Blood Collection Through Subclavian Vein Puncture in Mice
Summary
Here, we present a protocol to take blood samples from the subclavian vein of mice.
Abstract
The mouse is the foremost mammalian model for studying human disease and human health. However, blood sample collection from mice is challenging in research work. Tail blood collection is a popular method when a small amount of blood sample is needed. Orbital artery could be considered if a large amount of blood is needed but this blood collection method has ethical issues. Formerly, we demonstrated the feasibility and safety of blood sample collection through subclavian vein puncture in rats, and here we investigate whether this method could be used in mice. We report that this method is safe and practical for blood collection in mice. Blood collection through the subclavian vein puncture in mice can be a convenient method in daily research works.
Introduction
Blood sample collection from mice is essential in most research laboratories. The conventional approaches for blood collection in mice is tail cutting when less than 100 µL of sample is needed1. However, if more than 100 µL of blood are required at a nonterminal time point, retroorbital, submandibular bleeding or submental blood collection are the most commonly considered techniques2. In some occasions, the jugular vein catheterization through a surgical incision was adopted as an alternative method3.
Nevertheless, the above methods are harmful to the mice. To the best of our knowledge, the retroorbital method is not widely accepted because of the potential risk of complications4,5. Operation related trauma not only happens in the visible area6,7, but also deep within the orbit6. Besides, submandibular blood collection is stressful8 and might be associated with excessive bleeding2,9. Based on our prior research10,11, here we introduce a new strategy for blood collection from the subclavian vein in mice. The safety, feasibility, and obtained blood volume with this technique are presented and discussed.
Protocol
This study was approved by the Central South University Ethics Committee for Animal Research from The Second Xiangya Hospital (Changsha, China). The manuscript was prepared according to ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines12.
1. Material and animal
- Prepare required materials: 75% ethanol, adhesive tape, epilating agent, 2 mL tube, 1.0 mL syringe connected with needle (26G), electronic scale, heparin and saline (see Table of Materials).
- Animals: Prepare 10 Kunming mice, 6-8 weeks old and weighing 21.6-28.3 g (see Table of Materials). Maintain mice in accordance with the Guide for the Care and Use of Laboratory Animals13.
2. Anesthesia and animal positioning
- Weigh the mouse to calculate the required dosage of anesthetic agent.
- Inject sodium pentobarbital (60 mg/kg) through intraperitoneal injection to induce general anesthesia14 (Figure 1). Apply a sterile eye lubrication ointment at the start of the procedure to prevent damage to exposed eyes.
NOTE: Mice are considered to be sufficiently anesthetized when showing no motor response to testing of the pedal withdrawal reflex, tail pinch, or abdominal skin pinch. - Place the mouse in the operation table in a supine position and 2-4 cm away from the edge of the table to facilitate vein puncture (Figure 2). Fix the limbs in a comfortable position as shown in Figure 2. No intubation or mechanical ventilation is needed in the whole procedure.
- Apply epilating agent around the infraclavicular space with a cotton swab.
- Three minutes later, wash the epilating agent with a wet cotton swab to remove the fur and any visible dirt.
- Sterilize the infraclavicular space with 75% ethanol and then dry with clean gauze.
3. Subclavian vein puncture and blood collection
- Identify the location of the clavicle bone and superior sternal fossa with a finger.
- Locate the puncture site close to the middle of the clavicle bone and caudal to it. Put the left index finger lateral to the puncture site to fix the skin and subcutaneous tissue (Figure 3).
- Move the needle upward and cranially toward the superior sternal fossa. Once the needle enters the subcutaneous tissue, move the ring finger of the right hand backward to form negative pressure (Figure 3).
NOTE: During this step, the position of the operator´s right hand should be slightly lower than the operation table to make the needle move upward and superior to the horizontal plane. That is why the animal is placed near the edge of the table. - Move the syringe forward 3-4 mm but stop if there is no blood drain into the syringe. Then slowly draw back the syringe and keep negative pressure in it. In most occasions, the blood would enter the syringe when drawing back.
- Once the blood enters the syringe, fix the syringe and maintain negative pressure until the required volume (200 µL) of blood is collected in the syringe.
- After blood collection, withdraw the puncture needle, and press the puncture site with a cotton swab slightly for 1-2 minutes to stop bleeding. Then, return mice to the cage.
NOTE: Occasionally, one could not obtain the blood at his/her first attempt. Adjust the direction of the needle laterally and repeat steps 3.3-3.5. If 3 attempts fail to obtain blood sample, switch to the other side. - Transfer the blood sample into a heparinized tube.
4. Mice recovery
- While the pentobarbital anesthesia is in effect, speed recovery by providing heat support until the mouse is moving again.
Representative Results
We repeated this procedure in 10 Kunming mice (male n = 5, female n = 5, weight 25.4 ± 2.0 g). Nine procedures succeeded on the right side. One case failed within 3 attempts in the right side and blood collection succeeded on the left side. The time course (from puncture to obtaining required blood volume) ranged between 35-126 seconds (average 68.4 ± 26.4 s). Blood collection volume was set to around 200 µL (average 203 ± 11.6 µL). Blood collection succeeded within 1 to 4 attempts. Blood sampling succeeded on the first attempt in two mice and after 4 attempts in one mouse; the sampling took 2-3 attempts in the other mice. All data were illustrated in Table 1. All animals survived and recovered within 30 minutes after the blood sampling. There was no significant difference between sexes for all observed parameters (Table 1).
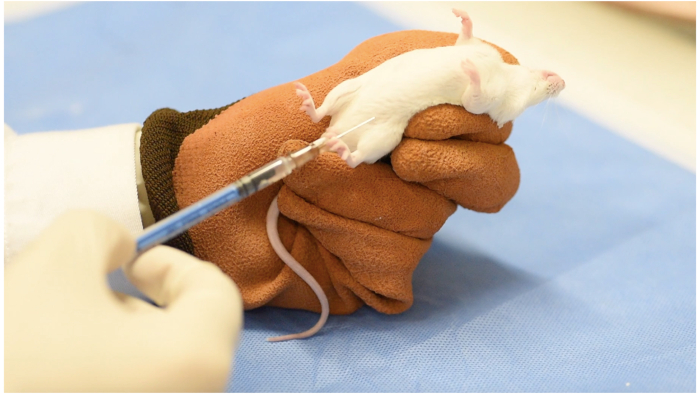
Figure 1: General anesthesia through intraperitoneal injection Please click here to view a larger version of this figure.

Figure 2: The supine position of mouse. Note the distance between margin and mouse is 2-4 cm. Please click here to view a larger version of this figure.
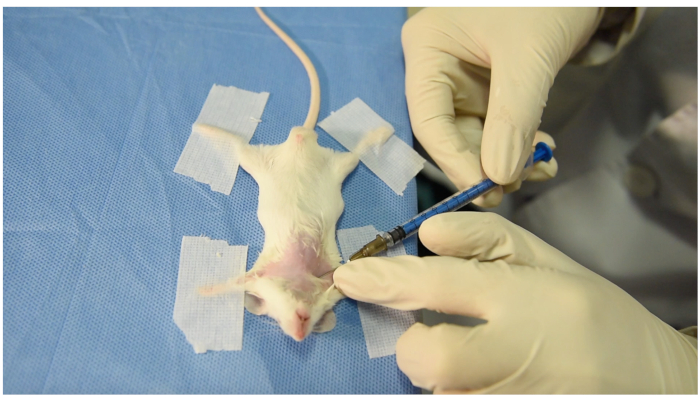
Figure 3: The puncture site and posture of operator's hands. Note that the ring finger of right hand moves backward to form negative pressure. The left hand is placed lateral to the puncture site to fix the skin and subcutaneous tissues. Please click here to view a larger version of this figure.
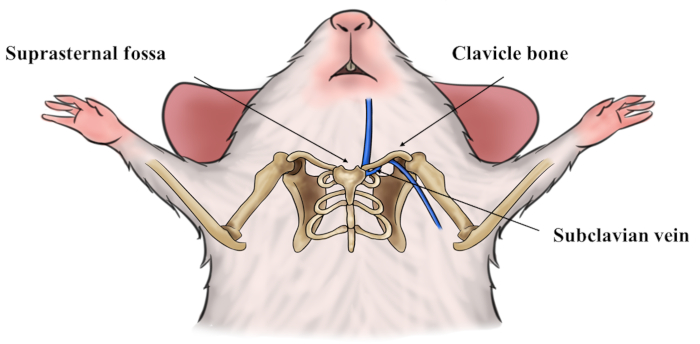
Figure 4: The location of the subclavian vein, clavicle bone and superior sternal fossa. The subclavian vein courses beneath the clavicle bone and drains into the superior vena cava under the superior sternal fossa. Please click here to view a larger version of this figure.
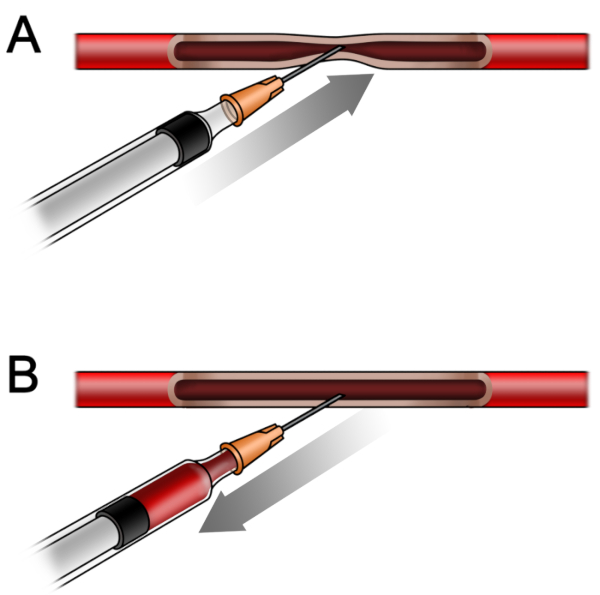
Figure 5: The relation between the vessel wall and needle during the procedure. (A) The walls of the subclavian vein attach to each other as the needle moves forward, so no blood could be drawn out. (B) While withdrawing the needle backward, the walls separate from each other and the blood could be drawn out. Please click here to view a larger version of this figure.
| variable | Total (n=10) | Male (n=5) | Female (n=5) | P Value |
| Body Weight (g) | 25.3 ± 2.2 | 25.3 ± 3.0 | 25.4 ± 1.3 | 0.926 |
| Blood Volume (μL) | 203.0 ± 11.6 | 208 ± 13.0 | 198 ± 8.4 | 0.187 |
| Time Course (s) | 68.4 ± 26.4 | 70.6 ± 35.1 | 66.2 ± 18.0 | 0.809 |
| Withdrawals | 2.3 ± 0.9 | 2.4 ± 1.1 | 2.2 ± 0.8 | 0.760 |
| There were no significant sex differences for all observed parameters, all P value >0.05. | ||||
Table 1: Observed parameters between the sexes
Discussion
This report represents an extension of previous research on blood sampling through the subclavian vein puncture in rats11. As the mouse is the most commonly used research animal, it would be valuable to see if this technique can also be applied to mice. The challenge comes from the relatively smaller diameter of the subclavian vein.
In the present research, we found that subclavian puncture in mice is a feasible and reliable way to collect blood. Compared to the conventional methods such as tail vein cutting or orbital blood collection, there are no ethical issues of the present method. All animals survived after this procedure without liquid supplements after around 200 µL of blood extraction, and animals could be used for further experimental studies. Theoretically, the total sample volume extraction could not reach more than 10% of total circulating blood volume each time and the total volume of an adult animal is 55 to 70 mL/kg body weight5. For the mice used in this research (21.6-28.3 g), the maximum volume extraction should be around 200 µL. We thus set the blood sampling amount around 200 µL for each mouse. The inability to measure blood pressure, heart rate, and other stress parameters are the main limitations of this report.
Sufficient general anesthesia is another important issue to guarantee the success of the puncture. The mice should remain quiet during the puncture procedure to avoid the risk of injuring the vessel by the needle. We found that pentobarbital sodium at 60 mg/kg for general anesthesia could satisfy ideal depth of anesthesia. A downside of this agent is the relatively long recovery time (averaging about 30 minutes). In some institutes, isoflurane inhalation is used for the short recovery time and should be taken into consideration as an alternative anesthesia choice15.
The location of subclavian vein is shown in Figure 4. Because of the thin wall and low pressure in the vein, the anterior and posterior wall of the subclavian vein would attach to each other under the pressure of a needle while the needle is moving forward (Figure 5A). While withdrawing the plunger slowly backwards, the attached walls will separate spontaneously, and the tip of needle could enter the true cavity of vessel and blood could drain into the syringe under sustained negative pressure (Figure 5B). Use of a vacuum blood collection system16 might also favor the blood collection. Due to the relatively small diameter of the mice subclavian vein, there can be failures even for an experienced operator. For a novice, we recommend subclavian vein puncture in rats as reported11. After several successful puncture attempts in rats, the success rate of subclavian vein puncture in mice could be significantly enhanced. Make sure that the exact puncture location is just caudal to the middle of the clavicle bone. The needle moving upward and cranial to the superior sternal fossa are other key points to guarantee a successful puncture.
In conclusion, subclavian vein puncture is a safe and effective method for blood collection in mice. Besides tail vein cutting and retro-orbital plexus blood collection, this method is feasible, safe, and suitable for observational researches at several time points in mice.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the grant from the National Natural Science Foundation of China No. 81670269, No. 81500355 and No. 81500226.
Materials
| 1.0 mL syringe | Shandong Weigao Group Medical Polymer Co., Ltd (Weihai, Shandong Province, China) | 20163151593 | |
| 75% ethanol | Department of Pharmacy, The Second Xiangya Hospital of Central South University | made in Department of Pharmacy,The Second Xiangya Hospital of Central South Univesity | |
| adhesive tape | 3M Deutschland GmbH (Kamen, Germany) | 1534-1 | |
| canvas gloves | for anesthesia | ||
| electronic scale | Dongguan Shengheng Electronics Co. Ltd (Dongguan, Guangdong Province, China) | KP-3000 | |
| epilating agent | France Yi Sha Cosmetics Co. Ltd (Guangzhou, Guangdong Province, China) | 8281744 | |
| heparin (used concentration 10 U/mL, 2 mL, 12500 IU) | Nanjing Xinbai Pharmaceutical Co. Limited (Nanjing, Jiangsu Province, China) | H32025851 | |
| mice | Hunan SJA Laboratory Animal Co. Ltd (Changsha, Hunan Province, China) | Kunming spcies | |
| needle, 26G, 0.45 mm x 16 mm | Shandong Weigao Group Medical Polymer Co. Ltd (Weihai, Shandong Province, China) | 20163151593 | |
| pentobarbital sodium (used solution 1%) | Merck | P-010-1ML | |
| physiological saline, 100 mL | Hunan Kelun Pharmaceutical Co. Ltd (Yueyang, Hunan Province,China) | H43020454 | |
| stastical software | International Business Machines | SPSS Statistics 25 | |
| tube, 2 mL | Hubei Jinxing Technology & Development Co. Ltd (Wuhan Hubei Province, China) | MCT-150-C | |
References
- McCosh, R. B., Kreisman, M. J., Breen, K. M. Frequent Tail-tip Blood Sampling in Mice for the Assessment of Pulsatile Luteinizing Hormone Secretion. Journal of Visualized Experiments. (137), (2018).
- Regan, R. D., Fenyk-Melody, J. E., Tran, S. M., Chen, G., Stocking, K. L. Comparison of Submental Blood Collection with the Retroorbital and Submandibular Methods in Mice (Mus musculus). Journal of the American Association for Laboratory Animal Science. 55 (5), 570-576 (2016).
- Park, A. Y., et al. Blood collection in unstressed, conscious, and freely moving mice through implantation of catheters in the jugular vein: a new simplified protocol. Physiological Reports. 6 (21), e13904 (2018).
- Heimann, M., Kasermann, H. P., Pfister, R., Roth, D. R., Burki, K. Blood collection from the sublingual vein in mice and hamsters: a suitable alternative to retrobulbar technique that provides large volumes and minimizes tissue damage. Laboratory Animals. 43 (3), 255-260 (2009).
- Parasuraman, S., Raveendran, R., Kesavan, R. Blood sample collection in small laboratory animals. Journal of Pharmacology & Pharmacotherapeutics. 1 (2), 87-93 (2010).
- Diehl, K. H., et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. Journal of Applied Toxicology. 21 (1), 15-23 (2001).
- van Herck, H., et al. Orbital sinus blood sampling in rats as performed by different animal technicians: the influence of technique and expertise. Laboratory Animals. 32 (4), 377-386 (1998).
- Tsai, P. P., et al. Effects of different blood collection methods on indicators of welfare in mice. Lab Anim (NY). 44 (8), 301-310 (2015).
- Holmberg, H., Kiersgaard, M. K., Mikkelsen, L. F., Tranholm, M. Impact of blood sampling technique on blood quality and animal welfare in haemophilic mice. Laboratory Animals. 45 (2), 114-120 (2011).
- Yang, H., et al. Safety and efficacy of a modified axillary vein technique for pacemaker implantation. Cardiology Plus. 3, 104-107 (2018).
- Yang, H., et al. Subclavian Vein Puncture As an Alternative Method of Blood Sample Collection in Rats. Journal of Visualized Experiments. (141), (2018).
- Kilkenny, C., Altman, D. G. Improving bioscience research reporting: ARRIVE-ing at a solution. Laboratory Animals. 44 (4), 377-378 (2010).
- Research, I. f. L. A. . Guide for the care and use of laboratory animals. , (1996).
- Redel, A., et al. Impact of ischemia and reperfusion times on myocardial infarct size in mice in vivo. Experimental Biology and Medicine (Maywood, N.J.). 233 (1), 84-93 (2008).
- Tsukamoto, A., Serizawa, K., Sato, R., Yamazaki, J., Inomata, T. Vital signs monitoring during injectable and inhalant anesthesia in mice. Experimental Animals. 64 (1), 57-64 (2015).
- Zou, W., et al. Repeated Blood Collection from Tail Vein of Non-Anesthetized Rats with a Vacuum Blood Collection System. Journal of Visualized Experiments. (130), (2017).

