Measurement of the Potential Rates of Dissimilatory Nitrate Reduction to Ammonium Based on 14NH4+/15NH4+ Analyses via Sequential Conversion to N2O
Summary
A series of methods to determine the potential DNRA rate based on 14NH4+/15NH4+ analyses is provided in detail. NH4+ is converted into N2O via several steps and analyzed using quadrupole gas chromatography–mass spectrometry.
Abstract
The importance of understanding the fate of nitrate (NO3−), which is the dominant N species transferred from terrestrial to aquatic ecosystems, has been increasing because global nitrogen loads have dramatically increased following industrialization. Dissimilatory nitrate reduction to ammonium (DNRA) and denitrification are both microbial processes that use NO3− for respiration. Compared to denitrification, quantitative determinations of the DNRA activity have been carried out only to a limited extent. This has led to an insufficient understanding of the importance of DNRA in NO3− transformations and the regulating factors of this process. The objective of this paper is to provide a detailed procedure for the measurement of the potential DNRA rate in environmental samples. In brief, the potential DNRA rate can be calculated from the 15N-labeled ammonium (15NH4+) accumulation rate in 15NO3− added incubation. The determination of the 14NH4+ and 15NH4+ concentrations described in this paper is comprised of the following steps. First, the NH4+ in the sample is extracted and trapped on an acidified glass filter as ammonium salt. Second, the trapped ammonium is eluted and oxidized to NO3− via persulfate oxidation. Third, the NO3− is converted to N2O via an N2O reductase deficient denitrifier. Finally, the converted N2O is analyzed using a previously developed quadrupole gas chromatography–mass spectrometry system. We applied this method to salt marsh sediments and calculated their potential DNRA rates, demonstrating that the proposed procedures allow a simple and more rapid determination compared to previously described methods.
Introduction
The artificial synthesis of nitrogen fertilizer and its widespread application have greatly perturbed the global nitrogen cycle. It is estimated that the transfer of reactive nitrogen from terrestrial to coastal systems has doubled since pre-industrial times1. A significant portion of fertilizers applied to a given field is washed away from the soil to rivers or groundwater, primarily as NO3− 2. This may cause environmental problems such as drinking water pollution, eutrophication, and the formation of hypoxia. NO3− in water environments is removed from or retained in the ecosystem via biological assimilation and various microbial dissimilatory processes. Denitrification and anammox are known to be major microbial removal processes for NO3−. Denitrification is the microbial reduction of NO3− to gaseous N products (NO, N2O, and N2) coupled with the oxidation of an electron donor, such as organic substances, thereby reducing the risk of the above-mentioned problems. Anammox also produces N2 from NO2− and NH4+; therefore, it removes inorganic N from an ecosystem. Conversely, DNRA works to retain N in an ecosystem; it is generally accepted that DNRA is performed primarily by fermentative bacteria or chemolithoautotrophic bacteria and that they reduce dissimilatory NO3− to bioavailable and less mobile NH4+.
Studies on DNRA have primarily been performed in marine or estuarine ecosystems, such as oceanic or estuarine sediments and water, salt or brackish marsh soil, and mangrove soil. Coastal or marine ecosystems are important as reservoirs for removing NO3− from terrestrial ecosystems, and in previous studies DNRA has been shown to contribute over a very wide range of NO3− removal (0–99%)3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18. Further, the existence of DNRA has been demonstrated in a wide range of environments including freshwater environments19, rice paddy soils20, and forest soils21. While these studies have shown that DNRA is potentially comparable to denitrification for NO3− removal, studies measuring the DNRA activity are still very limited compared to those measuring denitrification.
The DNRA rate has been evaluated using 15N-labeling techniques in conjunction with data analysis via analytical or numerical models. One analytical solution to calculate the DNRA rate is based on the increase in the 15N enrichment of the NH4+ pool after the addition of 15NO3− as a tracer. 15N-labeled NO3− is added to a sample and incubated, and the DNRA rate can then be calculated from the concentration and isotope ratio changes in NH4+ before and after a certain period of time. In this paper, a method to quantify the NH4+ concentration and the isotope ratio, which are required to calculate the DNRA rate, is described in detail. Basically, the method reported here is a combination of several previously reported techniques22,23,24,25,26 with modifications added to some procedures. The method is comprised of a series of five component procedures: (1) incubation of an environmental sample with the amendment of a stable isotope tracer, 15NO3−, (2) extraction and recovery of NH4+ using a “diffusion procedure” with modifications, (3) persulfate oxidation of NH4+ in the sample, consisting of indigenous NH4+ and 15NH4+ derived from 15NO3− via DNRA activity, into NO3− and 15NO3−, (4) subsequent microbial transformation of NO3− and 15NO3− to N2O isotopomers via the modified denitrifier method, and (5) quantification of the N2O isotopomers using gas chromatography–mass spectrometry (GC/MS). In the following section, first, the preparation for procedures (2) and (4) is described and then, subsequently, all five component procedures are described in detail.
Protocol
1. Preparation of a PTFE envelope for quantitatively capturing gaseous NH3
- Place a 60-mm piece of polytetrafluoroethylene (PTFE) tape (25 mm in width) on a small sheet of aluminum foil (approximately 300 mm x 450 mm in size, wiped with ethanol).
- Ash a glass fiber filter (10 mm in diameter with a pore size of 2.7 μm) at 450 °C for 4 h in a muffle furnace. Place the glass fiber filter a little above the midpoint of the longer axis of the tape (Figure 1a).
- Spot 20 µL of 0.9-mol/L H2SO4 on the center of a GF/D filter, and immediately fold the PTFE tape using two tweezers: flat-ended stamp tweezers and straight-ended tweezers. The following steps, steps 1.4–1.7, are shown in Figure 1 and should be conducted swiftly.
- Flip the PTFE tape over the GF/D filter at the dotted line shown in Figure 1a to form the shape shown in Figure 1b.
- Seal both sides by folding and then tightly pressing the edge with the tweezers (Figure 1c). Do not press too hard, and do not scratch the PTFE tape.
- Fold the open end with the tweezers, and then press the edge with the tweezers (Figure 1d).
- Seal the open end by tightly pressing the edge with the tweezers (Figure 1e). The GF/D filter should not be pressed during this procedure.
2. Preparing the biomass of a nitrous oxide reductase deficient denitrifier, Pseudomonas chlororaphis subsp. aureofaciens ATCC13985, for the denitrifier method
- Streak a 20% glycerol stock of Pseudomonas chlororaphis subsp. aureofaciens ATCC13985 on 1/4 strength tryptone soy broth (TSB) agar plates. Incubate the plates at 25 °C for 2–3 days.
- Transfer a singles colony of P. chlororaphis to a small test tube containing 5 mL of autoclaved TSB medium and culture aerobically (without shaking) for a day at 25 °C in dark until obtaining maximum growth; this will be used as the preculture.
- Transfer 3 mL of the preculture to a 1-L bottle with a silicon rubber stopper containing 1 L of freshly prepared autoclaved modified TSB supplemented with 10 mmol/L KNO323. Incubate the bottle while agitating using a stirrer under dark conditions at 25 °C. After cultivating for 8 h, replace the silicon rubber stopper with a screw cap and close tightly. Continue the cultivation in anoxia overnight.
- Centrifuge the culture at 18,800 x g for 15 min at 4 °C to obtain biomass pellets.
- Wash the packed biomass three times with 30 mL of Dulbecco’s phosphate-buffered saline (D-PBS(-), pH 7.5), to completely eliminate the NO3−. The conditions of the centrifugation are same as in Step 2.3.
- After washing, re-suspend the packed biomass with 30 mL of D-PBS(-). Use 1 mL of the suspension to determine the cell density by measuring OD600. Pipette 1 mL aliquots of the remaining suspension into sterile cryovials containing 0.8 mL of 45% glycerol. Preserve the prepared glycerol stocks at −80 °C until use (see section 6).
3. Elimination of oxygen, nitrite, and nitrate from the sample sediment
- Weigh 3.0 g (wet weight) of sediment into a 20 mL glass vial and add 9.0 mL of surface water to suspend it (25% w/w slurry).
- Seal the vial with a black butyl rubber stopper (washed with ion-exchanged water and sterilized via autoclaving) and an aluminum cap.
- Purge the suspension and replace the headspace air with Ar (>99.99%) at 0.6 L/min for 20 min using a manifold.
- Replace the Ar headspace gas with ultra-pure (>99.99995%) He by vacuuming for 90 s and charging He for 30 s. Repeat this procedure four times. Set the headspace gas pressure to 1.5 atm.
- Incubate the vials at 20 °C overnight with shaking at 150 rpm under dark conditions using a constant temperature shaker to eliminate the remaining oxygen, nitrate, and nitrite in the sediment suspension and headspace gas.
4. Time course experiment for determining DNRA rate
- Replace the headspace gas with fresh ultra-pure He using the same procedure as in step 3.5 but without the four repetitions.
- Add labeled and non-labeled substrates to each vial according to Table 1 through the butyl rubber stopper using a gastight syringe. Purge the previously prepared substrate solutions in adequately sized glass vials using ultra-pure He with the pressure of the headspace set to 1.5 atm to avoid air contamination. Avoid the unintentional injection of any amount of air during the injection procedures.
- Incubate vials at 20 °C with shaking at 150 rpm. Add the substrates to each vial according to Table 1 and incubate for 1 h, 3 h, and 5 h. After incubation is halted, subject the sediment suspension in the vials to the following procedures: steps 4.4–4.9.
- Remove the aluminum cap and butyl rubber stopper from each vial. Then, add KCl (ashed at 450 °C for 4 h) to the sediment suspension up to a final concentration of approximately 2 mol/L to ensure the recovery of NH4+ from the sediment. Close the vials with a butyl rubber stopper and an aluminum closure.
- Shake the sediment at 150 rpm for 1 h at 4 °C under dark conditions to extract the NH4+.
- Transfer the entire sediment suspension in the vial to a 50 mL plastic centrifuge tube, and centrifuge at 10,000 x g for 10 min at 4 °C.
- Rinse the internal wall of a freshly opened 10-mL disposable syringe, and attach it to a freshly opened disposable cellulose acetate membrane filter (pore size 0.22 µm, 25 mm in diameter). Then, rinse the membrane filter with 1 mL of supernatant. Place the rinsed syringe–filter unit onto a 20-mL wide-mouth polypropylene (PP) bottle.
- Filter the remaining supernatant through an acetate membrane filter into the 20-mL PP bottle. Store the extracts extracts at −20 °C until further analysis.
5. Capturing diffused NH4+ in 2M H2SO4 absorbed to the GF/D filter in the PTFE envelope and the persulfate oxidation of NH4+ to NO3−
- Prepare standard solutions of 14NH4Cl with concentration gradients of 0 μmol/L, 10 μmol/L, 40 μmol/L, 100 μmol/L, 200 μmol/L, 400 μmol/L, and 500 μmol/L. For the 15N ratio analysis, fix the total concentration of NH4+ to 200 μmol/L and prepare isotope ratios of 100:0, 99.5:0.5, 99:1, 93:7, 90:10, 50:50, and 10:90 with pure 14NH4Cl and 15NH4Cl standard solutions.
- Transfer 30 mg of MgO (ashed at 450 °C for 4 h) to a 20-mL glass vial, and place the PTFE envelope in the vial.
- Transfer 5 mL of a sample or standard into the vial containing the MgO and the PTFE envelope, and immediately close with a gray butyl rubber stopper. Seal with an aluminum cap. If the concentration of NH4+ is expected to exceed 500 μmol/L, dilute the sample to below 500 μmol/L.
- Shake the vials at 150 rpm for 3 h at 4 °C under dark conditions.
- Remove the aluminum cap and the butyl rubber stopper. Take the PTFE envelope out of the vial using point-ended tweezers, thoroughly rinse the envelope and the tweezers with ion-exchanged water, wipe them with a wiping paper, and then place the envelope on a fresh wiping paper.
- Open the PTFE envelope with a couple of tweezers (use of both the flat-ended and point-ended tweezers is recommended) in the exact reverse order of the folding performed in steps 1.4–1.7.
- Hold the peripheral area of the GF/D filter, where the H2SO4 is supposed to be unabsorbed, with the flat-ended tweezers, and transfer it into an 11-mL screw cap test tube with a PTFE -lined cap. Rinse the tweezers with ion-exchanged water, and wipe them with wiping paper.
- Repeat steps 5.5–5.7 for the remaining envelopes.
- Add 1 mL of ion-exchanged water to each of the test tubes, close the screw cap, and maintain it without shaking for at least 30 min at room temperature to completely elute the NH4+ from the GF/D filter. During this step, carry out the following step (step 5.10) in parallel.
- Prepare the persulfate-oxidizing solution (POR) reagent25,26.
- Because it cannot be stored, prepare the exact amount of POR needed for treating a single day of samples.
- To prepare POR to treat 50 samples, pour 100 mL of ion-exchanged water into a 200-mL screw cap bottle and add 1.52 g of NaOH (nitrogen compound analysis grade), 3 g of boric acid, and 5 g of K2S2O8 (nitrogen and phosphorus analysis grade) in that order. Immediately after adding each reagent, shake the solution until it is completely dissolved.
- If necessary, soak the bottle in warm water to help the dissolution of the chemicals; however, attention should be paid with respect to the contamination of NO3− because tap water normally contains NO3−.
- After Step 5.8, open the screw cap, add 2 mL of the POR reagent to the test tube, and close the tube tightly with a screw cap to prevent any loss or contamination during the following steps.
- Stand the test tubes on a rack, wrap them in double-layered aluminum foil, and autoclave them for 1 h at 121 °C. Keep the tubes in an upright position during this step and avoid rapid changes in temperature after finishing the autoclaving.
6. Determining the NO3− converted from NH4+ by the denitrifier method using quadrupole GC/MS
- Mix 100 mL of sterile 40-mmol/L phosphate buffer (pH 7.2) and 100 mL of sterile 30-mmol/L glucose aseptically (20-mmol/L phosphate and 15-mmol/L glucose; final).
- Add a 1/7.2 volume of glycerol stock of P. chlororaphis to 200 mL of the phosphate-buffered glucose solution in a 300-mL Erlenmeyer flask, and purge with an ultra-pure He (>99.995%) stream for 1 h.
- Dispense 2.0 mL of the denitrifier suspension into each 5-mL vial. Cap the vials with a gray butyl rubber stopper and an aluminum closure.
- Replace the headspace air with ultra-pure He by vacuuming for 3 min and charging the He for 1 min. Set the headspace gas positive pressure to 1.3 atm to avoid unintentional air contamination.
- Inject 1 mL of a sample or standard through the butyl rubber stopper using a 1-mL disposable syringe. Note the exact amount of the sample actually injected.
- Incubate the vials overnight at 25 °C under dark conditions.
- Inject 0.3 mL of 6-mol/LL NaOH to stop the denitrification and absorb the headspace CO2, which will otherwise seriously disturb the N2O analysis by GC/MS because CO2 and N2O have the same molecular weight.
- Determine the amounts of 44N2O, 45N2O, and 46N2O in the headspace gas using quadrupole GC/MS with a modified injection port25. The operating conditions used for the GC/MS analysis are shown in Table 2.
7. Data analysis
- Derive the calibration curve for the NH4+ concentration from the linear relationship between the known concentration of 14NH4+ and the measured signal intensities of the total produced 44N2O + 45N2O + 46N2O. Derive the calibration curve for the 15N content from the linear relationship between the known atom% (i.e., 15N/14N+15N) and the calculated atom% using a previously provided equation27. Calculate the concentration of 15NH4+ by multiplying the total NH4+ by the 15N ratio of NH4+.
- Calculate the potential DNRA rate using equations provided elsewhere28,29,30.
Representative Results
The representative results presented in this paper were derived from 15N-tracing experiments of salt marsh sediments. The sampled salt marsh was newly created in the aftermath of the 2011 Great East Japan Earthquake in the Moune area of Kesen-numa city in Miyagi Prefecture, Japan. In September 2017, surface sediments (0–3 cm) were collected at two sites in the subtidal and intertidal zones. First, immediately after collection, the sediment was sieved through a 4-mm mesh to remove plant roots, shells debris, and rubble and then homogenized. The samples were stored at 4 °C until the DNRA analysis was conducted.
The incubation procedures for the 15NO3− and the simultaneous determination of the 14NH4+ and 15NH4+ concentrations were carried out as described in the protocol section. An increase in the 15NH4+ concentration throughout the incubation period was observed for all sediments (Figure 2). We calculated the DNRA rates by dividing the accumulation rate of 15NH4+ by the isotope ratio of the NO3− pool29. The calculated rates were within the range of 24.8–177 nmol-N g−1 dry soil h−1 (Table 3) and were comparable to values found in previous studies. This range of obtained rates is higher than the reported values derived from similar environments including those from intertidal sediments17, salt marshes5,16, and other estuarine environments18,33,34, as well as from eutrophic environments such as a shallow river estuary in North Carolina31 and the Shanghai urban river networks32. Conversely, Fernandes et al.13 reported higher potential DNRA rates in organically rich mangrove soils in India. In general, DNRA is thought to be favored by a high ratio of available C to electron acceptors35,36,37. The samples demonstrating the representative results were taken from a salt marsh newly created by an earthquake, which had originally been used as a cultivation field. This particular characteristic of the samples may contribute to the observed high DNRA rate. Consistent with this speculation, the DNRA rate in the intertidal zone, which is rich in organic compounds (data not shown) compared to the subtidal zone, was higher than that in the subtidal zone (Figure 2, Table 3).
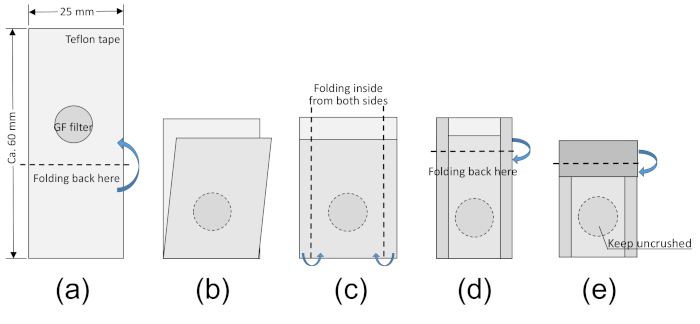
Figure 1: Preparation of a PTFE envelope for capturing gaseous NH3. The PTFE envelope used in the diffusion procedure is prepared by folding PTFE tape following the instructions shown in panels (A)–(E). The acidified filter inside the envelope captures the gaseous NH3. These steps should be conducted quickly. Detailed information is given in steps 1.2–1.7 in the protocol section. Please click here to view a larger version of this figure.
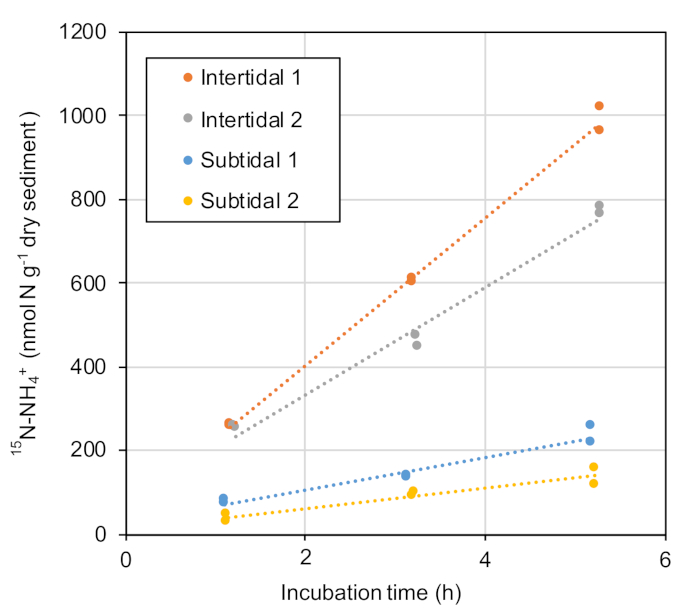
Figure 2: Change in the 15NH4+ concentration via the anaerobic incubation of sediments. The 15N tracer incubations of the sediment samples were conducted in duplicate. The concentration of 15N-NH4+ is shown in nmol per dry weight of sediment. Please click here to view a larger version of this figure.
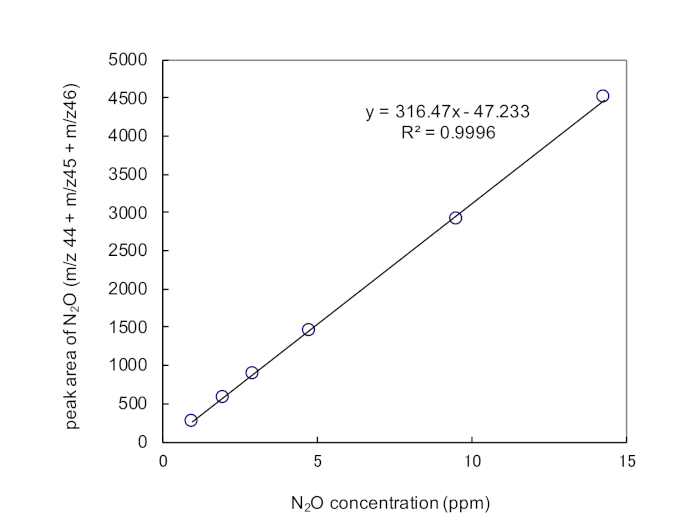
Figure 3: An example of calibration curve of low concentration N2O. Peak area of N2O was obtained by sum of the peak area of m/z 44, m/z 45, and m/z 46. Configurations for GC/MS analysis is shown in Table 2. Please click here to view a larger version of this figure.
| 15N-labeled and non-labeled substrates added to each vial | ||
| 100mM | 100mM | |
| NH4Cl | K15NO3 | |
| Volume (µL) of 100 mM stock solution added to each vial | 24 | 60 |
| Final concentration (µmol/L)* | >230§ | 570 |
| *shown values are calculated by assuming that water content of the sediment is 50% | ||
| §depending on the background ammonium concentration | ||
Table 1: Combinations of substrates amended to vials retaining approximately 11 mL of the sediment suspension. Samples were prepared in duplicate and subjected to further analyses after 1 h, 3 h, and 5 h of incubation.
| Equipment | |
| quadrupole GCMS | Shimadzu GCMS-QP2010 Ultra |
| column | CP-PoraBONDQ 25m; φ 0.32mm; film thickness, 5µm |
| Analytical conditions | |
| column temp | 40 °C |
| injection port temp | 100 °C |
| carrier gas stream | Total flow rate, 47.1 mL•min-1 flow rate in column, 2.10 mL•min-1 |
| sprit ratio | 20 |
| detection voltage | 1.5 kv |
| Sensitivity of N2O | |
| lower limit of detection (LOD)* | 1.42 pmol |
| lower limit of quantification (LOQ)* | 4.58 pmol |
| *LOD and LOQ were determined by a linear relationship among a serial dilution of N2O (0.97, 1.94, 2.91, 4.75, 9.50, 14.3 ppm) in He, corresponding responses in peak area, and S/N ratio. LOD and LOQ were calculated as concentrations equivalent to S/N=3 and S/N=10, respectively. |
Table 2: Conditions for the GC/MS analysis.
| sediment | DNRA rate | enrichment of NO3– * |
| nmol-N g-1 hr-1 | atom% | |
| Intertidal 1 | 177 | 99.9 |
| Intertidal 2 | 129 | 99.0 |
| Subtidal 1 | 39.3 | 99.9 |
| Subtidal 2 | 24.8 | 99.0 |
| *same as atom% of added KNO3; complete elimination and no nitrification under used incubation conditions was tested previously. |
Table 3: Potential DNRA rates of the tested intertidal and subtidal sediments.
Discussion
The concentration and isotope ratio of NH4+ for the DNRA analysis was quantified using several methods. The concentrations and isotope ratios of NH4+ are generally measured separately. The NH4+ concentration is typically measured using colorimetric methods including an autoanalyzer4,10,15,16,17. The isotope ratio measurement has wide variations depending on its method of NH4+ conversion, trapping, and instrumentation for the analysis. Typical methods include the following:
(1) The NH4+ in the sample is converted to NH3 via the addition of MgO or NaOH. After moving from the liquid phase to the gas phase, the NH3 is trapped on an acidified glass filter or in an acid solution. After drying, the filter is combusted and analyzed as N2 using an elemental analyzer/isotope ratio mass spectrometer (EA-IRMS)11,18,22,38. Alternatively, the captured NH4+ in an acid solution is collected on an adsorbent (e.g., zeolite) and is combusted and analyzed via EA-IRMS10,12.
(2) NH4+ is oxidized to N2 via hypobromite oxidation. The isotopic composition of the evolved N2 is measured via IRMS4,14,39 or membrane-introduction mass spectrometry (MIMS)16,17.
(3) The NH4+ concentration is determined using high-performance liquid chromatography without any conversion. Because the equilibrium of NH4+ and NH3 is slightly different for 15NH4+ and 14NH4+ near the pH of pKa, the 14NH4+ and 15NH4+ concentrations can be determined based on a small shift in the retention time6,19,40.
The method described in this paper is basically the same as approach (1) listed above, i.e., the step recovering NH4+ as NH3 on a glass filter under alkaline conditions; however, it is different with respect to the following sequential NH4+ conversion steps. These conversion steps are based on previous studies with added modifications to shorten the required time for a series of experiments. First, we made a few modifications to the original denitrifier method. After the biomass of P. chlororaphis is prepared as previously described23,24, we grow bacteria and preserve the concentrated cell suspension as a glycerol stock. This dense cell suspension can be directly used for the isotope analysis by mixing it with a buffer solution because the denitrifying activity has already been sufficiently induced. Even though further investigation is required, this modification may improve the reproducibility of the analysis because the presented method enables the mass-cultivation of denitrifier cells, which are directly available for analyses. We also modified the composition of the solution for suspending the bacterial cells, from a medium based on TSB to a phosphate-buffered glucose solution, to exclude the unnecessary components such as peptone in the original medium. This modification may reduce contamination by blank N because the phosphate-buffered glucose solution does not contain N, unlike the original medium used in previous studies; this should be tested via further analyses. In the step collecting NH4+ via the diffusion method, we shortened the incubation period and lowered the temperature to minimize the breakdown of organic N and any unfavorable conversion or loss of NH4+. The validity of this modification was checked using the linearity of the calibration curve. We also checked that the modified temperature and incubation period did not affect the recovery of NH4+ (data not shown).
Another advantage of this method is that NH4+ is ultimately converted to N2O, which has a low atmospheric background and can be measured using quadrupole GC/MS, which is less expensive and easier to manage than IRMS. Under the condition shown in table 2, CO2 (m/z 44) and N2O (m/z 44) are completely separated by GC; the retention time of these gases are 1.15 and 1.07 min, respectively. Since atmospheric concentration of N2O is on the order of ppb, N2O can be measured with negligible air interference even in low concentrations. The calibration curve of N2O passes almost through the origin, demonstrating the influence on the concentration of N2O due to atmospheric contamination is very limited (Figure 3). This method also has the advantage that it can quantify the 14NH4+ and 15NH4+ concentrations together; canonical methods, except approach (3) listed above, require individual analyses for the concentration and the isotope ratio.
Overall, the limits of detection and quantification for NH4+ using this method were approximately 0.03 μmol and 0.09 μmol, respectively, and these values are equivalent to 6 μmol/L and 18 μmol/L, if 5 mL of the sample solution (i.e., the sediment extract in this case) is used for the diffusion procedure as described in this paper. Even though using the colorimetric method is recommended to determine the NH4+ concentration of samples that have low NH4+, the proposed method effectively determines the NH4+ isotope ratio and the concentration in samples with relatively high concentrations of ammonium.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Naoto Tanaka for helping data collection and developing the protocol. The collection of samples was supported by JSPS KAKENHI Grant Number 17K15286.
Materials
| 15N-KNO3 | SHOKO SCIENCE | N15-0197 | |
| 15N-NH4Cl | SHOKO SCIENCE | N15-0034 | |
| 20 mL PP bottle | SANPLATEC | 61-3210-18 | Wide-mouth |
| Aluminum cap | Maruemu | 1307-13 | No. 20, with hole |
| Boric acid | Wako | 021-02195 | |
| Centrifuge | HITACHI | Himac CR21G II | |
| Deoxygenized Gas Pressure & Replace Injector | SANSIN INDUSTRIAL | IP-12 | |
| Disposable cellulose acetate membrane filter | ADVANTEC | 25CS020AS | Pore size 0.22 µm, 25 mm in diameter |
| Disposable syringe | Termo | SS-10SZ | 10 mL |
| Disposable syringe | Termo | SS-01T | 1 mL |
| Dulbecco’s Phosphate Buffered Saline (-) | NISSUI PHARMACEUTICAL | 5913 | |
| Gastight syringe | VICI Valco Instruments | 4075-15010 | Series A-2, 100 µL |
| GC/MS | shimadzu | GCMS-QP2010ultra | |
| GF/D | Whatman | 1823-010 | 10 mm in diameter |
| Glass vial | Maruemu | 0501-06 | 20 mL |
| Gray butyl rubber stopper | Maruemu | 1306-03 | No.20-S |
| H2SO4 | Wako | 192-04696 | Guaranteed Reagent |
| K2S2O8 | Wako | 169-11891 | Nitrogen and Phosphorus analysis grade |
| KCl | Wako | 163-03545 | Guaranteed Reagent |
| KNO3 | Wako | 160-04035 | Guaranteed Reagent |
| NaOH | Wako | 191-08625 | Nitrogen compounds analysis grade |
| NH4Cl | Wako | 017-02995 | Guaranteed Reagent |
| Plastic centrifuge tube | ASONE | 1-3500-22 | 50 mL, VIO-50BN |
| Pseudomonas chlororaphis subsp. aureofaciens | American Type Culture Collection (ATCC) | ATCC 13985 | Freeze-dried, the type strain of Pseudomonas aureofaciens |
| PTFE sealing tape | Sigma-Aldrich | Z221880 | 25 mm in width |
| Reciprocating shaker | TAITEC | 0000207-000 | NR-10 |
| Screw-cap test tube | IWAKI | 84-0252 | 11 mL |
| PTFE-lined cap for test tube | IWAKI | 84-0262 | |
| Tryptic Soy Broth | Difco Laboratories | 211825 |
References
- Gruber, N., Galloway, J. N. An Earth-system perspective of the global nitrogen cycle. Nature. 451 (7176), 293-296 (2008).
- Galloway, J. N., et al. The Nitrogen Cascade. Bioscience. 53 (4), 341-356 (2003).
- Rysgaard, S., Risgaard-Petersen, N., Sloth, N. P., Caumette, P., Castel, J., Herbert, R. Nitrification, denitrification, and nitrate ammonification in sediments of two coastal lagoons in Southern France. Coastal Lagoon Eutrophication and Anaerobic Processes (C.L.E.AN.). Developments in Hydrobiology. 117, 133-141 (1996).
- Christensen, P. B., Rysgaard, S., Sloth, N. P., Dalsgaard, T., Schwærter, S. Sediment mineralization, nutrient fluxes, denitrification and dissimilatory nitrate reduction to ammonium in an estuarine fjord with sea cage trout farms. Aquatic Microbial Ecology. 21, 73-84 (2000).
- Tobias, C. R., Anderson, I. C., Canuel, E. A., Macko, S. A. Nitrogen cycling through a fringing marsh-aquifer ecotone. Marine Ecology Progress Series. 210, 25-39 (2001).
- An, S. M., Gardner, W. S. Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Marine Ecology Progress Series. 237, 41-50 (2002).
- Gardner, W. S., et al. Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnology & Oceanography. 51 (1), 558-568 (2006).
- Preisler, A., et al. Biological and chemical sulfide oxidation in a Beggiatoa inhabited marine sediment. The ISME Journal. 1 (4), 341-353 (2007).
- Gardner, W. S., McCarthy, M. J. Nitrogen dynamics at the sediment-water interface in shallow, sub-tropical Florida Bay: why denitrification efficiency may decrease with increased eutrophication. Biogeochemistry. 95 (2-3), 185-198 (2009).
- Dong, L. F., et al. Changes in benthic denitrification, nitrate ammonification, and anammox process rates and nitrate and nitrite reductase gene abundances along an estuarine nutrient gradient (the Colne estuary, United Kingdom). Applied and Environmental Microbiology. 75 (10), 3171-3179 (2009).
- Koop-Jakobsen, K., Giblin, A. E. The effect of increased nitrate loading on nitrate reduction via denitrification and DNRA in salt marsh sediments. Limnology & Oceanography. 55 (2), 789-802 (2010).
- Dong, L. F., et al. Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnology & Oceanography. 56 (1), 279-291 (2011).
- Fernandes, S. O., Bonin, P. C., Michotey, V. D., Garcia, N., LokaBharathi, P. A. Nitrogen-limited mangrove ecosystems conserve N through dissimilatory nitrate reduction to ammonium. Scientific Reports. 2, 419 (2012).
- Behrendt, A., de Beer, D., Stief, P. Vertical activity distribution of dissimilatory nitrate reduction in coastal marine sediments. Biogeosciences. 10 (11), 7509-7523 (2013).
- Song, G. D., Liu, S. M., Marchant, H., Kuypers, M. M. M., Lavik, G. Anammox denitrification and dissimilatory nitrate reduction to ammonium in the East China Sea sediment. Biogeosciences. 10 (11), 6851-6864 (2013).
- Yin, G., Hou, L., Liu, M., Liu, Z., Gardner, W. S. A novel membrane inlet mass spectrometer method to measure 15NH4+15+ for isotope-enrichment experiments in aquatic ecosystems. Environmental Science & Technology. 48 (16), 9555-9562 (2014).
- Zheng, Y., et al. Tidal pumping facilitates dissimilatory nitrate reduction in intertidal marshes. Scientific Reports. 6, 21338 (2016).
- Bu, C., et al. Dissimilatory Nitrate Reduction to Ammonium in the Yellow River Estuary: Rates, Abundance, and Community Diversity. Scientific Reports. 7, 6830 (2017).
- Scott, J. T., McCarthy, M. J., Gardner, W. S., Doyle, R. D. Denitrification, dissimilatory nitrate reduction to ammonium, and nitrogen fixation along a nitrate concentration gradient in a created freshwater wetland. Biogeochemistry. 87 (1), 99-111 (2008).
- Shan, J., et al. Dissimilatory Nitrate Reduction Processes in Typical Chinese Paddy Soils: Rates, Relative Contributions, and Influencing Factors. Environmental Science & Technology. 50 (18), 9972-9980 (2016).
- Silver, W. L., Herman, D. J., Firestone, M. K. Dissimilatory nitrate reduction to ammonium in upland tropical forest soils. Ecology. 82 (9), 2410-2416 (2001).
- Holmes, R. M., McClelland, J. W., Sigman, D. M., Fry, B., Peterson, B. J. Measuring 15N–NH4+ in marine, estuarine and fresh waters: An adaption of the ammonia diffusion method for samples with low ammonium concentrations. Marine Chemistry. 60 (3-4), 235-243 (1998).
- Sigman, D. M., et al. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Analytical Chemistry. 73 (17), 4145-4153 (2001).
- Weigand, M. A., Foriel, J., Barnett, B., Oleynik, S., Sigman, D. M. Updates to instrumentation and protocols for isotopic analysis of nitrate by the denitrifier method. Rapid Communications in Mass Spectrometry. 30 (12), 1365-1383 (2016).
- Isobe, K., et al. Analytical techniques for quantifying 15N/14N of nitrate, nitrite, total dissolved nitrogen and ammonium in environmental samples using a gas chromatograph equipped with a quadrupole mass spectrometer. Microbes and Environments. 26 (1), 46-53 (2011).
- Miyajima, T., Tanaka, Y., Koile, Y. Determining 15N enrichment of dissolved organic nitrogen in environmental waters by gas chromatography/negative-ion chemical ionization mass spectrometry. Limnology and Oceanography. 3 (3), 164-173 (2005).
- Stevens, R. J., Laughlin, R. J., Burns, L. C., Arah, J. R. M., Hood, R. C. Measuring the contributions of nitrification and denitrification to the flux of nitrous oxide from soil. Soil Biology and Biochemistry. 29 (2), 139-151 (1997).
- Porubsky, W. P., Velasquez, L. E., Joye, S. B. Nutrient-replete benthic microalgae as a source of dissolved organic carbon to coastal waters. Estuaries and Coasts. 31 (5), 860-876 (2008).
- Huygens, D., et al. Mechanisms for retention of bioavailable nitrogen in volcanic rainforest soils. Nature Geoscience. 1 (8), 543-548 (2008).
- Rutting, T., Boeckx, P., Muller, C., Klemedtsson, L. Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosciences. 8 (7), 1779-1791 (2011).
- Song, B., Lisa, J. A., Tobias, C. R. Linking DNRA community structure and activity in a shallow lagoonal estuarine system. Frontiers in Microbiology. 5, 460 (2014).
- Cheng, L., et al. Dissimilatory nitrate reduction processes in sediments of urban river networks: Spatiotemporal variations and environmental implications. Environmental Pollution. 219, 545-554 (2016).
- Lisa, J. A., Song, B., Tobias, C. R., Hines, D. E. Genetic and biogeochemical investigation of sedimentary nitrogen cycling communities responding to tidal and seasonal dynamics in Cape Fear River Estuary. Estuarine, Coastal and Shelf Science. 167, A313-A323 (2015).
- Deng, F. Y., et al. Dissimilatory nitrate reduction processes and associated contribution to nitrogen removal in sediments of the Yangtze Estuary. Journal of Geophysical Research: Biogeosciences. 120 (8), 1521-1531 (2015).
- Tiedje, J. M., Zehnder, A. J. B. . Biology of Anaerobic Microorganisms. , 179-244 (1988).
- Tiedje, J. M., Sexstone, A. J., Myrold, D. D., Robinson, J. A. Denitrification: ecological niches, competition and survival. Antonie van Leeuwenhoek. 48, 569-583 (1982).
- Hardison, A. K., Algar, C. K., Giblin, A. E., Rich, J. J. Influence of organic carbon and nitrate loading on partitioning between dissimilatory nitrate reduction to ammonium (DNRA) and N2 production. Geochimica et Cosmochimica Acta. , 164 (2015).
- Sigman, D. M., et al. Natural abundance-level measurement of the nitrogen isotopic composition of oceanic nitrate: an adaptation of the ammonia diffusion method. Marine Chemistry. 57 (3-4), 227-242 (1997).
- Risgaard-Petersen, N., Rysgaard, S., Revsbech, N. P. Combined microdiffusion-hypobromite oxidation method for determining nitrogen-15 isotope in ammonium. Soil Science Society of America Journal. 59 (4), (1995).
- Gardner, W. S., Bootsma, H. A., Evans, C., John, P. A. S. Improved chromatographic analysis of 15N:14N ratios in ammonium or nitrate for isotope addition experiments. Marine Chemistry. 48 (3-4), 271-282 (1995).

