Providing Meaningful Environmental Enrichment and Measuring Saliva Cortisol in Pigs Housed on Slatted Flooring
Summary
This protocol demonstrates how to provide practical meaningful environmental enrichment for pigs which are housed on slatted flooring during the different stages of their lives, and how to collect saliva samples in a non-invasive manner for the measurement of cortisol concentrations, as a biomarker for acute stress.
Abstract
As pork is the most consumed meat worldwide, the welfare of animals in the swine industry has increasingly become a major public concern, which imposes a substantial pressure coming from customers, legislators and other stakeholders, to make management changes to improve the well-being of these animals. Several studies have demonstrated that providing environmental enrichment to pigs allows them to express their natural behavior, such as rooting and exploring, as well as nesting prior to farrowing, and is associated with reduced stress and improved production and welfare. However, many considerations should be taken into account when providing environmental enrichment, such as the type of floor, drainage and sewage systems, the pigs' stage in life, the material, as well as its hanging method, height and location within the pen. The objectives of this paper are (1) to give methodologic information on how to provide a relatively simple and practical meaningful environmental enrichment for pigs which are housed on slatted floors during the different stages of their life, and (2) to demonstrate how to collect saliva samples for the measurement of cortisol concentrations, as a biomarker for acute stress. Protocols include information regarding the use of jute, cotton ropes, straw in racks, as well as chewable silicone sticks devices as environmental enrichment in pens of farrowing and lactation, weaners and finishers. In addition, the use of cotton rope for a non-invasive saliva samples collection for cortisol concentrations analysis is detailed. The protocols provided are relevant for professionals aiming to improve and monitor animal welfare, in both research and industrial swine farming.
Introduction
Pork is the most consumed meat worldwide, with over 1.3 billion pigs being raised and slaughtered annually1,2. In recent years, the welfare of animals in the swine industry has increasingly become a major public concern, which imposes substantial pressure, coming from customers, legislators and other stakeholders, to make management changes to improve the well-being of these animals. Several studies demonstrated that providing environmental enrichment to pigs is associated with reduced stress and improved production and welfare, as it allows the pigs to express their natural behavior, such as rooting and exploring, as well as nesting prior to farrowing3,4,5,6,7.
Pigs are considered to be intelligent animals, and have a highly inquisitive nature; thus, if a suitable environment is not provided, pigs will likely demonstrate stereotypic behavior and direct manipulative social behavior towards pen mates, which may lead to tail biting, as well as other injuries and stress8,9. Therefore, providing a meaningful environmental enrichment is advised by professionals, and in some countries even imposed by regulations and legislations, such as the European Union Council Directive 2008/120/EC5.
Providing meaningful environmental enrichment can be challenging; it should fulfill the natural behavioral needs of the pigs in each stage of their lives, as well as take into consideration practical and technical limitations. Prior to farrowing, providing nesting material is associated with shorter farrowing duration as well as higher survival rate of the newborn piglets during farrowing and throughout the lactation period. Moreover, in free pens with environmental enrichment, maternal behavior is improved, as well as the cognitive performance and weight gain of the piglets3,10,11,12,13. After weaning, grouping pigs (weaners, growing or finishers) from different litters or pens can be stressful and cause aggressiveness towards other pen mates, which may lead to injuries14,15. Therefore, when mixing unfamiliar pigs, providing meaningful environmental enrichment can potentially reduce the occurrence of undesired behavior resulting from frustration and aggressiveness or redirection of rooting behavior.
According to the European Union Council Directive 2008/120/EC (established in 2001/93/EC), it is required that pigs have permanent access to a sufficient quantity of material, to enable proper investigation and manipulation activities, such as straw, hay, wood, sawdust, mushroom compost, peat or a mixture of these5,6. However, the use of these materials may be unmanageable on many farms, as it may cause blockage of the drainage and sewage systems, particularly on farms with slatted flooring. Still, according to the EU Council Directive, farmers can provide alternative enrichment materials, as long as it allows the pigs to perform proper investigation and manipulation activities.
Lack of environmental enrichment may potentially lead to frustration and stress3, which may activate the hypothalamic pituitary adrenal (HPA) axis. In pigs, as well as in humans and other animals, stress typically leads to the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland. ACTH binds to its receptors on the adrenal cortex and stimulates adrenal release of the glucocorticoid cortisol, which is considered as a major biomarker of stress and can be measured in blood, urine, saliva and hair. Saliva cortisol is a marker for acute stress, since it reflects its biologically active concentration in the blood4. It has the advantage of a non-invasive sample collection, without handling of the animals, by means of cotton ropes provided as environmental enrichment16,17. Hair cortisol is being used as a marker for chronic stress, since cortisol accumulates in the hair over time and can be extracted and measured18,19; however, it requires animal handling, the number of repeated sampling is limited by the hair growth rate, and the analysis is more cumbersome as it require long extraction process. Still, both saliva and hair cortisol may provide complementary information to asses animal welfare.
The objectives of this paper are (1) to give methodologic information on how to provide relatively simple and practical meaningful environmental enrichment for pigs which are housed on slatted floor during different stages of their lives (farrowing and lactation, weaners to finishers), and (2) to demonstrate how to non-invasively collect saliva samples for the measurement of cortisol concentrations, as a biomarker for acute stress.
Protocol
The study protocol was ethically approved by the Hebrew University's Institutional Animal Care and Use Committee (MD-16-14754-2). The study was conducted during 2017, at Lahav Animal Research Institute and the Hebrew University of Jerusalem, Israel.
1. Environmental enrichment protocol during farrowing and lactation period
NOTE: In this study, sows were housed in farrowing/lactation pens, under restraint for a limited time, from 3-5 days prior to the expected farrowing date and up to 10 days post-farrowing; thereafter, confinement bars were removed, to allow free movement and interaction between the sow and its piglets (illustrated in Figure 1A). However, the following protocol is also suitable for conventional farrowing crates, in which the sow is restrained throughout the entire lactation period.
- Hang the enrichment materials (jute and cotton ropes) in the farrowing pen before the sow is moved into it, in order to allow the sows' nesting-like behavior prior to farrowing.
- For each farrowing pen/crate, prepare two pieces of jute (20 cm wide, one-meter-long), and two pieces of 100% natural, uncolored cotton ropes (2-3 cm in diameter), approximately one-meter-long each.
- Tie the jute and cotton ropes (double overhand knot) to the pen/crates' bars, as illustrated in Figure 1, from its middle, allowing the two free ends of each piece to be hung towards the floor. The height of the jute and cotton ropes knots should be just lower than the sows' shoulder, while its free ends are just above the floor (Figure 1B-C).
- Sows are typically interested in the jute and cotton ropes; they often pull and chew it. Therefore, replace or refill materials when dirty or finished.
NOTE: In this study, materials were inspected every 4-5 days, and replaced or refilled when needed. - After piglets are born, hang the jute and cotton ropes so that they are reachable for both the sow and its piglets, in a safe place. Typically, when the materials are reachable for both, piglets imitate their dams from a very early age.
NOTE: As an addition, shredded paper can also be provided as environmental enrichment for the farrowing sows and piglets, on slatted floor. However, the compatibility of the drainage and sewage systems, should be critically examined on the specific farm.
2. Environmental enrichment protocol for weaners (when straw cannot be provided)
NOTE: In this study, after weaning, each two litters were grouped into one pen of weaners. Optimally, groups should remain static until slaughter, without mixing or introducing new pen mates into the pen, to avoid hierarchy-related struggles and injuries. Space allowance in the pen was according to European Council Directive 2008/120/EC and the Israeli legislation, with approximately 20 pigs per group, and 0.3 m2 of unobstructed floor area available per pig. For technical reasons, straw could not be provided for weaners, not even in racks. When straw can be provided, use the finishers' protocol from weaning to slaughter, as detailed in protocol section 3.
- For each pen, prepare one-meter-long pieces of 100% natural, uncolored cotton ropes, 2-3 cm in diameter; at least one piece for every 10 pigs. Tie the cotton ropes to a chain hung from the ceiling or to a hanging pole (to distance it from the wall), as demonstrated in Figure 2B.
- Position the ropes preferably at the center of the "active" area of the pen, close to the feeder and drinkers, and far from the pens' wall (as marked in Figure 2A) to allow 360˚ access, which is expected to prevent aggressive behavior due to competition.
- Hang the ropes at a height which allows the pigs to reach it with their mouths easily. Typically, the knot should be lower than the pigs' shoulder, while the free ends of the ropes should be just above the floor, without touching it (Figure 2).
- Replace or refill ropes when dirty or finished.
NOTE: In this study materials were inspected every 4-5 days, and replaced or refilled when needed. - In addition to the cotton ropes, provide chewable silicone sticks or similar chewable devices (Figure 2). The device used (see the Table of Materials) contains four chewable sticks which allows at least four pigs to interact with the device simultaneously. Provide one device for every 10 to 15 pigs.
- Hang the chewable device with a chain from the ceiling (included in the kit) to allow the device swinging movement; this increases the activity and challenge to the pigs, and also allows more pigs to participate at once.
- Position the device at the center of the active area of the pen, far from the pen's walls to allow 360˚ access, which is expected to prevent aggressive behavior due to competition. The end of the silicone sticks should be at a height of 10 to 20 cm above the floor.
- Change the silicone sticks when finished or if dirty.
NOTE: In this study silicone sticks required to be changed only when moved from the weaners' pens to the finishers' pens.
3. Environmental enrichment protocol for finishers
NOTE: When straw can be provided during the whole period, start using this protocol from weaning until slaughter, instead of in the finishers' pens only.
- Provide each pen with straw placed in a rack, in a hanged net, or in a tower, as illustrated in Figure 2C. When provided in such devices, only small amounts of the straw are available for the pigs at a time, and most of it is being chewed. Therefore, this enrichment can be used on slatted floors, when straw cannot be provided as bedding.
NOTE: In this study, straw was provided in commercial racks (Figure 2C). - Hang the straw racks at a height which is reachable for all pigs, usually just below the shoulder level. Attach them to the wall with the easiest access for as many pigs as possible, preferably within the "active area". Make sure the racks are properly and safely secured to the wall to avoid device breakage and injuries.
- Make sure racks are completely filled with straw at the beginning; an addition small amount of fresh straw can be added every 1-3 days. Alternatively refill when the rack is empty, typically every 7-10 days.
NOTE: Long straw is better than chopped straw to reduce aggressiveness in the group and to meet pigs behavioral needs for rooting and exploring20. - When applicable, consider placing a collecting tray or a mat underneath the rack, when the floor is slatted, to avoid straw from being scattered and blocking the drainage and sewage systems (Figure 2C). Since straw is provided for pigs mainly to fulfill their rooting and exploratory behavior, a mat can allow the presence of some free straw to be explored at the ground level.
- In addition to the straw, provide chewable devices, as detailed in section 2.
- When cotton ropes are used for saliva collection, as detailed in the next section, leave the ropes in the pens after saliva collection, as an additional enrichment. Other cotton ropes can also be provided, as detailed in section 2.
4. Saliva samples collection for cortisol concentrations analysis
NOTE: This section provides information how to non-invasively collect saliva for cortisol concentration analysis, as a biomarker for acute stress; however, saliva samples can also be used for analysis of other biomarkers and even for screening of potential pathogens. In addition, hair cortisol should be measured when applicable.
- Plan saliva sampling for the same time of the day, to avoid bias, as cortisol secretion changes along the day due to physiologic circadian rhythm, or due to feeding21. Sampling is typically easier before feeding, preferably before the second feeding of the day.
- Use uncolored, 100% cotton rope (2-3 cm in diameter). One rope is typically needed per 12-15 pigs.
NOTE: In this study, commercial, ready to use kits were used for collection of saliva samples. - Tie the rope close to the center of the pen, far from the pens' wall. Make sure that the cord which is used for hanging (between the cotton rope and the bar) is not reachable for chewing by the pigs, and that the free ends of the cotton ropes do not touch the floor, for hygiene reasons (Figure 3A).
- Unravel the free end of the rope, to increase the available chewable surface (Figure 3B).
- Leave the rope hanging for 15-30 min. Typically, pigs are interested in newly provided ropes and would chew it during this time. Typically, the vast majority of the pigs will approach the rope.
- To extract the saliva from the rope, put the free part of the rope into a sterile plastic bag (as illustrated in Figure 3D) and squeeze the rope by applying pressure from the outside of the bag, from top to bottom, until enough liquid (suggested at least 2 mL) accumulates at the bottom of the bag (Figure 3E), and then remove the rope from the bag.
NOTE: In this study a 30 cm x 40 cm bag was included in the kit. If possible, this step should be performed in the pen without cutting the hanging cord. In this way, the saliva extraction is usually easier, and the ropes can be left in the pen as an enrichment. If there is not enough saliva, a wringer can be used (was not required in this study). - To transfer the saliva from the bag into a sterile tube, pull the rope out and carefully cut the bottom corner of the bag above a 50 mL sterile plastic tube (although only 400 µL is usually required for each analysis in duplicates, at least 2 ml are recommended for collection; Figure 3F). Seal the tube, and mark it with a permanent marker or a pre-prepared sticker (animals/pen, date, time, farm, etc.).
- Store saliva samples in an insulated container with ice packs until delivered to the lab.
- In the lab, centrifuge saliva samples until dirt can be separated (for 8 min at 3000 x g). The clean saliva should be transferred with a clean disposable Pasteur pipette into pre-marked sterile tubes of smaller volume (e.g., 2 mL screw cap freezer tubes) for convenience. Samples can be analyzed immediately, or can be kept frozen at a temperature of -20 °C or below until analysis.
- Prior to cortisol concentration analysis, the relevant samples identity should be confirmed, and samples should be thawed at room temperature if frozen. Gently mix each sample, and then centrifuge it again before the analysis to remove possible leftover dirt that may interfere with the assay (8 min at 3000 x g).
- Perform cortisol concentrations analysis with a validated assay, according to the specific kit protocol.
NOTE: In this study, saliva cortisol samples were measured in duplicates by enzyme-linked immunosorbent assay according to the manufacturer guidelines. For pigs that were previously provided with ropes as enrichments, collecting saliva would be very easy as pigs show high interest in the ropes. However, for pigs that are unfamiliar with the ropes, it might take time for training; thus, collecting adequate saliva may not be possible from the first time. If needed, ropes can be soaked in apple juice for training (obviously, in such case, saliva cannot be used for analysis). If saliva samples are needed from individually housed pigs, the collection may be more challenging than from a group, and training sessions with apple juice-soaked ropes are typically required. Chewing of collection ropes may also be encouraged by sampling individuals one next to each other due to mimicking.
Representative Results
In the current study, 16 litters (170 piglets) were allocated randomly into two treatment groups; in one group, environmental enrichment was provided to eight litters ("Enriched Group", as described in protocol sections 1-3), while it was not provided to the other eight litters ("Non-enriched Group"). After weaning, each two litters were grouped into one group of about 20 pigs. Saliva samples were collected and analyzed for cortisol concentrations every two weeks in each pen, as described in protocol section 4.
In this study, when provided properly, sows, piglets and pigs showed interest in all enrichment devices. Sows used the jute and cotton ropes prior to farrowing, when provided. During the lactation period, when jute and cotton ropes were reachable for both the dam and her piglets, piglets imitated their dams from the age of 2-3 days old; however, when materials were not reachable for the sow, piglets started using the material later, typically after more than one week of age. In lactation and weaners' pens, the jute and cotton ropes were usually replaced or refilled every 4-5 days, while the chewable silicone sticks were replaced only once, when pigs were moved from weaners' pen to finishers' pen, at the age of 70 days. In the finishers' pens, no drainage and sewage blockages were recorded.
The odds to die during the whole period until slaughter was significantly higher among piglets/pigs in the non-enriched group as compared to the enriched group (OR = 3.38, 95% CI = 1.05-10.83; P = 0.004). Saliva cortisol samples were successfully collected during the study every two weeks, just before the second feeding of the day. The pigs in the enriched group showed high interest in the saliva collecting cotton ropes from the beginning; however, in the non-enriched group saliva volumes in the initial collection were typically lower (2-10 mL), but from the second or third collection sessions it was similar between the groups. Typically, a volume larger than 30-40 mL was easily extracted from each rope. Samples were stored frozen (-80 °C) until analysis. Cortisol concentrations, as measured by ELISA, were overall significantly lower in the enriched pigs, as compared to the non-enriched pigs (P = 0.0044; Figure 4).
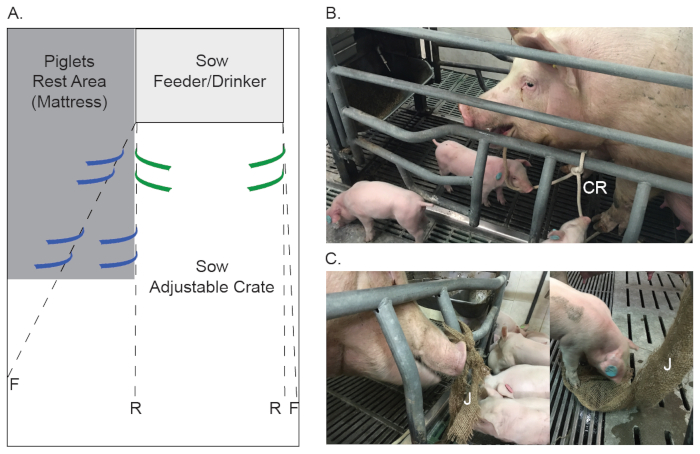
Figure 1: Environmental enrichment during farrowing and lactation period. (A) Designed farrowing pen illustration which demonstrates two stages of the confinement bars, either when the sow is restrained (stage R) or after confinement bars are removed (stage F) to allow free movement and interaction between the sow and her piglets. Suggested locations for the jute and cotton ropes before and after farrowing are marked in green and blue, respectively. (B) Images of a sow and her piglets interact with cotton ropes (CR) and jute (J) provided as environmental enrichment in a farrowing/lactation pen. Please click here to view a larger version of this figure.

Figure 2: Environmental enrichment for weaners and finishers. (A) A schematic illustration of a pen demonstrating the suggested locations for hanging the environmental materials in the "active area" (blue X). (B–C) Images of pigs enriched as suggested. (B) In the weaners' pen, cotton ropes (CR) provided as environmental enrichment or for saliva samples collection hung from the ceiling or from a pole (P) to distance it from the pen's wall, chewable silicone sticks device (BR) hung by a chain from the ceiling (HC). (C) In the finishers' pen, straw is provided in a rack (SR), a straw tower (ST) or a straw basket (SB); a mat (M) can cover the slatted floor underneath each of these straw devices. Please click here to view a larger version of this figure.
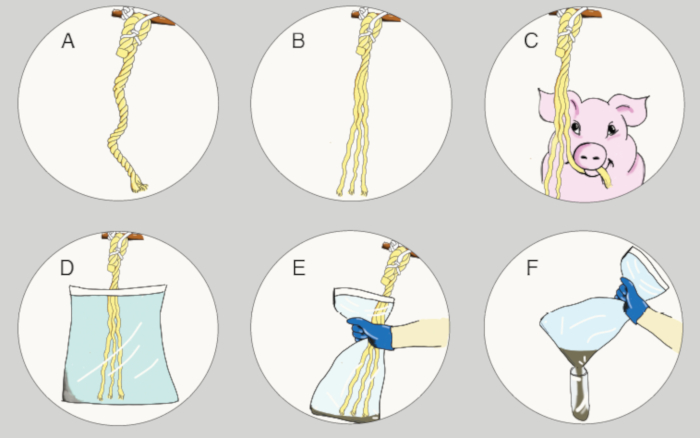
Figure 3: Saliva sampling process using cotton ropes. (A–F) The six main stages for the saliva sampling are illustrated: hanging the rope, unraveling the free end of the rope, allowing the pigs to chew it, saliva extraction from the rope into a plastic bag and then transferring it to a plastic tube. Please click here to view a larger version of this figure.
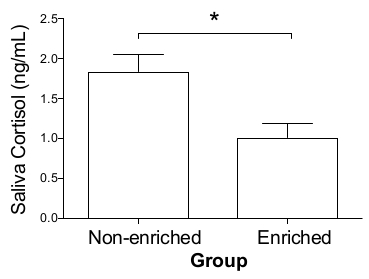
Figure 4: Saliva cortisol concentrations in enriched and non-enriched pigs. Saliva samples were collected every two weeks using cotton ropes, at the pens' level, from weaning to slaughter, from enriched and non-enriched pigs; cortisol concentrations were measured by ELISA. For this analysis, all samples were clustered together per group, without taking the sampling date into consideration. Cortisol concentrations were significantly lower in the Enriched Group, as compared to the Non-enriched Group (Mann Whitney test; P = 0.0044). Results are presented as mean ± SEM. Please click here to view a larger version of this figure.
Discussion
Herein, we describe protocols detailing how to provide simple and practical environmental enrichment for pigs which are housed on slatted floor during different stages of their life, and how to non-invasively collect saliva samples for the measurement of cortisol concentrations, as a biomarker for acute stress. Straw as a bedding is considered as one of the most suitable environmental enrichment for pigs, but may be impractical on slatted floors. However, relatively inexpensive alternatives such as jute, cotton ropes, straw in racks, and chewable devices can practically and safely be applied.
The range of saliva cortisol concentrations in this study are comparable to previous publications17,22,23. The significant differences in saliva cortisol concentrations between enriched- and non-enriched animals, is in accordance with the hypothesis that environmental enrichment may reduce acute stress in pigs at a point sample, as has been shown in previous studies, at least up to a certain age, as long as the circadian rhythm is not damaged by the prolonged chronic stress24,25. As noted, cortisol can be measured in blood, urine, and saliva as markers for acute stress, and in hair as a marker for chronic stress. A protocol for measuring hair cortisol was previously described by Meyers et al. and may be relevant when assessing animal welfare19. However, when looking for markers for acute stress, one should consider that any sampling, by itself, may increase cortisol release due to animal handling or pain caused during the procedure16,26. Hence, non-invasive saliva sampling, as suggested in the current study, is a relevant method, which can be used for cortisol analysis, as well as for the analysis of other biomarkers and even for screening of potential pathogens27.
The main challenges in collecting saliva according to this protocol may be when collecting it from pigs that are unfamiliar with the ropes, as they may avoid chewing the ropes, and therefore, training may be needed. Training can be performed by using ropes which are soaked in apple juice (in such cases, those saliva samples cannot be used for analyses). If saliva samples are needed from individually housed pigs, the collection may be even more challenging than from a group, and training sessions with apple juice-soaked ropes are typically required. Chewing the ropes may also be encouraged by sampling individuals next to each other due to mimicking. For the training of young piglets, ropes should be hung to be reachable for their mother to encourage learning and early chewing. Another possible obstacle when using cotton ropes for enrichment or for saliva collection is that it can be caught in ear tags of certain brands. If it occurs, either the ear tags or the enrichment method has to be replaced.
Using cotton ropes allows measuring saliva cortisol concentration at the group level, which provides applicable screening for this hormone. A possible disadvantage of this collection method is that it may not be a representative measurement of all individuals in the group, as it is affected by the amount of saliva absorbed in the rope from each individual pig. In order to minimize this disadvantage, it is advised to provide adequate number of ropes, to allow sufficient collection time, as well as to perform several repeated collections. As compared to the existing method of individually sampling, the collection itself is not stressful for the pigs, since it is given as an environmental enrichment, without handling of the pigs.
In summary, pigs can be environmentally enriched at all life stages, even on slatted floor, using jute, cotton ropes, straw in racks and chewable devices. The type of enrichment, the hanging method, as well as the height and location within the pen, are important for their effectiveness. Regarding saliva collection by cotton topes, the timing and the hanging recommendations are crucial for successful samplings. These protocols are relevant for professionals aiming to improve and monitor animal welfare, in both research and industrial swine farming.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Lahav CRO for conducting the research in their farm and to the farm's team for providing valuable technical help throughout the study. The study was funded by the Israel Chief Scientist, Ministry of Agriculture and Rural Development.
Materials
| Bite-Rite | Ikadan System USA Inc. | Consider ordering additional replaceable silicone sticks | |
| ELISA; Saliva Cortisol Kit | DRG International Inc. NJ, USA | Slv2930 | |
| HALM 60/80 CM | W. Domino A/S, DK | 49084/ 85 | |
| TEGO Swine Oral Fluids Kit | ITL BioMedical, USA | A100930 | Including everything needed for the saliva sampling protcol |
References
- McGlone, J. J. The Future of Pork Production in the World: Towards Sustainable, Welfare-Positive Systems. Animals (Basel. 3, 401-415 (2013).
- Kuberka, L., Cozzens, T., Mezoughem, C. Livestock and Poultry: World Markets and Trade. Available at: https://apps.fas.usda.gov/psdonline/circulars/livestock_poultry.pdf [accessed). USDA-FAS, Office of Global Analysis, Global Commodity Analysis Division. , (2018).
- Oostindjer, M., et al. Effects of environmental enrichment and loose housing of lactating sows on piglet performance before and after weaning. Journal of Animal Science. 88, 3554-3562 (2010).
- Munsterhjelm, C., et al. Environmental enrichment in early life affects cortisol patterns in growing pigs. Animal. 4, 242-249 (2010).
- Bracke, M. B. M., Koene, P. Expert opinion on metal chains and other indestructible objects as proper enrichment for intensively-farmed pigs. PLoS One. 14, (2019).
- Marcet Rius, ., M, , et al. Providing straw to allow exploratory behaviour in a pig experimental system does not modify putative indicators of positive welfare: peripheral oxytocin and serotonin. Animal. 12, 2138-2146 (2018).
- Giuliotti, L., Benvenuti, M. N., Giannarelli, A., Mariti, C., Gazzano, A. Effect of Different Environment Enrichments on Behaviour and Social Interactions in Growing Pigs. Animals. 9, (2019).
- Stafford, K. J. Tail biting: an important and undesirable behaviour of growing pigs. Veterinary journal. 186, 131-132 (2010).
- Sutherland, M. A. Welfare implications of invasive piglet husbandry procedures, methods of alleviation and alternatives: a review. New Zealand Veterinary Journal. 63, 52-57 (2015).
- Herskin, M. S., Jensen, K. H., Thodberg, K. Influence of environmental stimuli on nursing and suckling behaviour in domestic sows and piglets. Animal Science. 68, 27-34 (1999).
- Lawrence, A. B., et al. The Effect of Environment on Behavior, Plasma-Cortisol and Prolactin in Parturient Sows. Applied Animal Behaviour Science. 39 (94), 313-330 (1994).
- Baxter, E. M., Lawrence, A. B., Edwards, S. A. Alternative farrowing accommodation: welfare and economic aspects of existing farrowing and lactation systems for pigs. Animal. 6, 96-117 (2012).
- Oliviero, C., Heinonen, M., Valros, A., Halli, O., Peltoniemi, O. A. Effect of the environment on the physiology of the sow during late pregnancy, farrowing and early lactation. Animal Reproduction Science. , 365-377 (2008).
- Thomsen, R., Edwards, S. A., Rousing, T., Labouriau, R., Sorensen, J. T. Influence of social mixing and group size on skin lesions and mounting in organic entire male pigs. Animal. 10, 1225-1233 (2016).
- Rydhmer, L., Hansson, M., Lundstrom, K., Brunius, C., Andersson, K. Welfare of entire male pigs is improved by socialising piglets and keeping intact groups until slaughter. Animal. 7, 1532-1541 (2013).
- Heimburge, S., Kanitz, E., Otten, W. The use of hair cortisol for the assessment of stress in animals. General and Comparative Endocrinology. , 10-17 (2019).
- Morgan, L., et al. Effects of group housing on reproductive performance, lameness, injuries and saliva cortisol in gestating sows. Preventive Veterinary Medicine. , 10-17 (2018).
- Meyer, J. S., Novak, M. A. Minireview: Hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 153, 4120-4127 (2012).
- Meyer, J., Novak, M., Hamel, A., Rosenberg, K. Extraction and analysis of cortisol from human and monkey hair. Journal of Visualized Experiments : JoVE. , (2014).
- Lahrmann, H. P., Oxholm, L. C., Steinmetz, H., Nielsen, M. B., D’Eath, R. B. The effect of long or chopped straw on pig behaviour. Animal. 9, 862-870 (2015).
- Boumans, I., de Boer, I. J. M., Hofstede, G. J., Fleur, l. a., E, S., Bokkers, E. A. M. The importance of hormonal circadian rhythms in daily feeding patterns: An illustration with simulated pigs. Hormones and Behavior. 93, 82-93 (2017).
- Schonreiter, S., et al. Salivary cortisol as a stress parameter in piglets. Tierarztliche Praxis Ausgabe G: Grosstiere – Nutztiere. 27, 175-179 (1999).
- Cook, N. J., Hayne, S. M., Rioja-Lang, F. C., Schaefer, A. L., Gonyou, H. W. The collection of multiple saliva samples from pigs and the effect on adrenocortical activity. Canadian Journal of Animal Science. 93, 329-333 (2013).
- Reimert, I., Rodenburg, T. B., Ursinus, W. W., Kemp, B., Bolhuis, J. E. Responses to novel situations of female and castrated male pigs with divergent social breeding values and different backtest classifications in barren and straw-enriched housing. Applied Animal Behaviour Science. , 24-35 (2014).
- Avan de Weerd, H., Day, J. E. L. A review of environmental enrichment for pigs housed in intensive housing systems. Applied Animal Behaviour Science. 116, 1-20 (2009).
- Wright, K. D., Hickman, R., Laudenslager, M. L. Hair Cortisol Analysis: A Promising Biomarker of HPA Activation in Older Adults. Gerontologist. 55 Suppl 1, S140-S145 (2015).
- Decorte, I., et al. Detection of total and PRRSV-specific antibodies in oral fluids collected with different rope types from PRRSV-vaccinated and experimentally infected pigs. BMC Veterinary Research. 10, 134 (2014).

