Electrophysiological Recordings of Single-cell Ion Currents Under Well-defined Shear Stress
Summary
The goal of this protocol is to describe a modified parallel plate flow chamber for use in investigating real time activation of mechanosensitive ion channels by shear stress.
Abstract
Fluid shear stress is well known to play a major role in endothelial function. In most vascular beds, elevated shear stress from acute increases in blood flow triggers a signaling cascade resulting in vasodilation thereby alleviating mechanical stress on the vascular wall. The pattern of shear stress is also well known to be a critical factor in the development of atherosclerosis with laminar shear stress being atheroprotective and disturbed shear stress being pro-atherogenic. While we have a detailed understanding of the various intermediate cell signaling pathways, the receptors that first translate the mechanical stimulus into chemical mediators are not completely understood. Mechanosensitive ion channels are critical to the response to shear and regulate shear-induced cell signaling thereby controlling the production of vasoactive mediators. These channels are among the earliest activated signaling components to shear and have been linked to shear-induced vasodilation through promoting nitric oxide production (e.g., inwardly rectifying K+ [Kir] and transient receptor potential [TRP] channels) and endothelium hyperpolarizing factor (e.g., Kir and calcium-activated K+ [KCa] channels) and shear-induced vasoconstriction through an undetermined mechanism that involves piezo channels. Understanding the biophysical mechanism by which these channels are activated by shear forces (i.e., directly or through a primary mechano-receptor) could provide potential new targets to resolve the pathophysiology associated with endothelial dysfunction and atherogenesis. It is still a major challenge to record flow-induced activation of ion channels in real time using electrophysiology. The standard methods to expose cells to well-defined shear stress, such as the cone and plate rheometer and closed parallel plate flow chamber do not allow real time study of ion channel activation. The goal of this protocol is to describe a modified parallel plate flow chamber that allows real time electrophysiological recording of mechanosensitive ion channels under well-defined shear stress.
Introduction
Hemodynamic forces generated by the blood flow are well known to play major roles in endothelial and vascular function1,2. It is also well known that several types of ion channels acutely respond to changes in shear stress3,4,5 leading to the hypothesis that ion channels can be primary shear stress sensors. More recently, we and others showed that mechanosensitive ion channels play critical roles in several shear-stress sensitive vascular functions, including the vasoactive response to shear stress6,7,8, and developmental angiogenesis9. The mechanisms of the shear-stress sensitivity of ion channels, however, are almost totally unknown. This gap of knowledge is likely to be due to the technical difficulty of performing electrophysiological recordings under well-defined shear stress. In this article, therefore, we provide a step by step detailed protocol routinely performed in our lab to achieve this goal6,7,10,11.
The overall goal of this method is to allow the real-time investigation of ion channel mechanoactivation under well-defined shear stress in the physiological range. This is achieved by modifying a standard parallel plate flow chamber to allow an electrophysiological pipette to be lowered into the chamber and access cells grown on the bottom plate during the real time exposure to flow, providing a unique approach to achieve this goal6,7,11. In contrast, standard parallel plate flow chambers, described in prior publications can be used for the real time imaging analysis of cells exposed to shear forces12 or other non-invasive approaches13,14 but not for electrophysiology. Similarly, the cone and plate apparatus, another powerful approach to expose cells to shear stress15,16 is also not suitable for electrophysiological recordings. Thus, these flow devices do not allow the investigation of shear stress sensitivity of ion channels. The difficulty in performing electrophysiological recordings under flow is the main reason for the paucity of information about the mechanisms responsible for these crucial effects.
In terms of the alternative approaches to achieve the same goal, there are none that are as accurate or controlled. Some earlier studies attempted to record ion channel activity under flow by exposing cells to a stream of liquid coming from another pipette brought to the vicinity of a cell from above17,18. This is highly non-physiological, as the mechanical forces generated under these conditions have little in common with the physiological profiles of shear stress in the blood vessels. Similar concerns apply to the attempts to simulate physiological shear stress by perfusion of open chambers. As discussed in detail in our earlier study10, an open liquid-air interface creates multiple disturbances and recirculation, which are non-physiological. To address all these concerns, we have designed a modified parallel plate (MPP) flow chamber, also referred to as the “minimally invasive flow device” in our earlier studies6,7,10,11, made from acrylic and extensively used in our lab. However, in spite of the fact that the original description of the design has been published almost 20 years ago and is the only flow device that allows performing electrophysiological recordings under well-defined shear stress, this methodology has not been adopted by other labs and there are only very few studies that attempt to record currents under flow. We believe, therefore, that providing a detailed description for using the MPP flow chamber will be of great help to researches who are interested in mechanosensitive ion channels and vascular biology.
Protocol
The use of animals in our studies is approved by the University of Illinois at Chicago Animal Care Committee (#16-183).
1. Assembly of the Modified Parallel Plate Flow Chamber
NOTE: Please refer to Table 1 and Figure 1 for MPP flow chamber piece IDs. Please refer to Figure 1 for a schematic detailing the orientation of chamber pieces for assembly.
- To adhere the rectangular cover glass, piece D, to the bottom of piece C, first make the silicone elastomer solution by thoroughly mixing 500 µL silicone elastomer curing agent into 5 mL of silicone elastomer base.
- Apply a thin layer of the silicone elastomer solution around the edges of the rectangular space of piece C and gently place the rectangular cover glass piece D directly on the elastomer solution such that piece D completely covers the open rectangular space of piece C. Carefully wipe away excess silicone elastomer solution.
- Repeat step 1.2 for adhering the rectangular cover glass, piece F, to the bottom of piece E and allow the silicone elastomer solution to cure over night at room temperature.
NOTE: Once cured, the rectangular cover glass will remain adhered for up to six months before needing to be replaced. - Beginning with the bottom chamber piece, piece E, assemble the MPP flow chamber by sequentially placing each piece on top of the previous in the following order: piece E (bottom), piece C, piece B, piece A (top).
- Align the screw holes of each piece at the corners and tightly screw the pieces together to prevent leaks from occurring while administering flow to the MPP flow chamber.
2. Cell Preparation and Seeding into the MPP Flow Chamber
NOTE: Follow steps 2.1−2.7 for cultured endothelial cells. Follow the method detailed in steps 2.8−2.14 for isolating endothelial cells from the mouse mesenteric arterial arcade and preparation of freshly isolated endothelial cells.
- In a 6-well plate, place four to five 12 mm cover glass circles/well and seed cells between 10% and 30% confluency such that single cells can be accessed for electrophysiological recordings.
- Incubate cells under standard culture conditions (5% CO2, 37 °C) for no less than 2 h to allow cells to adhere and no more than 24 h as endothelial cells in authors’ experience become very flat and difficult to patch when seeded at sub-confluency for more than 24 h.
- Remove a cover glass containing adhered cells from a well of the 6-well plate, quickly rinse in phosphate-buffered saline (PBS), and transfer to a 35 mm Petri dish containing 2 mL electrophysiological bath solution (Table 2) prior to transfer to the MPP flow chamber.
- Transfer the cover glass circle to the rectangular cover glass, piece D, which is adhered to piece C of the MPP flow chamber being sure that adequate solution stays on the cover glass so that cells to not become exposed to air. Add the desired bath solution (~250 µL) to the cells so that the cover glass circle and cells are completely submerged in solution.
- Position the cover glass circle such that it rests in the half closest to the vacuum reservoir side so that cells will be in line with the slit openings of piece B. Ensure that the glass cover circle adheres to piece D through solution-glass adhesion so that application of flow to the chamber does not disrupt the position of the cover glass circle.
- Assemble the MPP flow chamber by screwing the pieces together in the appropriate order, as described in steps 1.4 and 1.5 and as shown in Figure 1. Transfer the chamber to the microscope stage and immediately perfuse the chamber with bath solution such that solution reaches the vacuum reservoir for aspiration (~10 mL).
- Identify a healthy cell for the experiment by identifying a cell with a dark border and obvious nucleus. Avoid cells that appear to be blebbing or cells that are in contact with other cells.
NOTE: In the authors’ laboratory, human aortic endothelial cells and primary mouse mesenteric endothelial cells in culture are used. However, any other type of adherent cell that is of interest to specific research needs can be used in the same way. - Wash the isolated arterial arcade in dissociation solution (Table 2). Transfer the arcade to a 2 mL centrifuge tube containing 2 mL of pre-warmed (37 °C) dissociation solution (recipe for dissociation solution shown in Table 2) containing neutral protease (0.5 mg/mL) and elastase (0.5 mg/mL). Incubate for 1 h at 37 °C with gentle shaking every 10 min.
- Remove 1 mL of the neutral protease/elastase dissociation solution and add 1 mg/mL collagenase type 1. Return the collagenase solution to the solution containing the arteries for a final collagenase type 1 concentration of 0.5 mg/mL. Incubate for 2−3 min at 37 °C.
- Using 5 grade forceps, quickly move the arcade onto a 35 mm diameter Petri dish containing a 750 µL drop of fresh, chilled dissociation solution. Further dissociate the tissue by using two 20 G syringe needles to mechanically liberate endothelial cells from enzymatically digested arteries.
- Using a 9” disposable Pasteur glass pipet, triturate the cell solution 10x before transferring the cells to a new 1.5 mL centrifuge tube using the glass pipet.
- Wash the Petri dish with another 750 µL of dissociation solution and transfer to the same tube. Using the glass pipette, further mechanically disperse the cells by pipetting at least 10x to create a single cell suspension being careful not to introduce bubbles that may damage endothelial cell integrity.
- Add 750 µL of the endothelial cell suspension (~500−1,000 cells) directly to piece D of the MPP flow chamber on the half closest to the reservoir vacuum side. Allow the endothelial cells to adhere between 45 min and 1 h at room temperature.
- Assemble the MPP flow chamber by screwing the pieces together in the appropriate order as described in steps 1.4 and 1.5 and as shown in Figure 1. Transfer the chamber to the microscope stage and immediately perfuse the chamber with bath solution such that solution reaches the vacuum aspiration (~10 mL). Identify accessible endothelial cells by their rough and round phenotype19,20.
NOTE: A variety of digestion methods and enzyme cocktails have been used to isolate endothelial cells from different arterial beds. See Table 3 for detailed descriptions of protocols that have been used by a variety of investigators to isolate endothelial cells for patch clamp electrophysiology of mechanosensitive ion channels. These methods are likely suitable for use in combination with the MPP flow chamber.
3. Controlling Shear Stress to the MPP Flow Chamber for Electrophysiological Recordings of Shear-activated Mechanosensitive Ion Channels
- Set-up a gravity perfusion system by connecting a 30 mL graduated syringe cylinder to a 3-way luer lock fitted with microbore tubing (internal diameter: 0.05 inch, outer diameter: 0.09 inch) suited for insertion into the 3 mm diameter inlet hole of piece A of the MPP chamber.
- Attach the graduated cylinder to the outer face of the Faraday cage surrounding the electrophysiology rig (Figure 2) using double-sided tape. Prior to inserting the tubing in the MPP chamber, pre-fill the syringe and tubing with bath solution (see Table 2 for bath solution used for investigating inwardly rectifying K+ channels in endothelial cells). Insert the tubing into the MPP flow chamber inlet hole of piece A.
- Pre-fill the MPP flow chamber with solution such that solution is being removed in the vacuum reservoir. Stop flow to the chamber and refill the graduated cylinder to the top mark. Calculate flow rates manually by allowing the solution to flow through the chamber and using a stop-watch to calculate mL/s at a given syringe cylinder height.
- Raise or lower the syringe to alter flow, and thus shear in the chamber, and continue this process until a desired level of shear stress is found.
- Calculate shear stress in a parallel chamber using the following equation21:
τ = 6µQ/h2w
where µ = fluid viscosity (g/cm·s), Q = flow rate (mL/s), and parallel plate chamber width (w = 2.2 cm) and height (h = 0.1 cm).
NOTE: In the current gravity perfusion system, at a syringe cylinder height (as measured from the top of the cylinder) of 57 cm above the microscope stage, the flow rate is 0.3 mL/s. The shear calculated in the chamber at this syringe cylinder height and flow rate is 0.7 dyn/cm2. It should also be noted that other perfusion systems, such as a peristaltic pump, can be used to control flow to the MPP flow chamber. However, these devices may add unwanted turbulence and influence stability of the electrophysiology measurements under flow, therefore, using the gravity perfusion system described here is recommended. - Transfer an assembled chamber containing adhered cells to the microscope stage of the electrophysiology rig and insert the tubing pre-filled with bath solution into the hole of piece A. Simultaneously fill the chamber and wash the cells with 10 mL of bath solution by turning the 3-way luer lock such that solution flows to the chamber.
- Once the desired patch configuration is successfully obtained allow channel currents to stabilize in a static bath at room temperature. Once currents have stabilized, apply shear in a step-wise fashion allowing increases in current to stabilize prior to the next step increase in shear stress.
NOTE: The authors find the most success with the perforated patch configuration when studying mechanoactivated ion channels in endothelial cells. To perform perforated whole-cell patch configurations, add 5 µL of a 60 mg/mL stock amphotericin B in dimethyl sulfoxide (DMSO) to 1 mL of 0.2 µm sterile filtered pipette solution. After generating a giga-ohm seal in the cell-attached configuration, perforated whole-cell patches form within 2−5 min. - Remove shear exposure to the cells by stopping flow to the chamber allowing mechanosensitive channel currents to return to baseline currents observed in the static bath.
- Isolate mechanosensitive ion channel currents of interest by altering solution valence (e.g., 60 mM K+ in bath solution with 0 Ca2+ in pipette solution to study inwardly rectifying K+ channels. Table 2 shows example solution recipes) and/or pharmacological inhibition of potentially contaminating current sources.
Representative Results
Multiple photographs showing different views of the MPP flow chamber on the microscope stage (upper panel) and a schematic representation of the MPP flow chamber (bottom panel) are shown in Figure 1. The schematic details the dimensions of the entire device and flow chamber. Figure 2 shows a photograph of the gravity perfusion system to the MPP flow chamber in our laboratory (upper panel). Also shown is a schematic representation of the flow system (bottom panel) intended to highlight the steps which isolate the cells in the flow chamber from the rush of solution from the perfusion system and from the force of the vacuum removal of solution.
A hallmark of mechanosensitive ion channels is an abrupt return to baseline levels upon cessation of mechanical stimulus3,6,7. Figure 3 shows as an example that removing the shear stress stimulus during electrophysiological recordings of Kir current from a freshly isolated endothelial cell results in a return to baseline currents initially recorded in a static bath. The return to baseline current levels after stopping flow to the MPP chamber occurred within ten seconds of flow cessation.
Whole-cell (WC) electrophysiological recordings, especially those taken from freshly isolated cells, often have obvious leak background currents that can mask channel activity. Some ion channels, such as those of the inwardly rectifying K+ channel family, have biophysical properties that allow for subtracting the leak background current for more accurate analysis. Figure 4 shows as an example the process from raw data (Figure 4A) to calculated and plotted linear outward leak (Figure 4B) to final, leak subtracted representative trace (Figure 4C). See a detailed explanation for calculating and subtracting leak from the raw perforated WC patch recording in the accompanying legend to Figure 4.
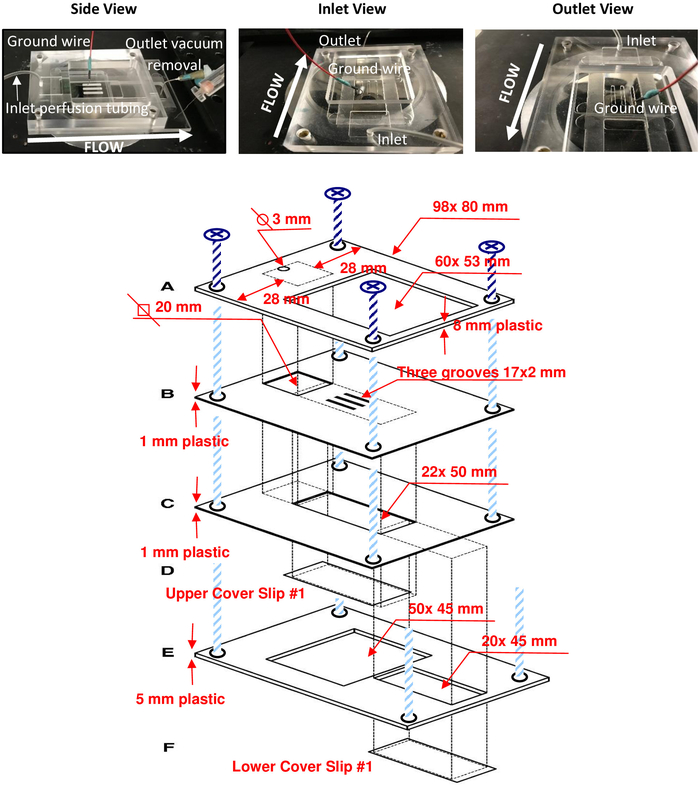
Figure 1: MPP flow chamber and detailed schematic. Photographs of the assembled MPP flow chamber (upper panel) show the chamber on the microscope stage from three different views: the side as viewed during experiments (left), from the perfusion inlet (middle), and from the vacuum outlet (right) which is out of view in the photograph. The direction of flow is labeled in each. Notice that the ground wire can easily fit into one of the 2 mm slits when bent at a 90° angle. A detailed schematic (bottom) shows exact dimensions for replication of the apparatus. Please click here to view a larger version of this figure.
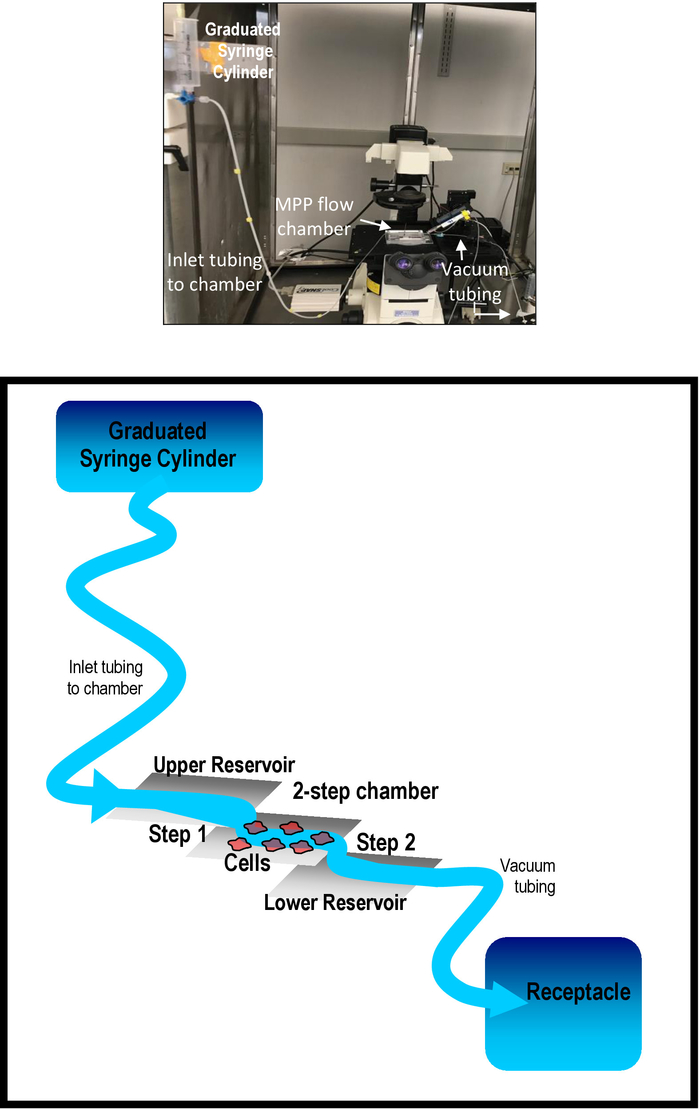
Figure 2: The gravity perfusion system. A labeled photograph of the gravity perfusion system in our laboratory is shown in the upper panel. The two-step MPP flow chamber and gravity perfusion system are detailed in the bottom panel. The separation of the flow chamber containing cells and the two upper and lower reservoirs is highlighted. Step 1 allows solution to flow from the upper reservoir of piece B to piece D of the chamber where cells are seeded. Step 2 allows solution to flow from piece D down to the lower reservoir, piece F. Please click here to view a larger version of this figure.
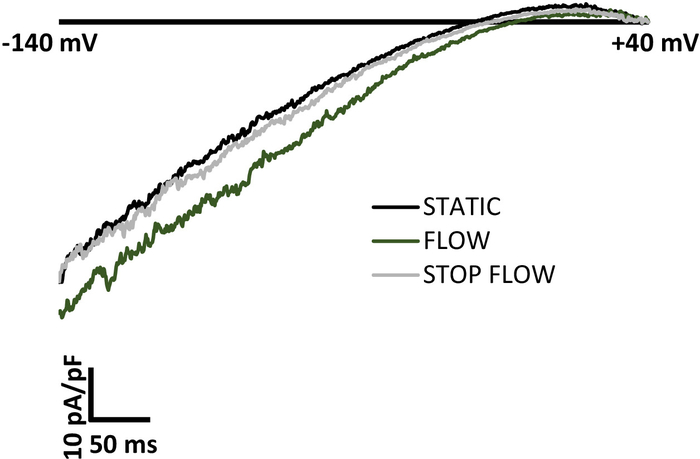
Figure 3: Representative traces of shear-induced current activation of Kir channels and return to baseline current levels upon removal of flow. A good positive control for mechanoactivation of ion channels is the return to baseline current levels initially observed in a static bath upon cessation of the mechanical stimulus. Inwardly rectifying K+ (Kir) channel currents are activated within seconds by shear stress when gravity solution is allowed to flow to the MPP flow chamber. Upon cessation of flow to the chamber, Kir currents quickly return to baseline static levels observed prior to flow. A ramp of -140 mV to +40 mV was applied to the patch over 400 ms. The bath solution contained 60 mM K+ and the reversal potential was ~-20 mV. The representative traces were generated from an endothelial cell freshly isolated from mouse mesenteric arteries. Please click here to view a larger version of this figure.
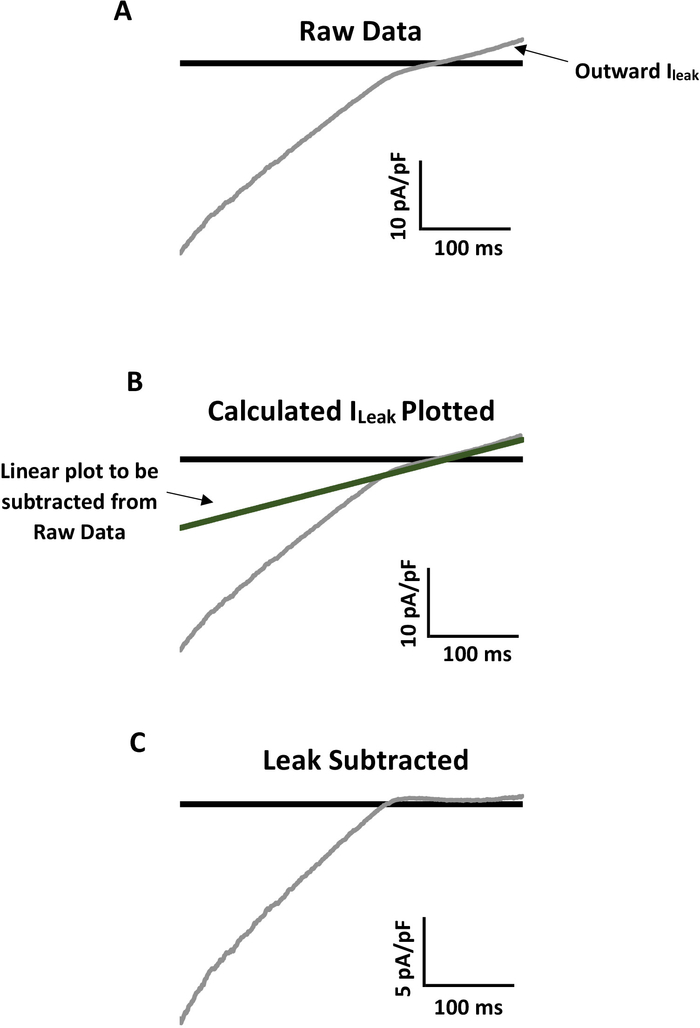
Figure 4: Example of leak subtraction for accurate analysis of mechanoactivated Kir current. (A) Representative raw recording of Kir current from a primary mouse mesenteric endothelial cell with notable linear outward leak current (Ileak). A ramp of -140 mV to +40 mV was applied to the patch over 400 ms. The bath solution contained 60 mM K+ and the reversal potential was ~-20 mV. (B) Ileak prevents analysis of real Kir channel activity. To subtract Ileak, first calculate the linear slope conductance of Ileak (Gslope = (Ia-Ib)/(Va-Vb)). Multiply Gslope by corresponding voltages of the entire raw trace to plot Ileak on the raw data. The line should overlay the outward linear leak exactly. (C) Subtract the plotted Ileak from the entire trace so that the linear outward current is ~0 pA/pF and the real Kir current can be analyzed. Notice in panel C that the inward Kir current is approximately half that of the original raw data trace in panel A. Please click here to view a larger version of this figure.
| Height (mm) | Width (mm) | Length (mm) | Additional information | ||
| Piece A | 8 | 80 | 98 | Contains a 3 mm diameter hole for inlet perfusion tubing (see Table of Materials) and 53 mm x 60 mm rectangular space for access to middle pieces and cells | |
| Piece B | 1 | 80 | 98 | Contains a space (20 mm diagonal) underneath perfusion hole of Piece A and three 2 mm x 17 mm slits for access to cells | |
| Piece C | 1 | 80 | 98 | Contains a 22 mm x 50 mm space where Piece D is adhered using the silicone elastomer solution (see Table of Materials) | |
| Piece D | 0.16 | 24 | 40 | Rectangular glass slide bottom of the flow chamber | |
| Piece E | 5 | 80 | 120 | Contains a 45 mm x 50 mm space to allow visualization of cells on Piece D. A 20 mm x 45 mm space is present for the reservoir vacuum outlet, Piece F, to be adhered | |
| Piece F | 0.16 | 24 | 50 | Rectangular glass slide bottom of vacuum outlet reservoir | |
| Assembled MPP | 15 | 80 | 120 | Flow chamber is separated from inlet perfusion and outlet vacuum by two steps to avoid disruption of well-defined laminar shear | |
| Flow Chamber of Assembled MPP | 1 | 22 | 42 | Piece D is the glass slide bottom of the flow chamber | |
Table 1: Dimensions of the MPP (assembled and disassembled) and additional information specific to each part of the device.
| Solution | Recipe (in mM) | pH | ||
| Dissociation | 55 NaCl, 80 Na-glutamate, 6 KCl, 2 MgCl2, 0.1 CaCl2, 10 glucose, 10 HEPES | 7.3 | ||
| (Cell isolation) | ||||
| Bath (Electrophysiology) | 80 NaCl, 60 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, 10 HEPES | 7.4 | ||
| Pipette (Electrophysiology) | 5 NaCl, 135 KCl, 5 EGTA, 1 MgCl2, 5 glucose, 10 HEPES | 7.2 | ||
Table 2: Examples of solutions with recipes used in the experiments.
| Endothelial cell isolation protocol | References | |
| Cocktail of neutral protease and elastase (0.5 mg/mL each; 1 h at 37 °C) followed by collagenase type I (0.5 mg/mL; 2.5 min) | 6,7 | |
| Gentle mechanical scraping of a 5-cm2 region located at the inner wall of the descending thoracic aorta | 11 | |
| NA | 17 | |
| NA | 3 | |
| Collagenase type IA (1 mg/mL) for 14 min at 37 °C | 8 | |
Table 3: Methodology to study mechanosensitive ion channels using electrophysiological techniques.
Discussion
The vascular system is constantly exposed to active hemodynamic forces, which activate mechanosensitive ion channels3,22 but the physiological roles of these channels in shear stress-induced mechanotransduction is only starting to emerge4,6,8. The mechanisms responsible for the mechanosensitivity of shear stress-activated channels remain unknown. The protocol detailed here describes the method for direct investigation of mechanosensitive ion channels exposed to laminar shear stress in real time.
The critical and essential step of this protocol is the use of a modified parallel plate flow chamber that has narrow openings to allow an electrophysiological pipette to be lowered into the chamber and access cells under flow. The general principle of this device is that if the openings are narrow enough, physiological shear stress (up to 15 dyn/cm2) can be achieved without solution overflow due to surface tension forces10. In order to perform these experiments successfully, it is critical: (i) to seed cells in the area of the bottom plate that is directly underneath the openings; (ii) establish a giga-ohm seal prior to the initiation of the flow or during very slow (<0.01 dyn/cm2) background flow; (iii) maintain a stable seal while stepping up the flow. Dimensions of the four-piece acrylic apparatus used in our laboratory are provided along with detailed schematics (Figure 1) such that the MPP flow chamber and flow system (Figure 2) can be replicated for use in any laboratory investigating mechanosensitive ion channels. These dimensions can also be modified to increase the area seeded by cells and to change the height of the flow channel, which would change the relationship between the flow rate and the shear stress. A decrease in the height of the flow chamber would result in higher shear stress for the same flow rate, which could be beneficial for achieving higher shear stresses. The chamber can also be modified to include a flow separation barrier that induces a local region of recirculating disturbed flow23.
The major advantages of using the MPP flow chamber include (1) real time investigation of shear-activated ion channels from endothelial cells in culture and cells freshly isolated from vascular tissue, (2) exposure of cells and the response of mechanosensitive ion channels to physiologically relevant levels of shear stress, and (3) easy assembly and disassembly with perfusion options for experimentation. With respect to other existing methods, the only other method that allows performing electrophysiological recordings under well-defined shear stress is seeding the cells inside a capillary end and inserting the recording pipette into the capillary opening, as was done in the early studies3,22. There are, however, multiple disadvantages compared to the method described here, such as the difficulty of seeding cells into capillaries, access to a very small number of cells that are close enough to the capillary end for the pipette to be able to reach them, and flow disturbances at the end of the flow channel (capillary in this case). Each of these disadvantages makes it difficult to perform electrophysiology under flow in cells cultured in the capillary opening and virtually impossible to patch or even seed cells freshly isolated from the vasculature. It is also impossible to introduce a step to generate an area of disturbed flow. All other existing methods that either employ open chambers24,25 or puffing solutions directly on a cell17,18 do not mimic the hemodynamic environment in the blood vessel.
The main limitation of this method, however, is a constraint to achieve shear stress at higher levels of the physiological range. Specifically, physiological shear stress levels have been estimated to reach up to 70 dyn/cm2 in healthy arteries26. In contrast, the highest shear stress level that we could achieve in the current configuration of chamber was 15 dyn/cm2, after which the surface tension forces become not sufficient to prevent the solution to spill out of the openings10. It is possible that decreasing the height of the flow channel might allow achieving higher shear stress levels. Maintaining a stable giga-ohm seal under high shear stress is another challenge but the success rate is reasonable with practice. We found that using perforated patch technique (including the antibiotic amphotericin B in the pipette solution as described above) yields more stable recordings than the standard whole cell configuration. Additionally, the MPP flow chamber and solutions used do not exactly replicate the shear of blood flow in arteries. Blood is a viscous, non-Newtonian fluid that we have not replicated in our in vitro experiments. Additionally, the chamber used here is a parallel chamber while shear stress in arteries is best calculated using the formula for shear in a cylinder.
There are important considerations and limitations for performing single-channel recordings under flow. This method is appropriate for a cell-attached configuration (pipette attached to the membrane of an intact cell), which yields stable seals. It is critical though to be aware that single channels whose activity is being recorded are not directly exposed to shear stress because they are shielded by the recording pipette, especially in the inside-out configuration27. We believe that the excised patch configurations are not the most reliable when used to test the sensitivity of ion channels to flow because the excised membrane is typically pulled into the recording pipette and thus is not exposed to a well-defined flow.
In terms of the type of cells that can be used in these experiments, vascular endothelial cells represent the most applicable cell type to studying shear stress and the mechanosensitive channels. Earlier studies focused primarily on cultured endothelial cells3,28 that are easily available. We have tested and extended the use of this method to endothelial cells freshly-isolated from mouse resistance arteries6,7. Other cell types should definitely be considered. Vascular smooth muscle cells, for instance, can become exposed to shear stress during disease states where the endothelium has been damaged and removed29,30. This represents an intriguing area of study whereby mechanosensitive channels that reside in smooth muscle, which would otherwise be uninfluenced by shear stress, would now contribute to potentially pathophysiological disease mechanisms. Furthermore, the transfection of vehicle cell lines like HEK or CHO with the gene encoding the channel of interest is an excellent platform for the biophysical analyses of channels in combination with use of the MPP flow chamber.
It is also important to note that while the original intention for the MPP flow chamber was for the real time investigation of ion channel mechanoactivation, the application of the device may extend beyond this goal. Specifically, the same approach can be used for studies that use electrode sensors, such as a nitric oxide (NO) sensor to determine the release of NO in response to shear stress. Therefore, we provide a generalized methodology for those with interest in investigating mechanically regulated biological processes in real time and promote further modification of the MPP chamber to meet specific research needs for those studying mechanosensitive processes in a variety of fields.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was funded by the National Heart, Lung, and Blood Institute (R01 HL073965, IL) and (T32 HL007829-24, ISF). The authors would also like to acknowledge the Scientific Machine Shop at the University of Illinois at Chicago for generating our latest MPP flow chambers.
Materials
| 0.2 µm sterile syringe filters | VWR | 28145-501 | Used for filtering electrophysiolgoical pipette solution |
| 5 grade forceps | Fine Scientific Tools | 1252-30 | Used for transferring digested arteries to fresh solution |
| 9" Pasteur Pipet | Fisher Scientifc | 13-678-20D | Used for mechanically disrupting digested arteries and transferring freshly isolated endohtelial cells |
| 12 mm diameter Cover glass circles | Fisher Scientifc | 12-545-80 | For use with studies involving cultured cells and multiple treatments. Cells adhered to the cover glass are used for patch clamp analyses |
| 24 x 40 mm Rectangluar Cover glass | Sigma-Aldrich | CLS2975224 | Cover glass to be added to MPP flow chamber pieces C (Figure 1) |
| 24 x 50 mm Rectangluar Cover glass | Sigma-Aldrich | CLS2975245 | Cover glass to be added to MPP flow chamber E (Figure 1) |
| 20 gauge syringe needles | Becton Dickinson and Co | 305175 | For use in mechanical disruption of digested mesenteric arteries |
| 35 mm Petri dish | Genesee Scientific | 32-103 | For use in mechanical disruption of digested mesenteric arteries |
| Amphotericin B solubilized | Sigma-Aldrich | A9528-50MG | Used for generating the perforated whole-cell patch configuration. |
| collagenase, type I | Worthington Biochemical | 100 mg – LS004194 | Enzyme used in our laboratory as a brief digestion following the initial cocktail of neutral protease and elastase |
| Dimethyl Sulfoxide (DMSO) | Fisher Scientifc | 67-68-5 | Solvent for Amphotericin B used in perforated whole-cell patch clamp |
| elastase, lyophilized | Worthington Biochemical | 25 mg – LS002290 | Enzyme used in our laboratory in a cocktail with neutral protease/dispase to begin digestion of arteries for endothelial cell isolation. |
| Falcon Tissue culture Plate, 6-well, Flat Bottom with Low Evaporation Lid | Corning | 353046 | For use with studies involving cultured cells and multiple treatments |
| neutral protease/dispase | Worthington Biochemical | 10 mg- LS02100 50 mg – LS02104 | Enzyme used in our laboratory in a cocktail with elastase to begin digestion of arteries for endothelial cell isolation |
| SylGard | World Precision Instruments | SYLG184 | Silicone elastomer for adhering the rectangular cover slip to the MPP flow chamber pieces C and E (Figure 1) |
| Tygon ND 10-80 tubing | Microbore Tubing | AAQ04133 | ID: 0.05 in, OD: 0.09 in, inlet perfusion tubing for adminsitering flow to the chamber |
References
- Green, D. J., Hopman, M. T., Padilla, J., Laughlin, M. H., Thijssen, D. H. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiological Reviews. 97 (2), 495-528 (2017).
- Gimbrone, M. A., Topper, J. N., Nagel, T., Anderson, K. R., Garcia-Cardena, G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Annals of the New York Academy of Sciences. 902, 230-239 (2000).
- Olesen, S. P., Clapham, D. E., Davies, P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 331 (6152), 168-170 (1988).
- Barakat, A. I., Lieu, D. K., Gojova, A. Secrets of the code: do vascular endothelial cells use ion channels to decipher complex flow signals?. Biomaterials. 27 (5), 671-678 (2006).
- Beech, D. J. Endothelial Piezo1 channels as sensors of exercise. Journal of Physiology. 596 (6), 979-984 (2018).
- Ahn, S. J., et al. Inwardly rectifying K(+) channels are major contributors to flow-induced vasodilatation in resistance arteries. Journal of Physiology. 595 (7), 2339-2364 (2017).
- Fancher, I. S., et al. Hypercholesterolemia-Induced Loss of Flow-Induced Vasodilation and Lesion Formation in Apolipoprotein E-Deficient Mice Critically Depend on Inwardly Rectifying K(+) Channels. Journal of the American Heart Association. 7 (5), (2018).
- Rode, B., et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nature Communications. 8 (1), 350 (2017).
- Li, J., et al. Piezo1 integration of vascular architecture with physiological force. Nature. 515 (7526), 279-282 (2014).
- Levitan, I., Helmke, B. P., Davies, P. F. A chamber to permit invasive manipulation of adherent cells in laminar flow with minimal disturbance of the flow field. Annals of Biomed Engineering. 28 (10), 1184-1193 (2000).
- Fang, Y., et al. Hypercholesterolemia suppresses inwardly rectifying K+ channels in aortic endothelium in vitro and in vivo. Circulation Research. 98 (8), 1064-1071 (2006).
- Shetty, S., Weston, C. J., Adams, D. H., Lalor, P. F. A flow adhesion assay to study leucocyte recruitment to human hepatic sinusoidal endothelium under conditions of shear stress. Journal of Visualized Experiments. (85), e51330 (2014).
- Man, H. S. J., et al. Gene Expression Analysis of Endothelial Cells Exposed to Shear Stress Using Multiple Parallel-plate Flow Chambers. Journal of Visualized Experiments. (140), e58478 (2018).
- White, L. A., et al. The Assembly and Application of ‘Shear Rings’: A Novel Endothelial Model for Orbital, Unidirectional and Periodic Fluid Flow and Shear Stress. Journal of Visualized Experiments. (116), e54632 (2016).
- Franzoni, M., et al. Design of a cone-and-plate device for controlled realistic shear stress stimulation on endothelial cell monolayers. Cytotechnology. 68 (5), 1885-1896 (2016).
- Dewey, C. F., Bussolari, S. R., Gimbrone, M. A., Davies, P. F. The dynamic response of vascular endothelial cells to fluid shear stress. Journal of Biomechanical Engineering. 103 (3), 177-185 (1981).
- Hoger, J. H., Ilyin, V. I., Forsyth, S., Hoger, A. Shear stress regulates the endothelial Kir2.1 ion channel. Proceedings of the National Academy of Sciences of the United States of America. 99 (11), 7780-7785 (2002).
- Moccia, F., Villa, A., Tanzi, F. Flow-activated Na(+)and K(+)Current in cardiac microvascular endothelial cells. Journal of Molecular and Cellular Cardiology. 32 (8), 1589-1593 (2000).
- Crane, G. J., Walker, S. D., Dora, K. A., Garland, C. J. Evidence for a differential cellular distribution of inward rectifier K channels in the rat isolated mesenteric artery. Journal of Vascular Research. 40 (2), 159-168 (2003).
- Hannah, R. M., Dunn, K. M., Bonev, A. D., Nelson, M. T. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. Journal of Cereberal Blood Flow and Metabolism. 31 (5), 1175-1186 (2011).
- Lane, W. O., et al. Parallel-plate flow chamber and continuous flow circuit to evaluate endothelial progenitor cells under laminar flow shear stress. Journal of Visualized Experiments. (59), e3349 (2012).
- Lieu, D. K., Pappone, P. A., Barakat, A. I. Differential membrane potential and ion current responses to different types of shear stress in vascular endothelial cells. American Journal of Physiology-Cell Physiology. 286 (6), C1367-C1375 (2004).
- Le Master, E., et al. Proatherogenic Flow Increases Endothelial Stiffness via Enhanced CD36-Mediated Uptake of Oxidized Low-Density Lipoproteins. Arteriosclerosis, Thrombosis, and Vascular Biology. 38 (1), 64-75 (2018).
- Kim, J. G., et al. Measurement of Ion Concentration in the Unstirred Boundary Layer with Open Patch-Clamp Pipette: Implications in Control of Ion Channels by Fluid Flow. Journal of Visualized Experiments. (143), e58228 (2019).
- Kim, J. G., et al. Fluid flow facilitates inward rectifier K(+) current by convectively restoring [K(+)] at the cell membrane surface. Scientific Reports. 6, 39585 (2016).
- Malek, A. M., Alper, S. L., Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. Journal of the American Medical Association. 282 (21), 2035-2042 (1999).
- Jacobs, E. R., et al. Shear activated channels in cell-attached patches of cultured bovine aortic endothelial cells. Pflugers Archiv. European Journal of Physiology. 431 (1), 129-131 (1995).
- Barakat, A. I., Leaver, E. V., Pappone, P. A., Davies, P. F. A flow-activated chloride-selective membrane current in vascular endothelial cells. Circulation Research. 85 (9), 820-828 (1999).
- Fitzgerald, T. N., et al. Laminar shear stress stimulates vascular smooth muscle cell apoptosis via the Akt pathway. Journal of Cellular Physiology. 216 (2), 389-395 (2008).
- Ueba, H., Kawakami, M., Yaginuma, T. Shear stress as an inhibitor of vascular smooth muscle cell proliferation. Role of transforming growth factor-beta 1 and tissue-type plasminogen activator. Arteriosclerosis, Thrombosis, and Vascular Biology. 17 (8), 1512-1516 (1997).

