Non-Invasive Modulation and Robotic Mapping of Motor Cortex in the Developing Brain
Summary
We demonstrate protocols for the modulation (tDCS, HD-tDCS) and mapping (robotic TMS) of the motor cortex in children.
Abstract
Mapping the motor cortex with transcranial magnetic stimulation (TMS) has potential to interrogate motor cortex physiology and plasticity but carries unique challenges in children. Similarly, transcranial direct current stimulation (tDCS) can improve motor learning in adults but has only recently been applied to children. The use of tDCS and emerging techniques like high–definition tDCS (HD-tDCS) require special methodological considerations in the developing brain. Robotic TMS motor mapping may confer unique advantages for mapping, particularly in the developing brain. Here, we aim to provide a practical, standardized approach for two integrated methods capable of simultaneously exploring motor cortex modulation and motor maps in children. First, we describe a protocol for robotic TMS motor mapping. Individualized, MRI-navigated 12×12 grids centered on the motor cortex guide a robot to administer single-pulse TMS. Mean motor evoked potential (MEP) amplitudes per grid point are used to generate 3D motor maps of individual hand muscles with outcomes including map area, volume, and center of gravity. Tools to measure safety and tolerability of both methods are also included. Second, we describe the application of both tDCS and HD-tDCS to modulate the motor cortex and motor learning. An experimental training paradigm and sample results are described. These methods will advance the application of non-invasive brain stimulation in children.
Introduction
Non-invasive brain stimulation can both measure and modulate human brain function1,2. The most common target has been the motor cortex, due in part to an immediate and measurable biological output (motor evoked potentials) but also the high prevalence of neurological diseases resulting in motor system dysfunction and disability. This large global burden of disease includes a high proportion of conditions affecting children such as cerebral palsy, the leading cause of lifelong disability affecting some 17 million persons worldwide3. Despite this clinical relevance and the diverse and increasing capacities of neurostimulation technologies, applications in the developing brain are only beginning to be defined4. Improved characterization of existing and emerging non-invasive brain stimulation methods in children are required to advance applications in the developing brain.
Transcranial magnetic stimulation (TMS) is a well-established neurophysiological tool being increasingly used for its non-invasive, painless, well-tolerated and safety profile in adults. TMS experience in children is relatively limited but steadily increasing. TMS delivers magnetic fields to induce regional activation of cortical neuronal populations in the brain with net outputs reflected in target muscle motor evoked potentials (MEP). Systematic application of single pulse TMS can define maps of the motor cortex in vivo. Seminal animal studies5 and emerging human TMS studies6 have shown how motor maps may help inform mechanisms of cortical neuroplasticity. Navigated motor mapping is a TMS technique that is used to map out the human motor cortex to interrogate functional cortical regions. Changes in motor map have been associated with plastic changes of the human motor system7. Recent advancements in robotic TMS technology have brought new opportunities to improve motor mapping efficiency and accuracy. Our group has recently demonstrated that robotic TMS motor mapping is feasible, efficient, and well tolerated in children8.
Transcranial direct current stimulation (tDCS) is a form of non-invasive brain stimulation that can shift cortical excitability and modulate human behaviors. There has been a multitude of studies examining the effect of tDCS in adults (>10,000 subjects) but less than 2% of studies have focused on the developing brain9. Translation of adult evidence to pediatrics applications is complex, and modified protocols are needed due to complex differences in children. For example, we and others have shown that children experience larger and stronger electric fields compared to adults10,11. Standardization of tDCS methods in children is important to ensure safe and consistent application, improve replication, and advance the field. Experience of motor learning modulation tDCS in children is limited but increasing12. Translational applications of tDCS to specific cerebral palsy populations are advancing towards late phase clinical trials13. Efforts toward more focal stimulation applied through high-definition tDCS (HD-tDCS) has only just been studied for the first time in children14. We demonstrated that HD-tDCS produces similar improvements in motor learning as conventional tDCS in healthy children14. Describing HD-tDCS methods will allow for replication and further applications of such protocols in children.
Protocol
All the methods described in this protocol have been approved by Conjoint Health Research Ethics Board, University of Calgary (REB16-2474). The protocol is described in Figure 1.
1. Non-invasive brain stimulation contraindications
- Screen all participants for contraindications for TMS15 and tDCS1 prior to recruitment.
2. Transcranial magnetic stimulation motor mapping
- Preparing MRI for navigated TMS
- Obtain each participant’s structural MRI (T1). If an MRI is unobtainable, use a template MRI from Montreal Neurological Institute.
- Import the MRI file in DICOM or NIFTI format to the neuronavigation software (see Table of Materials).
- TMS target trajectories
- Use the neuronavigation software to reconstruct Skin and Full Brain Curvilinear using the tabs.
- Select Nouveau, Skin, and Compute Skin. Ensure the nose and top of the head are included.
- Select Nouveau, and Full Brain Curvilinear. Enclose the green selection box outside of the brain but inside of the skull. Select Compute Curvilinear. Adjust the peel depth to 4.0-6.0 mm.
- Select Configure Landmarks. Place four landmarks at the tip of nose, nasion, and the notches of both ears of the reconstructed skin. Name the landmarks corresponding to their anatomy.
- Select the Targets tab to view curvilinear brain. Select Nouveau, and Rectangular Grid. Place uniform 12 x 12 coordinate grids with 7 mm spacing on the surface of the reconstructed brain over the “handknob” of the motor cortex (precentral gyrus)17.
- Use the Target Positioning Tool on the right to optimize the grid positioning for rotation, tilt, and curvature. Convert the grid-points into trajectories that will guide the robot to position the TMS coil. Adjust the angle of the trajectories so they are 45° to the longitudinal fissure of the brain.
- Use the SNAP tool to extrapolate and optimize the trajectories to curvilinear brain.
- Initialize and position the TMS robot arm and seat to Welcome position and calibrate the force plate sensor using Force sensor test.
- Preparing the participant for motor mapping
- Have the participants fill out a safety questionnaire18.
- Once the participants seated comfortably in the robot chair, adjust the backrest and neckrest. Ensure their feet are supported. Support their arms and hands with pillows to ensure their hands are in a resting position for the duration of the mapping session.
NOTE: Children and adolescents will need reminders throughout the session to keep their hands relaxed. - Clean the skin over the muscle of interest. Place Ag/AgCl surface electrodes on both hands and forearms of the participant, targeting four distal forelimb muscles, 1) the belly of the first dorsal interosseous (FDI), 2) abductor pollicis brevis (APB), 3) abductor digiti minimi (ADM), and 4) the wrist extensor (extensor carpi ulnaris).
- Connect the surface electrodes with electromyography (EMG) amplifier and data acquisition system and connect the amplifier to a data collecting computer with a compatible EMG software.
- Co-register the four landmarks on the head of the participant using the landmark pointer. Use the validation tab to ensure the participant’s head is properly registered.
- Determining motor mapping TMS intensity
- Select a grid-point closest to the participant’s “handknob”. Select the Align to Target button to align the TMS coil held by the robot to this target location. Select Contact on. Monitor the contact quality using the contact force indicator. Ensure the indicator is green or yellow.
NOTE: The red color on the contact indicator means there is too much force on the participant’s head. No color means the TMS coil is not in contact with the participant’s head. In these cases, adjust the force plate sensitivity. - Instruct the participant not to move outside the scope of the robot arm. Ensure the participant’s hand muscles are relaxed and remain still prior to contact.
- Select Align and Follow so the coil remains centered on the target if the participant moves.
- Use the TMS trigger button on the TMS machine to deliver 5-10 TMS pulses at an intensity between 40-60% maximum stimulator output (MSO). Repeat this step to 5-6 grid-points surrounding the “handknob”.
- Determine the grid-point that gives the largest and most consistent (hotspot) motor evoked potential (MEP) for the left or right FDI muscle.
- Determine the Resting Motor Threshold (RMT) as the lowest intensity that produces an MEP of at least 50 µV in the FDI muscle in 5/10 stimulations.
- Select a grid-point closest to the participant’s “handknob”. Select the Align to Target button to align the TMS coil held by the robot to this target location. Select Contact on. Monitor the contact quality using the contact force indicator. Ensure the indicator is green or yellow.
- Motor mapping
- Starting from the grid-point closest to the hotspot, deliver four single-pulse TMS pulses (1 Hz) at an interstimulus of 1 s and TMS intensity of 120% RMT. A responsive grid-point is determined by 2/4 MEPs >50 µV in any of the hand muscles.
- Move to the adjacent grid-point and repeat the above step.
- Continue sequentially in a linear fashion along responsive points until a non-responsive point is reached, which is the first border region of the map.
- Continue mapping to establish the border points in all four directions of the rectangular grid.
- Record all MEPs from all muscles using the EMG software for offline analysis.
- After 3-4 grid points, select Contact off and give the participant a break until they feel ready to continue.
- Throughout the mapping session, continuously check in with the participant to ensure they are comfortable and/or need a break.
- Use a hard copy version of the same grids to tack the simulation order for further analysis.
- Complete mapping using a robotic TMS as described here or manually (not described in this manuscript). If using a TMS Robot, it will move to the grid-point selected by the experimenter. The robot will accommodate for child head motion in near real time. This will alleviate any additional movement associated with a technician manually holding the coil on the participant’s head.
NOTE: If mapping using a TMS robot, ensure there is an experimenter beside the robot at all times during the session. If the robot is placed on a participant’s head and the participant suddenly moves, the robot will try to follow their head. If the participant must move, sneeze, scratch, or perform an activity involving the movement of their head, the robot arm must be moved to prevent the participant’s head from hitting the robot’s arm or TMS coil.
- Motor map creation
- Using a custom-made coding script, generate three-dimensional motor maps (Figure 2). Contact the authors for the script.
- Calculate motor map area and volume using responsive trajectory sites. Calculate center of gravity (COG) as weighted average of the motor representations of each coordinate location.
NOTE: Map area is calculated as the grid spacing (7 mm)2 multiplied by the total number of responsive sites. Map volume is calculated as the cumulative sum of grid spacing multiplied by the mean MEP amplitude at each responsive site. A user-friendly version of the script is being developed to share with the public as open source. Meanwhile, contact the corresponding author to get access to the script.
3. Conventional tDCS and HD-tDCS application
- Randomize the participants to one of three intervention groups (sham, conventional tDCS, HD-tDCS).
- Have the participant complete the Purdue Pegboard Test (PPT) three times using their left hand (non-dominant), establishing their baseline score.
- Inspect electrode quality to confirm the integrity of the tDCS sponge inserts and rubber electrodes.
- Turn on the conventional tDCS device by flipping the power switch to ON.
NOTE: Ensure the low battery light is not illuminated. If it is illuminated, change the batteries before starting the session.- For participants receiving conventional or sham tDCS, lightly soak two 25 cm2 sponge electrodes with saline. Ensure the entire electrode is covered but not dripping. Insert the rubber electrode into the saline soaked sponge electrodes and connect each electrode to the tDCS device.
- Locate the marked hotspot (Right M1) using the neuronavigation and mark it with a non-toxic marker. At the end of each tDCS, HD-tDCS or sham session, mark the hotspot again so that it is visible the next day.
- If randomized to conventional tDCS or sham tDCS, place one 25 cm2 saline-soaked sponge electrode over the participant’s marked hotspot (Right M1), serving as the anode. Place the other 25 cm2 saline-soaked sponge electrode on the contralateral supraorbital region, representing the cathode. Use a light plastic pediatric “headband” to hold electrodes in place.
NOTE: Ensure that there is no saline dripping from the electrode as it may shunt the current. - In the sham and conventional tDCS group, ensure “optimal” contact quality. If the contact quality is “sub-optimal”, inject a small amount of saline solution under the sponge electrodes, or ensure that there is minimal hair between the scalp and electrode.
NOTE: “Optimal” contact quality is achieved when more than half of the quality of contact indicator lights are on. If less than half of the contact indicator lights are on, the contact quality is sub-optimal. Do not start stimulation if only one of two of the indicator lights are on. - In the HD-tDCS group, refer to Villamar, M.F., et al.16 for the appropriate set-up.
- In the HD-tDCS group, set the device to the Scan setting to check the impedance at each electrode. Ensure the impedance is under 1 “quality unit” and described previously19,20. If contact quality is poor, remove the electrode and check that there is no hair obstructing the contact of the electrode, and that a continuous column of electrode gel is present between the scalp and electrode. If needed, apply more electrode gel.
- If randomized to conventional tDCS or sham tDCS, place one 25 cm2 saline-soaked sponge electrode over the participant’s marked hotspot (Right M1), serving as the anode. Place the other 25 cm2 saline-soaked sponge electrode on the contralateral supraorbital region, representing the cathode. Use a light plastic pediatric “headband” to hold electrodes in place.
- Set the tDCS and HD-tDCS device to the anode montage setting, 1 mA current strength, and 20 min duration.
- Ensure the participant is sitting comfortably and they understand the possible sensations they may experience (such as itchy or tingling sensations). Remind the participant to communicate if they feel any discomfort or if they have any questions.
- In the conventional tDCS and HD-tDCS groups, make sure the toggle is set to Active.
NOTE: For the sham group, the toggle should be set to Sham. This setting should be hidden from the participant. - Press the device’s Start button to start stimulation. Ensure the duration is set to 20 min, and the intensity to 1 mA.
NOTE: In the conventional tDCS and HD-tDCS groups, the current will ramp up over 30 s to 1 mA and continue for 20 min. In the sham tDCS group, current will be ramped up over 30 s to 1 mA and immediately ramped down over 30 s.
- In the conventional tDCS and HD-tDCS groups, make sure the toggle is set to Active.
- At 5 min, 10 min, 15 min, and 20 min, have the participant complete the PPT three times using their left hand.
- After 20 min, turn the device off after the intensity finishes ramping down to 0 mA.
NOTE: For participants receiving either conventional tDCS or HD-tDCS, the machine will automatically ramp down to 0 mA at 20 min. For participants receiving sham tDCS, the machine will automatically ramp up over 30 s to 1 mA and immediately ramp down to 0 mA over 30 s at 20 min. - Remove the electrodes from the participant’s head.
- For sham and conventional tDCS group, remove black electrodes from inside the sponges and rinse the sponge electrode with normal tap water.
- In the HD-tDCS group, take off the plastic electrode holder top and remove the electrodes. Remove the electrode cap from participants’ head. Rinse any gel in the electrode holder. Clean the electrode with a slightly damp paper towel. Wipe the electrode with a dry paper towel to remove any remaining gel.
- Have all participants complete the Transcranial Direct-Current Stimulation Side-effects and Tolerability questionnaire after each stimulation session.
- Have the participants complete the PPT three times using their left hand.
- Have the participants return the following day and for another four consecutive days (five days total) for non-invasive brain stimulation (sham, tDCS, or HD-tDCS) paired with motor learning (PPT). Repeat steps 3.2-3.13 on Day 2-4. On Day 5, have the participants begin with non-invasive brain stimulation (sham, tDCS or HD-tDCS) (steps 3.2-3.13 are repeated). After a break (45 min-~1.5 h since receiving stimulation), start robotic TMS motor mapping (steps 2.3-2.5.8).
NOTE: All participants received the same number of minutes for breaks between assessments. - After 6-weeks, invite the participants to return and perform the PPT without receiving any non-invasive brain stimulation (step 3.2 followed by robotic TMS motor mapping (step 2.5.8)).
- Have the participants return the following day and for another four consecutive days (five days total) for non-invasive brain stimulation (sham, tDCS, or HD-tDCS) paired with motor learning (PPT). Repeat steps 3.2-3.13 on Day 2-4. On Day 5, have the participants begin with non-invasive brain stimulation (sham, tDCS or HD-tDCS) (steps 3.2-3.13 are repeated). After a break (45 min-~1.5 h since receiving stimulation), start robotic TMS motor mapping (steps 2.3-2.5.8).
Representative Results
Using the methods presented here, we completed a randomized, sham-controlled interventional trial8. Right-handed children (n = 24, ages 12-18) with no contraindications for both types of non-invasive brain stimulation were recruited. Participants were specifically excluded in this study if on neuropsychotropic medication or if they were not naïve to tDCS. There were no dropouts.
Robotic TMS motor maps were obtained to acquire a baseline motor map and to serve as a potential mechanism to monitor neuroplastic and cortical excitability changes after motor learning paired with non-invasive brain stimulation. Using the methods described above, all participants received three robotic TMS motor maps, 1) baseline prior to non-invasive brain stimulation (sham, tDCS, or HD-tDCS), 2) day 5 (Post), and 3) at the 6-week follow up (retention time). All participants received bihemispheric motor mapping (3 participants received right hemispheric motor mapping only due to time constraints). Motor maps were completed on average in 18 min for unilateral motor maps and 36 min for bihemispheric mapping. Motor map area, volume, hotspot, and COG were computed and compared at the individual and group level. In our initial motor map analysis, motor map area and volume did not change significantly following the intervention. In our secondary analysis, measuring submaximal proportions of map area and volume resulted in significantly smaller variance (p<0.05).
All participants received one of three non-invasive brain stimulation interventions for a duration of 20 min (1 mA) for five consecutive days. We demonstrated that tDCS and HD-tDCS improve the rate of learning (number of pegs/day) (tDCS p=0.042, HD-tDCS p=0.049) over 5 days of training. The active intervention groups (tDCS and HD-tDCS) had larger improvements in daily average left hand PPT score (PPTL) at day 4 and 5 compared to sham (day 4 p≤0.043, day 5 p≤0.05) (Figure 3). The active intervention groups retained their motor skills (on the PPT) at 6-weeks post-training. However, there was significant skill decay in the sham group from post-training to the 6-week follow-up (p=0.034). This methodology has been replicated from a previous study21 and the datasets were combined (Figure 4). The replication data demonstrated similar results. There was a significant increase in the rate of learning observed in the tDCS and HD-tDCS group compared to the sham group (tDCS p = 0.001, HD-tDCS p = 0.012).
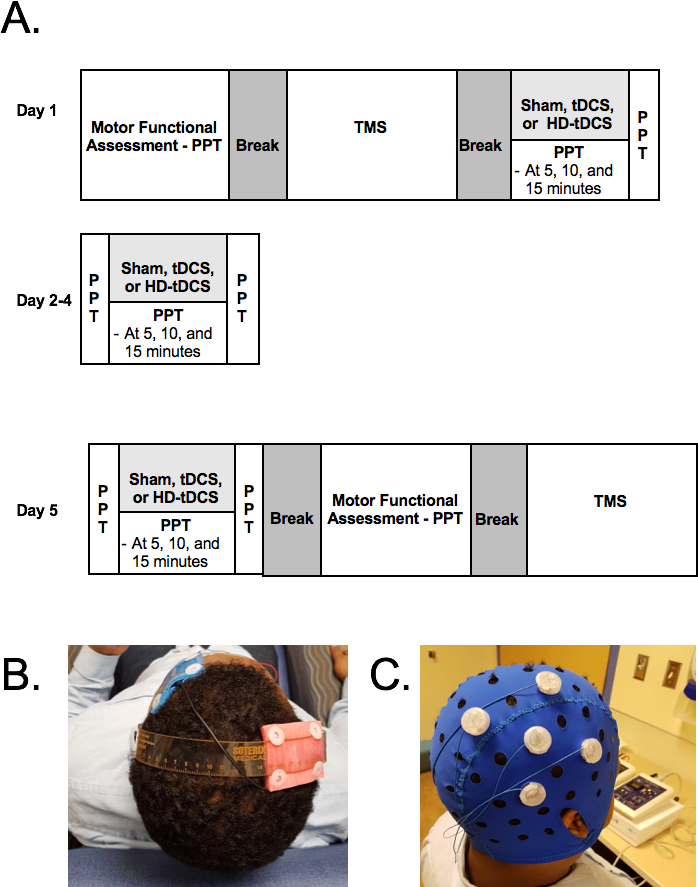
Figure 1: Trial protocol. PTT= Purdue pegboard Test, TMS= TMS motor mapping tDCS= transcranial direct current stimulation, HD-tDCS = High-definitional tDCS. Please click here to view a larger version of this figure.
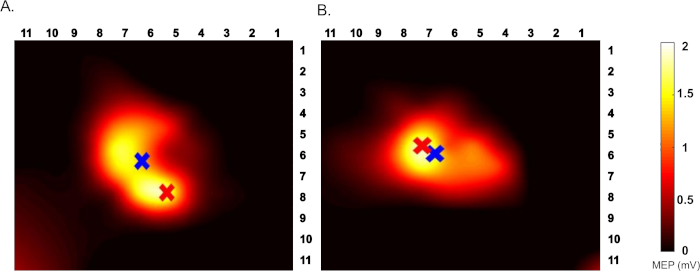
Figure 2: An example TMS motor map. Top view of left FDI motor map (A) Pre and (B) post HD-tDCS intervention. Red cross indicates hotspot, blue cross indicates COG. The color bar indicates the range of MEP from 0-2 mV. Please click here to view a larger version of this figure.
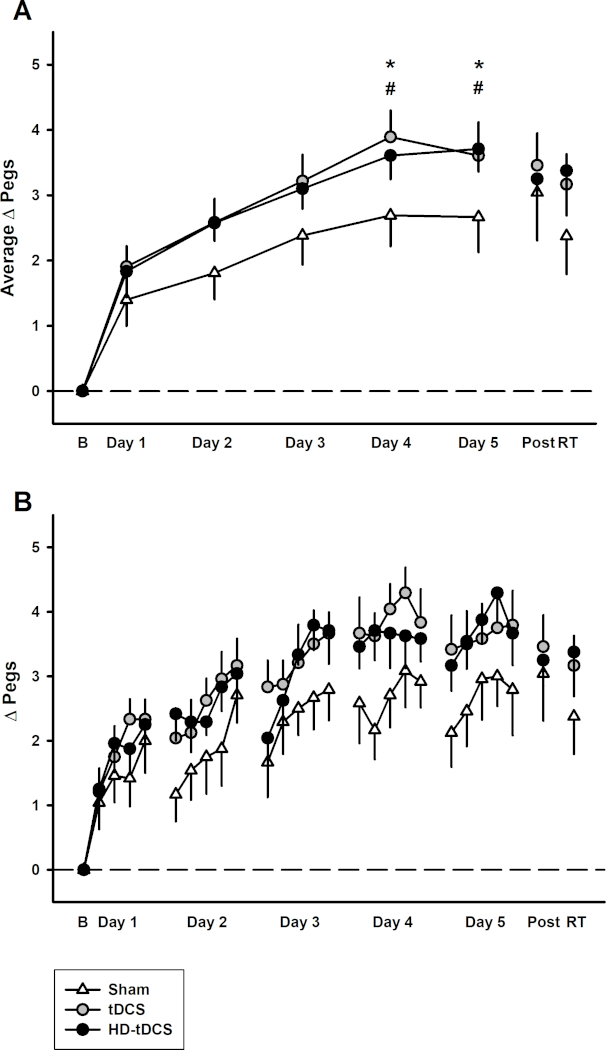
Figure 3: Motor learning observed in sham, tDCS and HD-tDCS groups. This figure has been republished from Cole & Giuffre et al. 2018. (A) Mean daily change in left hand Purdue Pegboard score from baseline in sham (white triangles), tDCS (grey circles), and HD-tDCS (black circles), (n = 24). (B) Daily mean score at each time point of PPTL. *p<0.05 for tDCS vs. sham, # p<0.05 for HD-tDCS vs. sham. Error bars indicate standard error. Please click here to view a larger version of this figure.
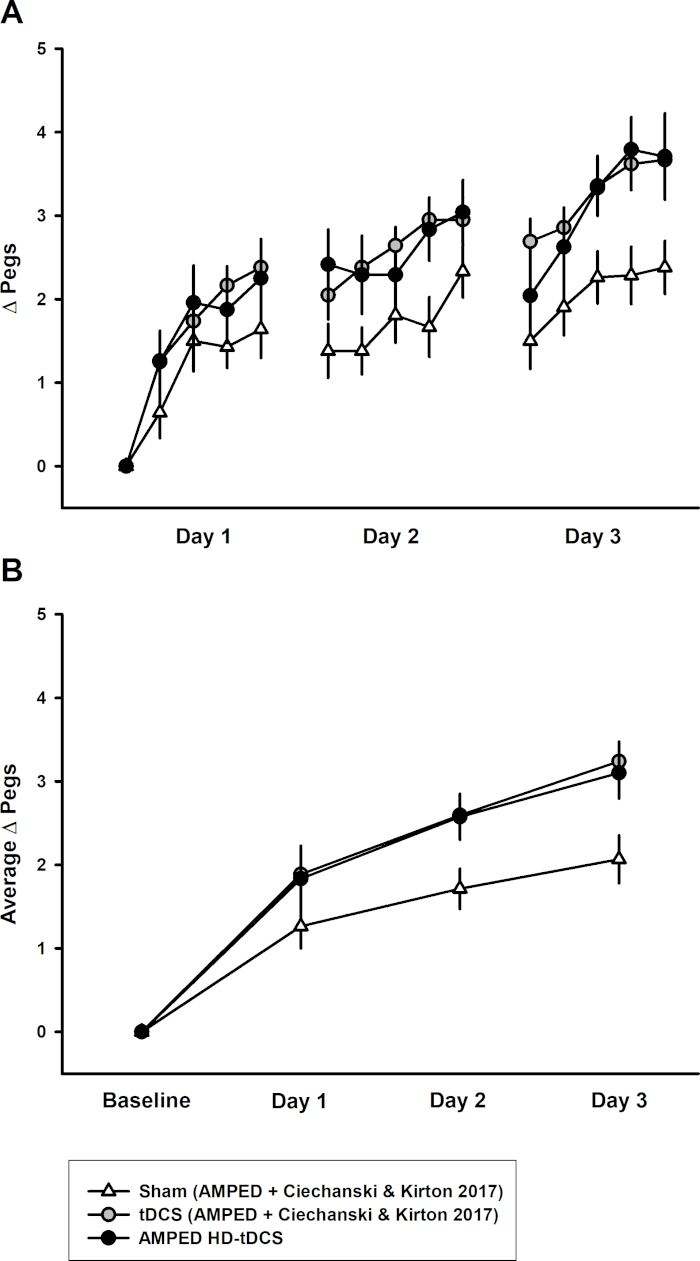
Figure 4: Replication of methods – combined PPTL dataset for 3 days of training. This figure has been republished from Cole & Giuffre et al. 2018). (A) The learning curves for sham (white triangles, n = 14), tDCS (gray circles, n = 14), and HD-tDCS (black circles, n = 8) groups. (B) Mean daily learning for sham, tDCS, and HD-tDCS from the combined studies. Error bars indicate standard error. Please click here to view a larger version of this figure.
Discussion
TMS has also been explored in clinical pediatric populations, including perinatal stroke22 and cerebral palsy, where TMS motor maps were successfully created in children with cerebral palsy to explore mechanisms of interventional plasticity. Using an established protocol8, TMS motor maps were successfully collected in typically developing children, and are currently being collected in an ongoing multicenter clinical trial for children with perinatal stroke and hemiplegic cerebral palsy (NCT03216837). Describing TMS motor mapping methods will allow for replication and further applications of protocols in healthy children and children with movement disorders.
Robotic motor mapping improves TMS coil placement accuracy and reduces human error when compared to manual techniques23,24. This technique is more advantageous for pediatric populations who have increased head movements and lower tolerability for long sessions12. Although motor mapping using a TMS robot has been reported in adults, our group is the first to apply this technique in a pediatric population. New motor mapping methodologies that use statistical weighting and interpolation25,26 can be used to decrease acquisition time if combined with robotic TMS. As such, methodologies should be further explored in the developing brain.
We outline a succinct approach to apply tDCS, HD-tDCS, and TMS in a healthy pediatric population. There are a variety of critical steps to consider in the application of non-invasive brain stimulation in children. It is crucial that children and/or their parents confirm that the participant has no contraindications for non-invasive brain stimulation. It is important for participants to feel comfortable and safe. Encourage the participants to ask questions throughout the session as it is necessary to continuously obtain feedback throughout the session, especially in a pediatric population. As well, it is important to inspect the quality of the electrodes and the quality of the participants’ scalp, as this precludes safe application of tDCS. It is vital to have the correct anodal montage, current intensity, and duration of stimulation selected on the machine before starting the stimulation. There are specific considerations for conventional tDCS and HD-tDCS. In HD-tDCS, it is crucial to rotate the electrode chosen to be in the center anodal position with the surrounding electrodes to decrease the amount of electrode breakdown. It is vital to have the correct connection of the cables to the anodal and cathodal ports on the 1×1 tDCS machine in conventional tDCS to allow for the correct polarity to be applied. Previous literature has demonstrated the importance of using saline solution to improve tolerability of the stimulation27. The most common sensation described in our study was itching (56%)14. We have reported no adverse effects in our population using our methods described12,14.
There are a variety of different modifications to make when perfecting the application of tDCS and HD-tDCS. It is important to have good contact quality to decrease the resistance of the current across the scalp. If the contact quality is poor, more saline solution can be applied to decrease the resistance in conventional tDCS. However, it is important to first ensure that good electrode contact with the scalp is present. In HD-tDCS, it is essential that the scalp be exposed to allow for better quality of electrode. Hair may need to be further brushed out of the way and more electrode gel applied to improve the contact quality. Ensure that the contact quality is continuously monitored throughout the session.
Current modeling studies have suggested a difference in current strength experienced across age groups depending on white matter and CSF volume10,11. A limitation of this method is that we did not perform prospective current modeling on each participant to apply a current strength that would induce comparable neuronal electric field strength across participants.
This method is an important next step in the application of non-invasive brain stimulation in pediatrics. We have extended our training period from three days to five days and observed similar improvements in skill. HD-tDCS has only been applied in a pediatric population using our method and we have demonstrated that there is similar motor skill learning to conventional tDCS. HD-tDCS induces a more focal current, improving targeting and implication28. The methods described in this paper will allow for the replication and further study of HD-tDCS in children.
These methods are currently being extended to a perinatal stroke population. The tDCS and HD-tDCS protocol has been adapted to this population and training time has been extended to further develop clinical trials in perinatal stroke. It is crucial to optimize the application of tDCS in pediatrics to advance therapeutic application in children with perinatal stroke and therefore improve motor function outcomes. For TMS motor mapping, it is important to ensure that the participant is comfortably seated, with their arms and hands in a relaxed position. Following full motor mapping session, only 15% of the participants experienced mild self-limiting headache.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This study was supported by the Canadian Institutes of Health Research.
Materials
| 1×1 SMARTscan Stimulator | Soterix Medical Inc. | https://soterixmedical.com/research/1×1/tdcs/device | |
| 4×1 HD-tDCS Adaptor | Soterix Medical Inc. | https://soterixmedical.com/research/hd-tdcs/4×1 | |
| Brainsight Neuronavigation | Roge Resolution | https://www.rogue-resolutions.com/catalogue/neuro-navigation/brainsight-tms-navigation/ | |
| Carbon Rubber Electrode | Soterix Medical Inc. | https://soterixmedical.com/research/1×1/accessories/carbon-ruber-electrode | |
| EASYpad Electrode | Soterix Medical Inc. | https://soterixmedical.com/research/1×1/accessories/1×1-easypad | |
| EASYstraps | Soterix Medical Inc. | https://soterixmedical.com/research/1×1/accessories/1×1-easystrap | |
| EMG Amplifier | Bortec Biomedical | http://www.bortec.ca/pages/amt_16.htm | |
| HD1 Electrode Holder | Soterix Medical Inc. | https://soterixmedical.com/research/hd-tdcs/accessories/hd1-holder | Standard Base HD-Electrode Holder for High Definition tES (HD-tES) |
| HD-Electrode | Soterix Medical Inc. | https://soterixmedical.com/research/hd-tdcs/accessories/hd-electrode | Sintered ring HD-Electrode. |
| HD-Gel | Soterix Medical Inc. | https://soterixmedical.com/research/hd-tdcs/accessories/hd-gel | HD-GEL for High Definition tES (HD-tES) |
| Micro 1401 Data Acquisition System | Cambridge Electronics http://ced.co.uk/products/mic3in | ||
| Purdue Pegboard | Lafayette Instrument Company | ||
| Saline solution | Baxter | http://www.baxter.ca/en/products-expertise/iv-solutions-premixed-drugs/products/iv-solutions.page | |
| Soterix Medical HD-Cap | Soterix Medical Inc. | https://soterixmedical.com/research/hd-tdcs/accessories/hd-cap | |
| TMS Robot | Axilium Robotics | http://www.axilumrobotics.com/en/ | |
| TMS Stimulator and Coil | Magstim Inc | https://www.magstim.com/neuromodulation/ |
References
- Woods, A. J., et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical Neurophysiology. 127 (2), 1031-1048 (2016).
- Nitsche, M. A., et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. Journal of Cognitive Neuroscience. 15 (4), 619-626 (2003).
- Oskoui, M., Coutinho, F., Dykeman, J., Jetté, N., Pringsheim, T. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Developmental Medicine & Child Neurology. 55 (6), 509-519 (2013).
- Zewdie, E., Kirton, A. TMS Basics: Single and Paired Pulse Neurophysiology. Pediatric Brain Stimulation: Mapping and Modulating the Developing Brain. , 475 (2016).
- Nudo, R. J., Milliken, G. W., Jenkins, W. M., Merzenich, M. M. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. The Journal of Neuroscience. 16 (2), 785-807 (1996).
- Friel, K. M., Gordon, A. M., Carmel, J. B., Kirton, A., Gillick, B. T. Pediatric Issues in Neuromodulation: Safety, Tolerability and Ethical Considerations. Pediatric Brain Stimulation: Mapping and Modulating the Developing Brain. , 475 (2016).
- Nudo, R. J., Plautz, E. J., Frost, S. B. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle & Nerve. 24 (8), 1000-1019 (2001).
- Grab, J. G., et al. Robotic TMS mapping of motor cortex in the developing brain. Journal of Neuroscience Methods. , (2018).
- Bikson, M., et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimulation. 9 (5), 641-661 (2016).
- Kessler, S. K., Minhas, P., Woods, A. J., Rosen, A., Gorman, C., Bikson, M. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PloS One. 8 (9), e76112 (2013).
- Ciechanski, P., Carlson, H. L., Yu, S. S., Kirton, A. Modeling Transcranial Direct-Current Stimulation-Induced Electric Fields in Children and Adults. Frontiers in Human Neuroscience. 12, 268 (2018).
- Ciechanski, P., Kirton, A. Transcranial Direct-Current Stimulation (tDCS): Principles and Emerging Applications in Children. Pediatric Brain Stimulation: Mapping and Modulating the Developing Brain. , 475 (2016).
- Kirton, A., et al. Transcranial direct current stimulation for children with perinatal stroke and hemiparesis. Neurology. 88 (3), 259-267 (2017).
- Cole, L., et al. Effects of High-Definition and Conventional Transcranial Direct-Current Stimulation on Motor Learning in Children. Front Neurosci. , (2018).
- Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A. Screening questionnaire before TMS: an update. Clinical Neurophysiology. 122 (8), 1686 (2011).
- Villamar, M. F., Volz, M. S., Bikson, M., Datta, A., Dasilva, A. F., Fregni, F. Technique and considerations in the use of 4×1 ring high-definition transcranial direct current stimulation (HD-tDCS). Journal of Visualized Experiments. (77), e50309 (2013).
- Yousry, T. A., et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 120 (Pt 1), 141-157 (1997).
- Garvey, M. A., Mall, V. Transcranial magnetic stimulation in children. Clinical Neurophysiology. 119 (5), 973-984 (2008).
- Borckardt, J. J., et al. A pilot study investigating the effects of fast left prefrontal rTMS on chronic neuropathic pain. Pain Medicine (Malden, Mass.). 10 (5), 840-849 (2009).
- Villamar, M. F., et al. Focal modulation of the primary motor cortex in fibromyalgia using 4×1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. The Journal of Pain. 14 (4), 371-383 (2013).
- Ciechanski, P., Kirton, A. Transcranial Direct-Current Stimulation Can Enhance Motor Learning in Children. Cerebral Cortex. 27 (5), 2758-2767 (2017).
- Kirton, A., Andersen, J. Brain stimulation and constraint for hemiparesis after perinatal stroke: The PLASTIC CHAMPS trial. European Journal of Paediatric Neurology. 19 (S1), S10 (2015).
- Ginhoux, R., et al. A custom robot for Transcranial Magnetic Stimulation: First assessment on healthy subjects. 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). , 5352-5355 (2013).
- Grau, C., et al. Conscious brain-to-brain communication in humans using non-invasive technologies. PloS One. 9 (8), e105225 (2014).
- Julkunen, P. Methods for estimating cortical motor representation size and location in navigated transcranial magnetic stimulation. Journal of Neuroscience Methods. 232, 125-133 (2014).
- van de Ruit, M., Perenboom, M. J., Grey, M. J. TMS brain mapping in less than two minutes. Brain Stimulation. 8 (2), 231-239 (2015).
- Dundas, J. E., Thickbroom, G. W., Mastaglia, F. L. Perception of comfort during transcranial DC stimulation: effect of NaCl solution concentration applied to sponge electrodes. Clinical Neurophysiology. 118 (5), 1166-1170 (2007).
- Alam, M., Truong, D. Q., Khadka, N., Bikson, M. Spatial and polarity precision of concentric high-definition transcranial direct current stimulation (HD-tDCS). Physics in Medicine and Biology. 61 (12), 4506-4521 (2016).

