Shootward Movement of CFDA Tracer Loaded in the Bottom Sink Tissues of Arabidopsis
Summary
The goal of this protocol is to show how to load the CFDA into different sites of the bottom parts of Arabidopsis. We then present the resulting distribution pattern of CF in the shoots.
Abstract
The symplastic tracer 5(6)-carboxyfluorescein diacetate (CFDA) has been widely applied in living plants to demonstrate the intercellular connection, phloem transport and vascular patterning. This protocol shows bottom-to-top carboxyfluorescein (CF) movement in the Arabidopsis by using the root-cutting and the hypocotyl-pinching procedure respectively. These two different procedures result in different efficiencies of CF movement: about 91% appearance of CF in the shoots with the hypocotyl-pinching procedure, whereas only about 70% appearance of CF with the root-cutting procedure. The simple change of loading sites, resulting in significant changes in the mobile efficiency of this symplastic dye, suggests CF movement might be subject to the symplastic regulation, most probably by the root-hypocotyl junction.
Introduction
Many fluorescent tracers with a range of spectral properties, such as 5(6)-carboxyfluorescein (CF)1, 8-hydroxypyrene-1,3,6-trisulphonic acid2, Lucifer yellow CH (LYCH)3, Esculin and CTER4, have been developed and applied in plants to monitor symplastic movement and phloem activity. Generally, a symplastic dye is loaded into a cut in the target tissue and the sequential dispersion of the reporter into other parts of plant will demonstrate the intercellular communication. Although the mechanism of dye absorption is not fully understood, the principle underlying CF movement inside live cells has been widely acknowledged. The ester form of CF (CF diacetate, CFDA) is non-fluorescent, but membrane-permeable. This property allows rapid membrane diffusion of the dye into cells. Once inside live cells, intracellular esterases remove the acetate groups at the 3' and 6' position of CFDA, releasing the fluorescent and membrane-impermeable CF (Figure 1, alternatively refer Wright et al.2); CF can then move through the plasmodesmata to other parts of plants.
A well-established procedure with CFDA is that it can be loaded into source leaves and used to monitor the phloem streaming and phloem unloading in the sink tissues of many species, e.g., as CF unloading in the Arabidopsis root5, phloem unloading during potato tuberization6, phloem unloading in the Nicotiana sink leaves7, and so on. By similar loading approaches, other studies have adopted this dye to demonstrate the symplastic connection between host and parasite8,9, or to reveal the symbiotic relationships10,11.
Another way to make use of this dye is to load it into specific cells or single cell by microinjection to determine its distribution pattern. Such sophisticated techniques have greatly facilitated our deeper understanding of plasmodesmata-mediated intercellular communication, particularly in the development of the concept of symplastic domain12,13. For example, the microinjection of CFDA into cotyledon cells of Arabidopsis resulted in the dye-coupling pattern in the hypocotyl epidermis but non-coupling in the underlying cells or in the root epidermis, therefore the hypocotyl epidermis forms a symplastic domain14. Similar domains, such as the stomatal guard cells15, sieve element-companion cells16, root hair cells14 and root cap17,18 have been identified by microinjection technique. Most surprisingly, some domains allow tracer molecules to move in a certain direction. Take the trichome domain for example, microinjection of a fluorescent probe into the supporting epidermal cell leads to the flow of tracer into the trichome domain, however, the reverse injection does not hold true19. A recent report has also found similar situations in the symplastic domains of the Sedum embryo20. Thus, all above cases imply that swapping of loading sites may lead to novel insights into symplastic communication. Our previous experiment aiming to dissect the route of root-to-shoot mobile silencing identified a novel symplastic domain, or the HEJ (Hypocotyl-epicotyl junction) zone, which was further verified through the root-loading (non-canonical sink-loading) CFDA experiment21. Here, we further elaborate the root-to-shoot CF movement by using a simple method and recover a potential symplastic domain by shifting the loading sites. Furthermore, this procedure may be adapted to differentiate genetic backgrounds that have altered root-to-shoot long-distance transport.
Protocol
1. Arabidopsis vertical growth in MS medium
- The interior of laminar flow cabinet needs to be treated with 30 min UV light and 15 min airflow before usage. Make sure to close the glass door when UV light is on. Spray all tools and plates with 70% ethanol before placing them in the cabinet.
- Prepare the Murashige and Skoog (MS) medium in a standard 90 mm-diameter Petri dish under a laminar flow cabinet.
NOTE: The MS medium contains the following components: 20.6 mM NH4NO3, 18.8 mM KNO3, 1.25 mM KH2PO4, 1.5 mM MgSO4·7H2O, 3 mM CaCl2·2H2O, 0.1 mM MnSO4·H2O, 1.03 µM Na2MoO4·2H2O, 0.1 mM H3BO3, 30 µM ZnSO4·7H2O, 0.1 µM CuSO4·5H2O, 0.1 µM CoCl2·6H2O, 1.39 µM KI, 97 µM FeSO4·7H2O, 114.5 µM ethylenediaminetetraacetic acid (EDTA), 4.07 µM nicotinic Acid, 2.44 µM pyridoxine HCl, 0.15 µM thiamine HCl, 2.68 mM glycine, 555 µM myo-inositol, 87.7 mM sucrose and 10 g/L agar. - Moisturize the sterilized tips or toothpicks by touching the tip or toothpicks to the MS medium, then dip the sterilized seeds one by one onto the fixed position of each Petri dish indicated by the seeding card.
- Seal the Petri dish with paraffin film and sticky tape and place it vertically on a clear stand in a growth room (22 °C, 70% moisture) with a day cycle of 16 h of light and 8 h of darkness (lighting from 6 am to 10 pm). The Arabidopsis plant is ready for CFDA loading on day 9-13 after sowing.
NOTE: The following procedure is performed in the afternoon from 2 pm to 5 pm.
2. CFDA loading with the root cutting procedure
- Prepare fresh CFDA working solution immediately before use. Dilute the 1 mM CFDA stock solution stored in -20 °C freezer with sterile ultrapure water to a working concentration of 5 µM.
- Cut the micro-porous paraffin membrane film (see Table of Materials) into small pieces of 3 mm x 3 mm size.
- Uncover the growing plants in the room at 22 °C and clear the excess moisture with a paper towel. Place the small film pieces below each root.
NOTE: From this to step 2.6, the whole process should be completed within 15 min. - Lift the plants onto the Petri dish lid. Cut the roots in a position about 5-10 mm below the root-hypocotyl junction. Place the shoot part back on to the film on the medium.
- Carefully apply 0.25 µL of CFDA onto the cutting end of each root under a dissecting microscope. Avoid splashing to other parts of plant.
- Cover the plate and leave the plants under light for 2-3 h (22 °C) in the growth room.
- Rinse the stained plants three times sequentially in three separate beakers filled with distilled water, then observe the plants under a stereo-fluorescence microscope with zoom from 1.4x to 3.3x (see the Table of Materials). For fluorescence detection, use a high efficiency filter cube (470/20 nm excitation, 495 nm split and 525/50 nm emission) mounted to transmit the fluorescence signal.
3. CFDA loading with hypocotyl-pinching procedure
- Prepare fresh CFDA working solution immediately before use. Dilute the 1 mM CFDA stock solution stored in -20 °C freezer with sterile ultrapure water (see the Table of Materials) to a working concentration of 5 µM.
- Cut the micro-porous paraffin membrane film (see Table of Materials) into small pieces of 3 mm x 3 mm size.
- Uncover the growing plants in the room at 22 °C and clear the excess moisture with a paper towel.
NOTE: From this to step 3.7, the whole process should be completed within 15 min. - Lay a small piece of film under the root-hypocotyl junction of each plant.
- Use forceps to gently pinch the hypocotyl near to the root-hypocotyl junction under a dissecting microscope.
- Carefully apply 0.1 µL of CFDA onto the wound site under a dissecting microscope. Avoid splashing to other parts of plant.
- Cover the plate, and leave the plants under light (22 °C) for 2-3 h.
- Rinse the stained plants three times sequentially in three separate beakers filled with distilled water, then observe the plants under a stereo-fluorescence microscope with zoom from 1.4x to 3.3x (see the Table of Materials). For fluorescence detection, mount a high efficiency filter cube (470/20 nm excitation, 495 nm split and 525/50 nm emission) to transmit the fluorescence signal.
Representative Results
Symplastic movement is often subject to environmental fluctuations. Perturbation of the plant growing state, and even the process of tissue preparation will affect the size exclusion limit of plasmodesmata, thus affecting the symplastic transport22. To improve the staining efficiency, we confine our operation in the growth room, where the temperature and moisture is tightly controlled, and also perform the whole procedure as quickly as possible (ideally within 10-15 min after lifting the lid of Petri dish). These precautions during an experiment can effectively reduce the rates of unsuccessful shoot staining.
We described two slightly different procedures to demonstrate CF shoot-ward movement. Normally, both procedures can lead to CF staining in the shoots about 2 h after feeding (Figure 2). Nevertheless, the two procedures produce different staining efficiencies. The hypocotyl-pinching procedure results in 91% staining efficiency, whereas the root-cutting procedure produces 70% (Welch's t-test, p < 0.001) (Figure 3). We also tried loading the dye by a root-pinching method and found an even lower staining efficiency compared with root-cutting method, suggesting that the approach to load the dye in the root does not account for the staining difference between the root and hypocotyl loading sites (Figure 3). The CF signal is mainly found in the vasculature, but only few plants show the half-leaf pattern (Figure 2) as seen in other macromolecule movement patterns21. Once the CF signal is spread to the shoot, it can be maintained for more than 72 h and the signal cannot unload further to other cell types; this is consistent with previously published results17.
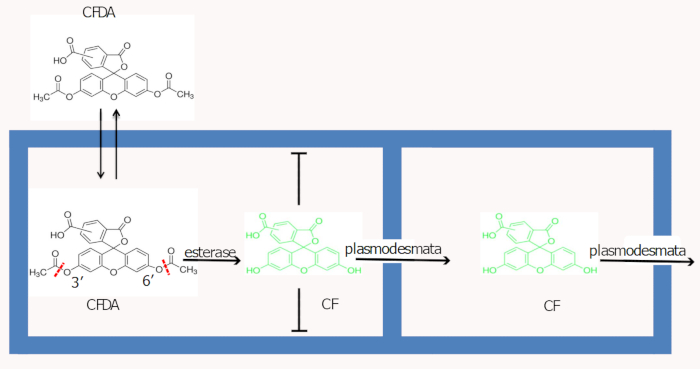
Figure 1: A schematic illustration for CFDA uptake and CF movement in the plant's cells. Please click here to view a larger version of this figure.
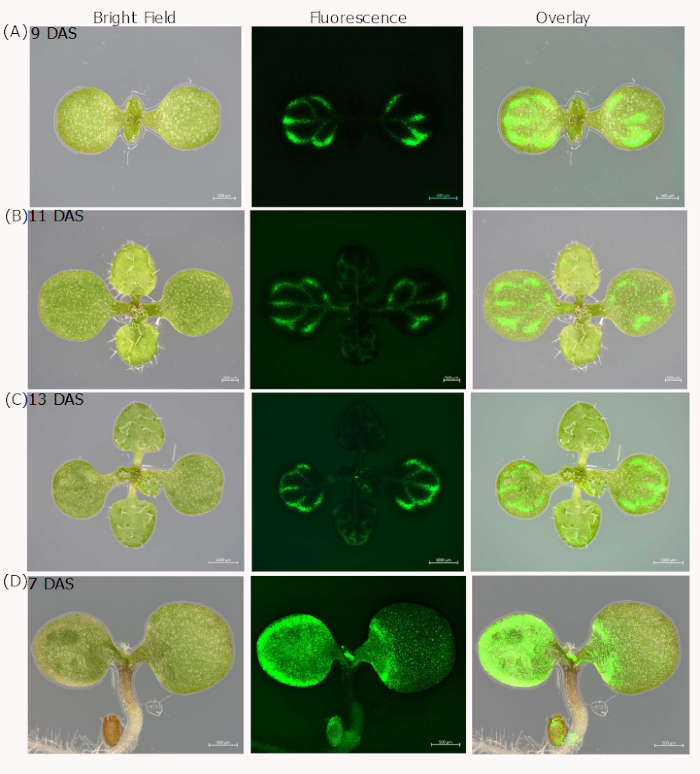
Figure 2: CF signal in the shoots of 9, 11 and 13 day after sowing (DAS) Arabidopsis plants. CF signal can be detected in both cotyledons and true leaves (A, B, C). In the majority of plants, the CF signal is observed in the vasculature after 2-3 hours of loading with either the root-cutting or hypocotyl-pinching method. Only in a very rare cases (less than 0.5%), the hypocotyl-pinching method can generate a partial staining pattern in the cotyledon of 7 DAS Arabidopsis (D). Please click here to view a larger version of this figure.
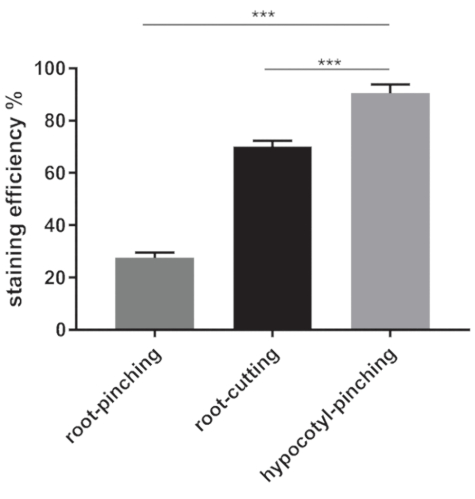
Figure 3: The staining efficiencies with the root-loading method (root-cutting and root-pinching) and hypocotyl-pinching method. The staining efficiency in 9 DAS plants was determined in three independent experiments (n = 26 in the root-pinching experiment; n = 335 in the root-cutting experiment; n = 522 in the hypocotyl-pinching method). Error bar indicates standard error. *** indicates p < 0.001. Please click here to view a larger version of this figure.
Discussion
Emerging studies have shown that plants can rapidly respond to external stimuli23, including manipulation introduced to the experimental procedures22. In our initial experiment, our oversight of this knowledge often leads to staining failure. With these lessons, we suggest that the following precautions should be kept in mind when performing this experiment: (1) the seeds after harvest should be kept in a storage cabinet set to a low temperature and low moisture; (2) manipulation of plants, particularly the exposure to air in the cabinet, should be kept to a minimum time; (3) the experimental conditions should be kept constant, e.g., all the procedures should be performed in a growth room.
Another aspect in this experiment that needs to be pointed out is that the loading volume of CFDA should be kept as small as possible. Excess solution often leads to artifacts in which the excess CFDA solution can diffuse up to the shoot through capillary action, thereby tinting the trichomes of young sink leaves. Although a washing step before imaging can diminish this artifact, the best approach is to load a minimum amount to avoid complication caused by excess solution.
With these technical precautions resolved, the root-to-shoot movement of CFDA can be stably observed as shown in Figure 2. When plants grow older, say over 24 days, the Arabidopsis plants seem to lose the ability to transmit CF to the shoot, for which we have not yet found an exact explanation. One possible clue comes from its intracellular accumulation. According to the reports by Wright et al.2, CF is liable to be sequestrated by vacuoles, therefore, the intracellular free CF over the course of translocation reduces gradually to sequestration in the larger vacuoles of aging plants.
One obvious feature of this procedure is the distribution pattern of CF in the shoot. This vascular pattern is reminiscent of those by loading the dye in the source leaf1,24,25, thus leading to an illusion that the dye also moves through the phloem. In fact, the bottom-to-top translocation of CF may not be achieved through the phloem given the fact that the root is a strong sink tissue and the shootward movement of CFDA goes against phloem streaming. Rather, this process may be facilitated by xylem transport as shown by Botha et al.25. Briefly, CFDA can be taken up through xylem vessels, processed in parenchyma cells and further translocated to the sieve element of phloem stream25. Therefore, the loading experiment in this way may not reflect the activity of phloem, but the symplastic movement of CF must occur as the strong fluorescence can be detected. In other words, this bottom-to-top CF movement may result from the combined xylem transport and symplastic transport through parenchyma cells.
Symplastic transport is often subject to symplastic domain formation13, and in certain circumstances it displays unidirectional transport19,20. One way for a quick check is to shift loading sites. Indeed, when we elaborated this process using the same method, we found the simple change of loading site, from root side to the hypocotyl side proximal to root-hypocotyl junction, would result in significant change of CFDA mobile efficiency (Figure 3). The CF mobile differentiation due to the distinct loading sites would suggest that the root-hypocotyl junction is another symplastic domain, where the symplastic barrier is formed for the root-derived shootward signals. Further experimental design with other molecules is needed to explore this possibility.
So far, this simple method can provide stable shootward movement of CFDA. This feature can be further explored to distinguish plants with compromised or enhanced root-to-shoot movement which has seldom been studied.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was funded by National Natural Science Foundation of China (31671257) and Hubei Collaborative Innovation Center for Grain Industry (LXT-16-18).
Materials
| KNO3 | Sinopharm Chemical Reagent | 10017218 | |
| KH2PO4 | Sinopharm Chemical Reagent | 10017608 | |
| MgSO4·7H2O | Sinopharm Chemical Reagent | 10013018 | |
| CaCl2·2H2O | Sinopharm Chemical Reagent | 20011160 | |
| MnSO4·H2O | Sinopharm Chemical Reagent | 10013418 | |
| Na2MoO4·2H2O | Sinopharm Chemical Reagent | 10019818 | |
| Boric Acid | Sinopharm Chemical Reagent | 10004818 | |
| ZnSO4·7H2O | Sinopharm Chemical Reagent | 10024018 | |
| CuSO4·5H2O | Sinopharm Chemical Reagent | 10008218 | |
| CoCl2·6H2O | Sinopharm Chemical Reagent | 10007216 | |
| KI | Sinopharm Chemical Reagent | 10017160 | |
| FeSO4·7H2O | Sinopharm Chemical Reagent | 10012118 | |
| EDTA | Sinopharm Chemical Reagent | 10009717 | |
| NaOH | Sinopharm Chemical Reagent | 10019718 | |
| KOH | Sinopharm Chemical Reagent | 10017018 | |
| Sucrose | Sinopharm Chemical Reagent | 10021418 | |
| Myo-inositol | MACKLIN | I811835 | |
| Nicotinic Acid | MACKLIN | N814565 | |
| Pyridoxine HCl | MACKLIN | V820447 | |
| Thiamine HCl | MACKLIN | T818865 | |
| Glycine | MACKLIN | G800880 | |
| Agar powder | Novon | ZZ14022 | |
| Fluorescence Microscope | Zeiss | Axio Zoom V16 | |
| Dissecting microscope | SDPTOP | SRE-1030 | |
| 200μl pipette | Dragon Laboratory Instruments | 713111110000-20-200ul | |
| 2.5μl pipette | Eppendorf | 3120000011 | |
| Fine forceps | TWEEZERS | ST-15 | |
| Parafilm | PARAFILM | PM-996 | |
| Stainless steel double-sided blade | Gillette | Platinum-Plus Double-Edge Blade | |
References
- Grignon, N., Touraine, B., Durand, M. 6(5)Carboxyfluorescein as a tracer of phloem sap translocation. American Journal of Botany. 76, 871-877 (1989).
- Wright, K. M., Oparka, K. J. The fluorescent probe HPTS as a phloem-mobile, symplastic tracer: an evaluation using confocal laser scanning microscopy. Journal of Experimental Botany. 47 (3), 439-445 (1996).
- Oparka, K. J., Prior, D. A. Movement of Lucifer Yellow CH in potato tuber storage tissues: A comparison of symplastic and apoplastic transport. Planta. 176 (4), 533-540 (1988).
- Knoblauch, M., et al. Multispectral Phloem-Mobile Probes: Properties and Applications. Plant Physiology. 167 (4), 1211-1220 (2015).
- Oparka, K. J., Duckett, C. M., Prior, D. A. M., Fisher, D. B. Real-time imaging of phloem unloading in the root tip of Arabidopsis. The Plant Journal. 6 (5), 759-766 (1994).
- Viola, R., et al. Tuberization in Potato Involves a Switch from Apoplastic to Symplastic Phloem Unloading. The Plant Cell. 13 (2), 385-398 (2001).
- Roberts, A. G., et al. Phloem Unloading in Sink Leaves of Nicotiana benthamiana: Comparison of a Fluorescent Solute with a Fluorescent Virus. The Plant Cell. 9 (8), 1381-1396 (1997).
- Péron, T., et al. New Insights into Phloem Unloading and Expression of Sucrose Transporters in Vegetative Sinks of the Parasitic Plant Phelipanche ramosa L (Pomel). Frontiers in Plant Science. 7 (2048), (2017).
- Spallek, T., et al. Interspecies hormonal control of host root morphology by parasitic plants. Proceedings of the National Academy of Sciences of the USA. 114 (20), 5283-5288 (2017).
- Complainville, A., et al. Nodule initiation involves the creation of a new symplasmic field in specific root cells of medicago species. The Plant Cell. 15 (12), 2778-2791 (2003).
- Bederska, M., Borucki, W., Znojek, E. Movement of fluorescent dyes Lucifer Yellow (LYCH) and carboxyfluorescein (CF) in Medicago truncatula Gaertn. roots and root nodules. Symbiosis. 58 (1-3), 183-190 (2012).
- Robards, A. W., Lucas, W. J. Plasmodesmata. Annual Review of Plant Physiology and Plant Molecular Biology. 41 (1), 369-419 (1990).
- Roberts, A. G., Oparka, K. J. Plasmodesmata and the control of symplastic transport. Plant, Cell & Environment. 26 (1), 103-124 (2003).
- Duckett, C. M., Oparka, K. J., Prior, D. A. M., Dolan, L., Roberts, K. Dye-coupling in the root epidermis of Arabidopsis is progressively reduced during development. Development. 120 (11), 3247-3255 (1994).
- Palevitz, B. A., Hepler, P. K. Changes in dye coupling of stomatal cells of Allium and Commelina demonstrated by microinjection of Lucifer yellow. Planta. 164 (4), 473-479 (1985).
- van Bel, A. J. E., Kempers, R. Symplastic isolation of the sieve element-companion cell complex in the phloem of Ricinus communis and Salix alba stems. Planta. 183 (1), 69-76 (1991).
- Erwee, M. G., Goodwin, P. B. Symplast domains in extrastelar tissues of Egeria densa Planch. Planta. 163 (1), 9-19 (1985).
- Oparka, K. J., Prior, D. A. M., Wright, K. M. Symplastic communication between primary and developing lateral roots of Arabidopsis thaliana. Journal of Experimental Botany. 46 (2), 187-197 (1995).
- Christensen, N. M., Faulkner, C., Oparka, K. Evidence for Unidirectional Flow through Plasmodesmata. Plant Physiology. 150 (1), 96-104 (2009).
- Wróbel-Marek, J., Kurczyńska, E., Płachno, B. J., Kozieradzka-Kiszkurno, M. Identification of symplasmic domains in the embryo and seed of Sedum acre L. (Crassulaceae). Planta. 245 (3), 491-505 (2017).
- Liang, D., White, R. G., Waterhouse, P. M. Gene silencing in Arabidopsis spreads from the root to the shoot, through a gating barrier, by template-dependent, non-vascular, cell to cell movement. Plant Physiology. 159 (3), 984-1000 (2012).
- Radford, J. E., White, R. G. Effects of tissue-preparation-induced callose synthesis on estimates of plasmodesma size exclusion limits. Protoplasma. 216 (1-2), 47-55 (2001).
- Kollist, H., et al. Rapid Responses to Abiotic Stress: Priming the Landscape for the Signal Transduction Network. Trends in Plant Science. 24 (1), 25-37 (2019).
- Haupt, S., Duncan, G. H., Holzberg, S., Oparka, K. J. Evidence for Symplastic Phloem Unloading in Sink Leaves of Barley. Plant Physiology. 125 (1), 209-218 (2001).
- Botha, C. E. J., et al. A xylem sap retrieval pathway in rice leaf blades: evidence of a role for endocytosis?. Journal of Experimental Botany. 59 (11), 2945-2954 (2008).

