overlap in their channel signal14.
- Prepare an IdU stock solution. Dissolve IdU powder at 10 mg/mL in 0.1 N NaOH at 60 °C. Aliquot IdU stock solution into microcentrifuge tubes and freeze at -20 °C for long-term storage.
- Adjust the pH of IdU solution to 7.5 with 12 N HCl immediately before use. Test with a pH strip on a discard aliquot to ensure the solution is at pH 7.5.
NOTE: Prolonged time (>5 min) of IdU at pH 7.5 will result in precipitation and a fresh aliquot of IdU is needed when this happens. - Use appropriate personal protective equipment (e.g., gloves, lab coat, and safety glasses) and work in a safety cabinet when handling NaOH or HCl solutions.
- Adjust the pH of IdU solution to 7.5 with 12 N HCl immediately before use. Test with a pH strip on a discard aliquot to ensure the solution is at pH 7.5.
- Prepare a 2x paraformaldehyde (PFA) fixing solution. Combine 5 mL of 10x PBS (pH 7.5) and 1 mL of 16% PFA with 44 mL of pure molecular grade dH2O (3.2% final concentration).
NOTE: A stock of PFA can be prepared as previously published15 or purchase 16% stocks free of contaminating metals.- Use appropriate personal protective equipment and work in a safety cabinet when handling PFA powder and solutions.
- Prepare barium (Ba2+) free 1x PBS by combining 5 mL of metal-free 10x PBS (pH 7.5) with 45 mL of pure molecular grade dH2O.
NOTE: The quality of PBS and other reagents is very important to avoid contaminating metals that can introduce background noise during analysis of mass cytometric data. - Prepare a 100 mM cisplatin stock solution. Dissolve 300.5 mg of cisplatin powder in 10 mL of DMSO. Store in aliquots at -80 °C. Prepare a 10 mM cisplatin working solution for experiments to be used over 1 d.
- Prepare epidermis/dermis separation solutions. Prepare a 20x dispase stock solution by dissolving 30 mg in 1 mL of HBSS or PBS. Filter sterilize through a 0.22 µm filter and store in aliquots at -20 °C. Prepare a 1x type IV collagenase stock solution at 1 mg/mL in HBSS, filter sterilize through a 0.22 µm filter, and store aliquots at -20 °C.
2. Labeling of S phase by IdU incorporation
- Weigh mice. Use P1-P3 neonatal pups or 8-10 weeks old adult mice and use male or female mice from a C57BL/6, FvB, or 129 backgrounds. Determine a dose at 0.1 mg IdU (pH 7.5)/g body weight. For example, a 25 g mouse requires 0.25 mL of IdU.
- Administer the dose of IdU by an intraperitoneal injection and wait for 2 h before harvesting cells. Use a tuberculin syringe to inject neonatal pups.
NOTE: A dose of 2 mg/mL of IdU can be used with tissue culture cells with a 1 h incubation at 37 °C before harvesting and labeling for mass cytometry.
3. Isolation of cells for labeling
- Thaw dispase and collagenase stock solutions. Dilute the 20x dispase stock solution to 1x (1.5 mg/mL) in sterile HBSS or PBS. Keep on ice until ready to use.
- Combine 2 volumes of dispase with 1 volume of collagenase in a total volume that allows the skin to float freely. For example, digestion of 5 neonatal mouse skins can be floated on 8 mL of 1x dispase with 4 mL of collagenase in a 100 mm Petri dish.
- Follow approved methods to euthanize experimental mice (e.g., isoflurane overdose/toe pinch check or CO2 inhalation/cervical dislocation). Clean the adult ear skin with an iodine solution (see Table of Materials) and rinse with sterile water when isolated cells are to be used for tissue culture.
- Surgically remove the ear at the base (Figure 1A). Rinse ears in sterile PBS when isolated cells is to be used for tissue culture. Place on a dry 100 mm Petri dish.
- Separate carefully anterior from posterior skin by initially creating a pocket in the middle or edge of the cut area using fine forceps and pulling the two skin flaps apart ( Figure 1B-D). Proceed with both the anterior and posterior skins.
NOTE: Detailed instructions to dissect neonatal mouse skin are provided by Litchi et al.16 In addition, the use of a dissecting scope may assist in the separation of anterior from posterior skin and identification of epidermis/dermal sides of dissected skin: hair is visible on the epidermis side whereas the dermis will have a gelatinous appearance. - Carefully place the anterior and posterior skins of the ear with the dermis side of the skin touching the dispase/collagenase solution ( Figure 1E). Use 1 mL for both the anterior and posterior skin of a single ear per well of a 12 well culture plate. Incubate at 37 °C for 1 h. Alternatively, float skins on 1x dispase solution at 4 °C for 16-18 h.3
NOTE: Work in a tissue culture hood with sterile technique when cells are to be cultured. - Place the digested skin with the epidermis side touching the surface of a clean Petri dish. Flatten out the skin and gently slide the dermis off the epidermis by working from center to the edges in a circular pattern.
- Discard the dermis or digest it further to liberate fibroblasts for tissue culture or analysis16.
NOTE: The dermis will be darker in appearance and have a gelatinous and sticky composition. The dermis should easily come off. Failure or difficulty in removing the dermis suggests insufficient tissue digestion. However, extended incubation in digestion buffer will reduce cell viability. The epidermis will remain on the Petri dish and will have a bleached appearance. - Gently lift off the epidermis by grabbing at the edges and peel it off the surface of the Petri dish. Carefully place the epidermis on pre-warmed cell detachment solution (see Table of Materials) for 5 min at 37 °C or 20 min at RT. Use 500 µL of cell detachment solution for 2 adult ear epidermises per well of a 12 well culture plate and use 750 µL of cell detachment solution for a single neonatal epidermis per well of a 6 well culture plate.
- Grasp the epidermis using sterile forceps and scrub by dragging the epidermis against the bottom of the dish to dissociate cells. Add 1 mL of DMEM containing 1% FBS (0.01 mL) and pass through a 40 μm cell sieve into a collection tube. Rinse well with an additional 2 mL of DMEM and add to the cell suspension.
- Centrifuge at 120 x g for 5 min. Aspirate the supernatant carefully.
- Resuspend cell pellet in 1-2 mL of DMEM containing 1% FBS. Determine the cell and % live cell counts using Trypan Blue and a hemocytometer or automated counting device. Pellet cells as described in step 3.11.
NOTE: Cells can be cultured in appropriate tissue culture media after Step 3.12.17
4. Cisplatin labeling to determine live/dead cells
- Resuspend 1-3 x 106 cells per 1 mL of DMEM containing 25 µM cisplatin (2.5 µL of stock/mL). Incubate for 1 min and quench by pipetting with an equal volume of FBS (e.g., 1 mL).
- Centrifuge at 120 x g for 5 min. Decant supernatant into a beaker containing diluted bleach and invert tubes to drain remaining solution onto a paper towel. Tap gently to liberate solution remaining on pellet and sidewall of the tube.
- Resuspend cell pellet in 2 mL of Ba+2-free PBS. Pellet cells as described in Step 4.2.
NOTE: It is recommended to fix cells if they cannot be stained immediately.
5. Fixation of cisplatin labeled cells (optional)
- Resuspend 1-3 x 106 cisplatin labeled cells in 1 mL of Ba+2-free PBS. Vortex cells under continuous low power and add dropwise 1 mL (1 volume) of the 2x PFA fixation buffer. Incubate at RT for 10 min on a rocking platform.
- Pellet cells as described in step 4.2, but centrifuge at 500 x g for 5 min.
- Wash cells with 2 mL of Ba+2-free PBS and pellet cells as described in Step 5.2. Repeat Step 5.3 once.
- Resuspend the pellet in 2 mL of Ba+2-free PBS. Store at 4°C for <3 d. Add FBS to 3% (e.g., 0.3 mL of FBS/mL of cells) of the total volume if storing longer >3 d, then mix and freeze at -80 °C.
NOTE: Fixation may affect the detection of certain epitopes. The effects of fixation on antibody signal need to be determined empirically.
6. Barcoding of samples (optional but recommended)
- Pellet cells as described in Step 5.2 and then resuspend 1-3 x 106 cells in 1 mL of 1x Fixation buffer (see Table of Materials). Incubate at RT for 10 min. Pellet cells as described in Step 5.2.
- Wash cells with 1 mL of Barcode permeabilization buffer (see Table of Materials). Pellet cells as described in Step 5.2. Repeat Step 6.2 once.
- Add 100 µL of Barcode permeabilization buffer to barcodes (see Table of Materials)18 and mix immediately while the cells from Step 6.2 are pelleting.
- Resuspend the cell pellet in 800 µL of Barcode permeabilization buffer. Add barcode solution to cells, mix and incubate at RT for 30 min. Pellet cells as described in Step 5.2. Wash cells in 2 mL of Cell staining buffer (see Table of Materials) and pellet again.
NOTE: Up to 20 samples can be labeled with various combinations of Palladium metals18. Other barcoding strategies are described elsewhere15.
7. Labeling of cells for mass cytometry
- Resuspend 1-3 x 106 cells in 1 mL of Nuclear Antigen Staining buffer working solution (see Table of Materials). Combine samples into a single tube for subsequent steps when using barcoded samples. Incubate at RT for 30 min. Pellet as described in Step 5.2.
- Resuspend 1-3 x 106 cells in 2 mL of Nuclear Antigen Staining permeabilization buffer (see Table of Materials). Scale as necessary. For example, use 6 mL of buffer for 10 combined samples with a cell count of 9 x 106 cells. Pellet as described in Step 5.2.
- Repeat Step 7.2. Gently vortex the cell pellet in the residual volume left in the tube.
- Add 50 µL of intracellular antibody cocktail per 1-3 x 106 cells. Scale as necessary. For example, use 150 μL of the antibody cocktail for 10 combined samples with a cell count of 9 x 106 cells. Mix and incubate at RT for 45 min. Add 2 mL of Cell Staining buffer and pellet as described in step 5.2.
- Resuspend 1-3 x 106 cells pellet in 2 mL of Cell Staining buffer. Pellet cells as described in Step 5.2. Repeat Step 7.5 once.
NOTE: Other buffer solutions can be used for staining extracellular membrane or cytoplasmic markers and experimenter may have to optimize staining if there is a need to detect epitopes in different cellular locations. Cells can also be fixed in PFA after Step 7.5 when using live cells for staining. - Resuspend 1-3 x 106 cells in 1 mL of intercalation solution and store for 1-3 d at 4 ˚C.
- Store cells at -80 °C in DMSO containing the solution for >3 d. Pellet cells as described in Step 5.2 if cells are in intercalation solution. Resuspend pellet (1-3 x 106 cells) in 1 mL of Cell staining buffer and pellet cells as described in Step 5.2. Resuspend pellet in 1 mL of 10% DMSO/90% FBS19, transfer to a cryovial, place in an isopropanol-freezing container, and store at -80 °C.
- Pellet cells as described in Step 5.2. Wash 1-3 x 106 cells with 2 mL of Cell Staining buffer, pelleting as described in Step 5.2. Perform two additional washes with 2 mL water, pelleting as described in Step 5.2.
- Dilute EQ calibration beads 1:9 in water (stock solution at 3.3 x 105 beads/mL).
- Resuspend the cell pellet at a concentration of 1 x 106 cells/mL with diluted EQ bead solution. Filter cells through 35 µm strainer cap flow tubes.
- Run samples on the mass cytometer and acquire data. The data will be deposited in a Flow Cytometry Standard (FCS) file format.
8. Processing and analysis of mass cytometry data files
- Normalize FCS files. Use a free program available at https://github.com/nolanlab/bead-normalization/releases/latest20
- Deconvolute barcoded FCS files. Separate out the pooled barcode population into separate barcoded files with a free program available at https://github.com/nolanlab/single-cell-debarcoder/releases/latest18
- Analyze normalized FCS files. Use commercial21,22 or freeware programs (see web.stanford.edu/group/nolan/resources.html).
Representative Results
Table 1 shows the expected cell yields and viability from adult mouse ear (Figure 1) and neonatal skin under non-pathological conditions. The table also shows representative data of animals from a mixed C57/126 background. It is expected that the skin of other strains would result in similar cell yields and viabilities. The approximate yield is dependent on the surface area of skin and indicates that neonatal skin would be a better choice for experiments that require larger numbers of cells (Table 1). Low yields or reduced viability (<50%) would indicate issues with digestion or experimental conditions that reduce skin integrity or induce of cell death. Culturing cells with appropriate media and supplements can help assess the quality of KC preparations17.
Following normalization20 and deconvulation18 of FCS files, gating of data will define epidermal cell populations of interest and demark individual cell cycle phases (Figure 2). In bivariate plots comparing the 191Ir vs 193Ir channels, intact cells can be gated in the upper right quadrant (Figure 2A). Plotting event length vs 191Ir and selecting for a tight cluster of cells near the 191Ir axis demarks single cells. Viable cells are selected in a plot of 195Pt vs 193Ir plot by gating for cells with low 195Pt levels. The sample shown in Figure 2A has debris and increased presence of cisplatin labeled non-viable cells. This may indicate that the sample was over-digested, harshly handled, or left on ice or RT for too long before staining for mass cytometry. Profiles from tissue-cultured cells typically give higher percentages of 195Pt negative cells (Figure 2B). Alternatively, the presence of 195Pt positive cells may be expected if the induction cell death is a feature of the experimental model and/or conditions.
Ideally, markers that define the population of interest should be included in the analysis of specific cell types. For example, immune cells that are expected to be present in crude KC suspensions can be detected by the addition of a CD4523 antibody, which detects hematopoietic cells (Figure 2C). With regards to the proliferation antibody panel13, plotting IdU vs CYCLIN B1 resolves cell cycle phases (Figure 2C) similar to standard BrdU/PI flow plot (Figure 2D), allowing for the detection of S-phase, G0/G1 and G2/M cell populations. In IdU vs pHH3 plots, high pHH3 positivity identifies M phase cells. Lastly, gating G0/G1 cells on high pRB signal can distinguish G0 (quiescent) from cells in G1 (Figure 2C, left most panel).
Having identified the different cell cycle phases, it is then possible to construct a graphical representation of cell cycle profiles for different cell types or experimental conditions. For example, distinct cell cycle profiles emerge when comparing CD45- vs CD45+ cell populations (Figure 3A) in the KC suspension shown in Figure 2. As expected hyperplastic KCs found in the CD45- group had fewer cells in G0 and more cells in S, G2, and M phases3. In contrast, immune cells from the same sample had notable populations in G0 and G1. In addition to cell cycle profiles, the characterization of gene expression in a cell cycle-dependent manner is also possible. For example, phospho proteins that help define EGFR and mTOR/PI3K signaling were analyzed in the data presented for Figure 3A. Analysis of the G1 phases of CD45- vs CD45+ cells revealed a similar profile for EGFR and mTOR/PI3K signaling proteins, although there appeared to be slight differences in the percentage of pS6 and pEGFR positive cells (Figure 3B). Similar approaches can be adapted to characterize the expression of other signaling pathway proteins or cellular processes at different phases of the cell cycle.
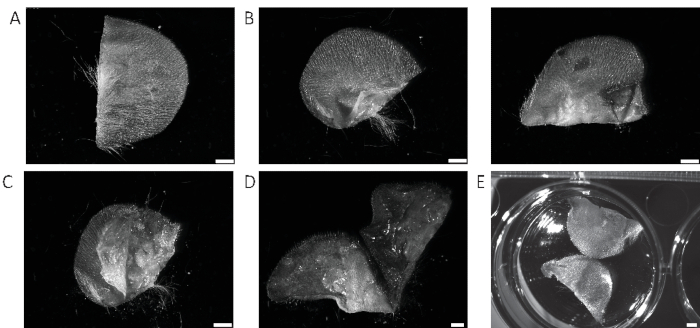
Figure 1: Preparation of mouse ear skin for digestion. (A) First, remove the ear from the animal and lay flat on a clean Petri dish. (B) Grasp one side of the ear at either the middle or edge using curved precision forceps. (C) Flip over and using another pair of forceps and gently pull the halves of the ear apart until the ear tears on the crease. (D) Continue pulling until the sides separate into two halves. (E) Float the ear halves on dissociation media with the dermis side touching the solution. Scale bar = 2 mm. Please click here to view a larger version of this figure.
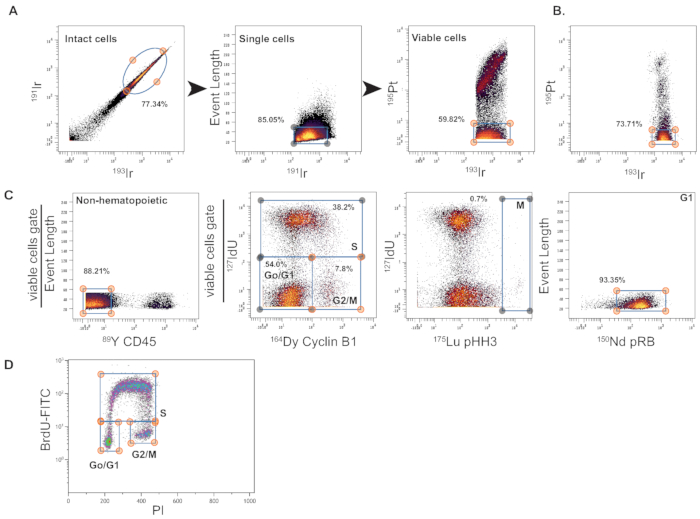
Figure 2: Gating strategy for mass cytometry data. The data in this figure was generated from an experimental animal treated with the phorbol ester TPA as previously described3. TPA is a PKC activator that induces skin inflammation24. (A) The panels show the initial gating strategy after normalization and deconvolution of barcoded data. By gating on event length, 191Ir,193Ir, and 195Pt channels, it is possible to select for intact, single, and viable cells. (B) Human SCC SRB-P93 cells treated with DMSO and were then stained for mass cytometry. Data was gated as in (A) and the plot shows the levels of live cells in this sample. (C) The inclusion of lineage markers allows for the selection of cell populations of interest. For this example, viable cells from (A) were gated on event length vs. CD45 to exclude hematopoietic cells. Gating CD45- cells for incorporation of IdU vs CYCLIN B1 allows the identification of S, G0/G1, and G2/M cell populations. Selection of pHH3 high cells defines M phase, whereas the analysis of G0/G1 for pRB positivity identifies cells in G1. (D) The plot shows the representative results of cell cycle analysis by the detection of BrdU incorporation and PI (DNA content) with fluorescence-based flow cytometry. The data shown is from human Colo163 SCC cells grown with BrdU for 1 h as previously described3. Please click here to view a larger version of this figure.
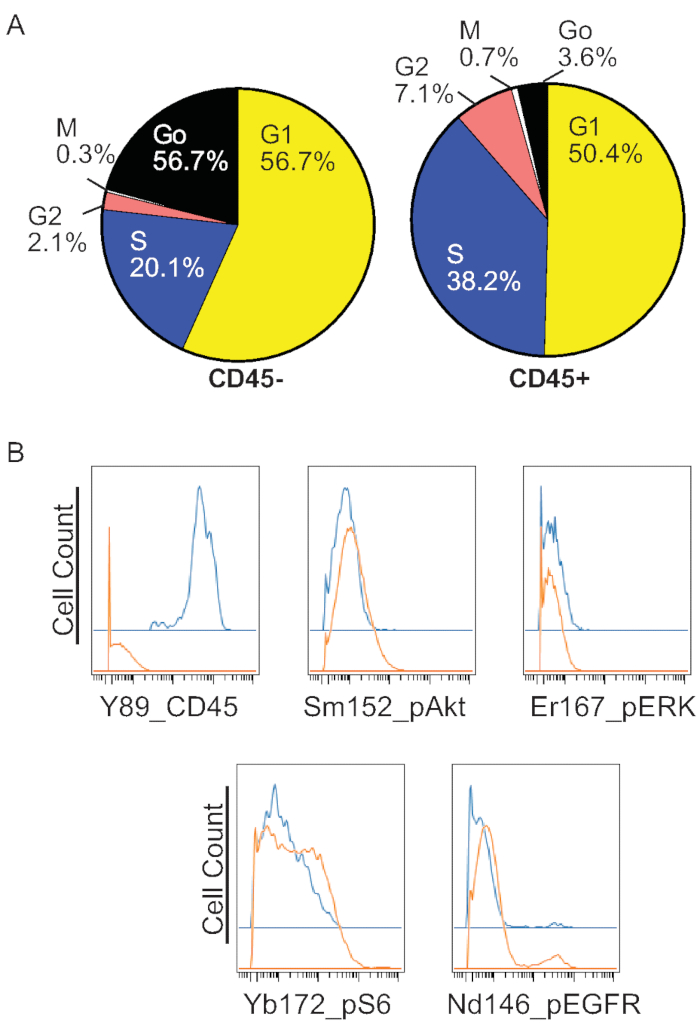
Figure 3: Characterization of cell cycle profiles and phase-specific protein expression. (A) The cell cycle profiles were determined in the sample from Figure 2 for CD45- vs CD45+ cells. (B) Analysis of the G1 phases with phospho-specific antibodies in CD45-(orange) and CD45+(Blue) cells revealed similar expression profiles for markers of the EGFR and mTOR/PI3K signaling pathways. Please click here to view a larger version of this figure.
| Tissue | Area | Cell yield | Viability |
| Mouse ear | 225-230 mm3 | 1-2×106 | 70-90% |
| Neonatal skin | 1000-1100 mm3 | 5-10×106 | 80-99% |
Table 1: Typical Cell yields and viability from adult mouse ear and neonatal skin. Cell counts and viability by Trypan blue exclusion were averaged from ear KCs harvested from n=7 8-10 week old adult mouse ears or n=7 P3 neonatal skins.
Discussion
The protocol outlined in this paper can be completed in about 8 h. The end result is a suspension of cells enriched in KCs that can be analyzed for protein expression in a cell cycle-dependent manner. Several previous studies have outlined methods to isolate KCs from human and mouse skin16,25. These studies also include protocols for the isolation of KCs for flow cytometry26. However, a detailed protocol has not been previously described that combines the isolation of KCs with the analysis of protein expression and cell cycle dynamics using mass cytometry.
The greatest hurdle to obtaining high-quality results in this protocol is the quality of live cells used. In the literature, the suggested conditions and enzymes for epidermis/dermis separation vary greatly16,25,26,27. However, it is thus recommended to keep the time of digestion of skin to the shortest possible duration that allows effective separation of the epidermis from the dermis. This protocol presents digestion with dispase and type IV collagenase that allows facile separation of both ear and mouse neonatal skin within 1 h. This results in sufficient cell yields with high cell viability. Another factor that can affect the quality of cells is the location of the skin selected for digestion. It is assumed that KCs behave similarly from different anatomical locations in the body. However, the skin at different sites may have different properties and stem cell composition28,29. Nevertheless, the use of neonatal and ear skin3,30 is preferred because the isolation of KCs is more difficult from areas of skin with a high density of hair follicles (e.g., back skin). A higher degree of manipulation or tissue dissociation may be required for efficient isolation of KCs from hairy areas of the mouse26. The experimental conditions prior to isolation of KCs may also affect the quality of isolated cells. Skin lesions or conditions that affect skin barrier or skin integrity may reduce the separation of epidermis from the dermis. This could result in reduced yields of viable cells or increased contamination of non-KC cells (e.g., immune cells). Lastly, isolated KCs are susceptible to clumping. They can also lose cell viability if left too long on ice or at RT before processing for mass cytometry or other single cell analyses.
The staining methodology for mass cytometry is similar to that used for fluorescence flow cytometry but with key differences. For example, the detection of DNA replication is accomplished by direct detection of IdU instead of by the use of antibodies against nucleotide analogs like BrdU. Direct detection of IdU greatly simplifies the identification of S-phase cells, as antibody detection of BrdU can be an involved procedure. Another difference is the use of cisplatin for the discrimination of dead and live cells. Cisplatin preferentially labels dead cells. This step is similar to the use of PI or other stains for dead/live FACS experiments. The cisplatin staining step is important because dead cells may bind antibodies and thereby generate false positive signals. Mass cytometry also uses antibodies to detect the expression of phase-specific marker in place of measuring DNA content. This allows for facile discrimination of individual cell cycle phases. Lastly, the mass cytometry data (FCS) files need to be normalized using the spike in calibration beads due to sample signal deterioration over time in the CyTOF instrument during the same run and between runs.
Staining, data acquisition, and analysis for mass cytometry has been well reviewed by recent publications14,15. The application of this technology to the analysis of hematopoietic and immune cells is well documented. This is evident by a large collection of commercially available metal-tagged antibodies and the predominance of immune-related publications using this technology. The immediate application of mass cytometry in the skin would be for the analysis of immune cells. This is particularly relevant for mouse skin models in diseases like cancer where inflammation has an important role. For non-immune cell analysis, the lack of commercially available metal-tagged antibodies can be a hindrance. The advantage of commercial reagents is that they can be used without considerable optimization as would be the case for in house metal-tagged antibodies. However, there are metal-tagged antibodies against commonly used fluorescent tags (e.g., FITC, PE, and APC) that would allow an experimenter to add additional markers to their staining panel. This workaround can also be applied to the analysis of skin cells in different species where there may be a limited number of commercial metal-tagged antibodies that show reactivity across species. Nevertheless, this workaround also requires an additional staining step after the cisplatin staining and some optimization. An additional consideration for building a successful panel are discussed in the literature14. Another disadvantage of mass cytometry is that data collection efficiency of mass cytometers can be 40-60% of the input cell number. As such, the analysis of rare cell populations (e.g., stem cells) from bulk samples may require a larger number of cells, pooling of samples for effective detection, or other approaches to enrich the cell population of interest prior to staining for mass cytometry24.
Mass cytometry is a powerful emerging technology whose use will continue to grow. Currently, the application of this technology is focused on the use of metal-tagged antibodies, but it was recently extended for the detection of mRNA31. Moreover, there is the potential of labeling other cellular components or metabolites with metal tags, which would expand the range of application for mass cytometry in the skin and other tissues from mice, human, and other species. In summary, this protocol may then extend the experimental tools available for skin biologists to correlate KC protein expression in the various cell cycle phases.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Support for this work came from the Department of Dermatology, the Gates Center for Regenerative Medicine at the University of Colorado and University of Colorado (UC) Skin Disease Center Morphology and Phenotyping Cores (NIAMS P30 AR057212). The authors acknowledge the UC Cancer Center Flow Cytometry Shared Resource and support grant (NCI P30 CA046934) for the operation of the mass cytometer and are grateful for Karen Helm and Christine Childs at the core for their expert advice on flow and mass cytometric techniques.
Materials
| 12-well plate | Cell Treat | 229512 | |
| Intercalator solution | Fluidigm | 201192A | 125 µM – Ir intercalator solution |
| Paraformaldehyde | Electron Microscopy Sciences | 30525-89-4 | 16 % PFA |
| Strainer cap flow tubes | Fisher/Corning | 352235 | 35 µm pore size |
| Cell sieve | Fisher | 22363547 | 40 μm pore size |
| Cell detatchment solution | CELLnTEC | CnT-ACCUTASE-100 | Accutase |
| Iodine Solution | ThermoFisher/Purdue | 67618-151-17 | Betadine 7.5%-iodine surgical scrub |
| Barcode permeabilization buffer | Fluidigm | 201060 | Cell-ID 20-Plex Pd Barcoding Kit |
| Barcodes | Fluidigm | 201060 | Cell-ID 20-Plex Pd Barcoding Kit |
| pH strips | EMD | 9590 | colorpHast |
| DMEM | Hyclone | SH30022.01 | Dulbecco’s Modified Eagle Media |
| Fine forceps | Dumont & Fils | 0109-5-PO | Dumostar #5 |
| Curved precision forceps | Dumont & Fils | 0109-7-PO | Dumostar #7 |
| Calibration Beads | Fluidigm | 201078 | EQ Four Element Calibration Beads |
| HBSS | Gibco | 14175-095 | Hank's Balanced Salt Solution |
| Water | Fisher | SH30538.03 | Hyclone Molecular Biology grade water |
| Iododeoxyuridine | Sigma | I7125 | IdU |
| Cryo vials | ThermoFisher | 366656PK | internal thread |
| Cell Staining buffer | Fluidigm | 201068 | Maxpar Cell Staining buffer |
| Fix & Perm buffer | Fluidigm | 201067 | Maxpar Fix & Perm buffer |
| Fix I buffer | Fluidigm | 201065 | Maxpar Fix I buffer |
| Phosphate buffered saline | Rockland | MB-008 | Metal free 10x PBS |
| isopropanol-freezing container | ThermoFisher | 5100-0001 | Mr.Frosty |
| Sodium hydroxide | Fisher | BP359-500 | NaOH |
| Petri dish | Kord-Valmark | 2900 | Supplied by Genesee 32-107 |
| 15 mL conical | Olympus/Genesee | 28-101 | |
| 50 mL conical | Olympus/Genesee | 28-106 | |
| 6-well plate | Cell Treat | 229506 | |
| Cisplatin | Sigma | 479306 | |
| Dispase II | Sigma/Roche | 4942078001 | |
| DMSO | Sigma | D2650 | |
| FBS | Atlanta Biologicals | S11150 | |
| Hydrochloric acid | Fisher | A144-212 | |
| Nuclear Antigen Staining permeabilization buffer | Fluidigm | 201063 | |
| Nuclear Antigen Staining buffer | Fluidigm | 201063 | |
| Trypan blue | Sigma | T8154 | |
| Tuberculin syringe | BD | 309626 | |
| Type IV Collagenase | Worthington Bioscience | CLSS-4 |
References
- Guzinska-Ustymowicz, K., Pryczynicz, A., Kemona, A., Czyzewska, J. Correlation between proliferation markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in colorectal cancer. Anticancer Research. 29 (8), 3049-3052 (2009).
- Ladstein, R. G., Bachmann, I. M., Straume, O., Akslen, L. A. Ki-67 expression is superior to mitotic count and novel proliferation markers PHH3, MCM4 and mitosin as a prognostic factor in thick cutaneous melanoma. BioMedCentral Cancer. 10, 140 (2010).
- Ryan, W. K. Activation of S6 signaling is associated with cell survival and multinucleation in hyperplastic skin after epidermal loss of AURORA-A Kinase. Cell Death & Differentiation. , (2018).
- Magaud, J. P. Double immunocytochemical labeling of cell and tissue samples with monoclonal anti-bromodeoxyuridine. Journal of Histochemistry & Cytochemistry. 37 (10), 1517-1527 (1989).
- Dolbeare, F., Gratzner, H., Pallavicini, M. G., Gray, J. W. Flow cytometric measurement of total DNA content and incorporated bromodeoxyuridine. Proc Natl Acad Sci U S A. 80 (18), 5573-5577 (1983).
- Toth, Z. E., Mezey, E. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. Journal of Histochemistry & Cytochemistry. 55 (6), 545-554 (2007).
- Stack, E. C., Wang, C., Roman, K. A., Hoyt, C. C. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 70 (1), 46-58 (2014).
- Kraemer, P. M., Petersen, D. F., Van Dilla, M. A. DNA constancy in heteroploidy and the stem line theory of tumors. Science. 174 (4010), 714-717 (1971).
- Dolbeare, F., Gratzner, H., Pallavicini, M. G., Gray, J. W. Flow cytometric measurement of total DNA content and incorporated bromodeoxyuridine. Proceedings of the National Academy of Sciences U S A. 80 (18), 5573-5577 (1983).
- Bandura, D. R. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Analytical Chemistry. 81 (16), 6813-6822 (2009).
- Bjornson, Z. B., Nolan, G. P., Fantl, W. J. Single-cell mass cytometry for analysis of immune system functional states. Current Opinion in Immunology. 25 (4), 484-494 (2013).
- Behbehani, G. K., Bendall, S. C., Clutter, M. R., Fantl, W. J., Nolan, G. P. Single-cell mass cytometry adapted to measurements of the cell cycle. Cytometry A. 81 (7), 552-566 (2012).
- Brodie, T. M., Tosevski, V. High-Dimensional Single-Cell Analysis with Mass Cytometry. Current Protocols in Immunology. 118, 5.11.11-15.11.25 (2017).
- McCarthy, R. L., Duncan, A. D., Barton, M. C. Sample Preparation for Mass Cytometry Analysis. Journal of Visual Experimentation. 122, (2017).
- Lichti, U., Anders, J., Yuspa, S. H. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nature Protocols. 3 (5), 799-810 (2008).
- Zhang, L. Defects in Stratum Corneum Desquamation Are the Predominant Effect of Impaired ABCA12 Function in a Novel Mouse Model of Harlequin Ichthyosis. PLoS One. 11 (8), e0161465 (2016).
- Zunder, E. R. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nature Protocols. 10 (2), 316-333 (2015).
- Sumatoh, H. R., Teng, K. W., Cheng, Y., Newell, E. W. Optimization of mass cytometry sample cryopreservation after staining. Cytometry A. 91 (1), 48-61 (2017).
- Finck, R. Normalization of mass cytometry data with bead standards. Cytometry A. 83 (5), 483-494 (2013).
- Trowbridge, I. S., Thomas, M. L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annual Review of Immunology. 12, 85-116 (1994).
- Torchia, E. C., Boyd, K., Rehg, J. E., Qu, C., Baker, S. J. EWS/FLI-1 induces rapid onset of myeloid/erythroid leukemia in mice. Molecular and Cellular Biology. 27 (22), 7918-7934 (2007).
- Liu, Z. A Simplified and Efficient Method to Isolate Primary Human Keratinocytes from Adult Skin Tissue. Journal of Visual Experimentation. (138), (2018).
- Jensen, K. B., Driskell, R. R., Watt, F. M. Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis. Nature Protocols. 5 (5), 898-911 (2010).
- Germain, L. Improvement of human keratinocyte isolation and culture using thermolysin. Burns. 19 (2), 99-104 (1993).
- Fluhr, J. W. Impact of anatomical location on barrier recovery, surface pH and stratum corneum hydration after acute barrier disruption. British Journal of Dermatology. 146 (5), 770-776 (2002).
- Webb, A., Li, A., Kaur, P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation. 72 (8), 387-395 (2004).
- Torchia, E. C., et al. A genetic variant of Aurora kinase A promotes genomic instability leading to highly malignant skin tumors. Recherche en cancérologie. 69 (18), 7207-7215 (2009).
- Frei, A. P. Highly multiplexed simultaneous detection of RNAs and proteins in single cells. Nature Methods. 13 (3), 269-275 (2016).

