Imaging Features of Systemic Sclerosis-Associated Interstitial Lung Disease
Summary
Here, we present practical recommendations for performing thoracic high-resolution computed tomography for diagnosing and assessing systemic sclerosis-related interstitial lung disease.
Abstract
Early diagnosis of systemic sclerosis-related interstitial lung disease (SSc-ILD) is important to enable treatment to be administered with minimal delay. However, diagnosing SSc-ILD is challenging because key symptoms are non-specific. High-resolution computed tomography (HRCT) of the chest is recognized as a sensitive imaging method for diagnosing and assessing SSc-ILD. Exposure of patients to ionizing radiation may be considered as a limitation, although methodological steps may be taken to moderate this. We present practical recommendations for performing HRCT scans and interpreting the results. Key features of SSc-ILD on HRCT include a non-specific interstitial pneumonia (NSIP) pattern with peripheral ground-glass opacities and extensive traction bronchiectasis. Despite similarities between SSc-ILD and idiopathic pulmonary fibrosis (IPF), HRCT can be used to differentiate between these conditions: in SSc-ILD compared with IPF, there is a greater proportion of ground-glass opacity and fibrosis is less coarse. A dilated, air-filled esophagus with diameter >10 mm, suggestive of esophageal dysmotility is commonly seen in SSc-ILD. Pulmonary artery size greater than the adjacent ascending aorta suggests coexistent pulmonary hypertension. Nodules must be monitored due to the increased risk of lung cancer. A large extent of disease on HRCT (≥20%) or a high fibrosis score suggests an increased risk of mortality. HRCT is central to diagnosing SSc-ILD, and serial assessments can be helpful in monitoring disease progression or treatment response.
Introduction
Systemic sclerosis (SSc) is a complex, heterogeneous, autoimmune disease. It may be manifested as vasculopathy, Raynaud’s phenomenon and fibrosis of the skin and internal organs1. SSc is classified into subtypes as follows: limited cutaneous, diffuse cutaneous, sine scleroderma (without skin involvement), and SSc overlap syndrome1.
SSc is not inherited in Mendelian fashion, but genetic factors appear to influence susceptibility to the disease. Incidence rates differ between ethnic groups and are increased among individuals with a family history of the disease2,3. Environmental risk factors also appear to exist, with high exposure to silica or organic solvents appearing to increase the occurrence of SSc4. The global prevalence of SSc is around 1 in 10,0001. More females than males are affected by SSc, with reported female:male ratios ranging between 3:1 and 8:1, and the age group with the highest incidence of the disease is 45–54 years5.
The lung is the second most commonly affected visceral organ in patients with SSc6. There are two main pulmonary manifestations of SSc: interstitial lung disease (ILD), and pulmonary hypertension7. ILD is usually fibrotic; it occurs in approximately 80% of patients with SSc and is more common in diffuse cutaneous scleroderma than in the limited form of the disease1,8. Pulmonary hypertension may manifest as isolated pulmonary arterial hypertension (PAH, which has a prevalence of 13–35% in SSc) or pulmonary hypertension resulting from left ventricular involvement/diastolic dysfunction or ILD/hypoxemia7. Antibody profiles differ between patients with SSc-ILD and those with SSc-PAH. For example, the presence of anti-Scl-70 antibodies is associated with SSc-ILD8, while anticentromere antibodies are more common in SSc patients with PAH than in those without PAH9.
The symptoms of SSc-ILD include dyspnea, coughing, chest pain, and exercise limitation. ILD is a major contributor to morbidity in SSc10,11,12. As a consequence, annual all-cause healthcare costs have been reported to be higher in patients with SSc-ILD than in those with SSc and no ILD: $31,285–55,446 versus $18,513–23,268, respectively13.
SSc-ILD is the leading cause of mortality in patients with SSc, accounting for 30–35% of deaths in this group10,14. Median survival among patients with SSc-ILD has been reported to be 5–8 years10,15; by comparison, approximately 76% of the overall population with SSc survive for more than 10 years from disease onset16. Significant predictors of mortality in SSc-ILD include age, forced vital capacity (FVC), baseline diffusing capacity of the lung for carbon monoxide (DLCO), extent of disease on high-resolution computed tomography (HRCT), presence of pulmonary hypertension and levels of Krebs von den Lungen 6 (KL-6) antigen17,18.
Early diagnosis is important to enable treatment to be administered with minimal delay and, in patients with a progressive phenotype, disease progression may potentially be slowed. However, diagnosing SSc-ILD is challenging because non-specific symptoms of cough, dyspnea, and fatigue can be mistaken for other aspects of SSc, such as cardiac disease and musculoskeletal involvement. Evaluations for diagnosing ILDs include: clinical presentation, history, smoking status, lung function, imaging, and in some cases, lung biopsy. Affirmation of SSc-ILD diagnosis requires several investigations, which are often used in combination19. The most frequently used assessments include pulmonary function tests and HRCT20,21,22,23. Other imaging methods, such as chest radiography and radiation-sparing imaging (e.g., magnetic resonance imaging [MRI], lung ultrasound) may also be employed22. Pulmonary function tests are used to assess the severity of the ILD and monitor its course. However, the use of pulmonary function tests alone is of limited use for diagnosing SSc-ILD24,25. HRCT of the chest is viewed as the most sensitive non-invasive means of facilitating differential diagnosis of SSc-ILD19. Baseline HRCT results, as well as changes over time, can be used to predict the future course of lung disease and potential response to therapy26.
Exposure to radiation with HRCT is sometimes considered as a limiting factor for regular screening27,28; limiting the number of slices is a potential method for reducing the radiation risk, and the dose may also be reduced by decreasing either the voltage or the current29,30,31. Alternatively, different assessment methods may be considered. For example, MRI appears to have some potential for evaluation and follow-up of ILD patients22. In one study using T2-weighted MRI images with respiratory synchronization, HRCT was performed in parallel as the ‘gold-standard’ assessment; 100% sensitivity and 60% specificity were reported with MRI for determining the presence of ILD32. Similar agreement between MRI and HRCT in the detection and categorization of ILD was reported in another study33. Despite the promising results, MRI is currently a research methodology and it is not yet ready for generalized clinical use.
Here, we provide a practical overview of the interpretation of imaging results, with a focus on HRCT, for diagnosing lung involvement in SSc, determining prognosis, and also exploring future developments that may improve imaging methods and interpretation of results. HRCT images from representative cases are included in the paper.
Protocol
1. HRCT scanning
- Perform volumetric HRCT acquisition scanning of the chest36. Contrast agents are not required36,37:
- Obtain the following acquisitions with parameters shown in Table 136,37.
- Acquire a supine inspiratory scan (volumetric) from the lung apices to the lung base.
- Acquire a supine expiratory scan (sequential with 10–20 mm gaps) from 2 cm below the lung apices to the lung base.
- Acquire a prone inspiratory optional (sequential with 10–20 mm gaps) from the carina to the lung base.
- Give breathing instructions to the patient before each acquisition36,37. For an inspiratory scan, say “Take in a deep breath….and let it out. Take in another deep breath….and let it out. Take in another deep breath, and hold your breath in. Keep holding your breath”37.
- Obtain inspiratory scans at full inspiration35,36.
- Use the thinnest collimation, shortest rotation time and highest pitch to ensure that motion-free images are obtained36. Suggested scanning parameters are detailed in Table 137.
- For optimal quality of volumetric scans, obtain thin section (<2 mm) images with high-spatial resolution reconstruction35,36.
- Review scans immediately after acquisition and repeat if either motion artifact is present or inadequate inspiration has occurred37.
2. Reporting
- Prepare an interpretive report.
- Share the report and HRCT images with the patient’s care team and add them to the patient’s medical records.
Representative Results
Diagnosis
Key features of SSc-ILD on HRCT commonly include a non-specific interstitial pneumonia (NSIP) pattern with peripheral ground-glass opacities and extensive traction bronchiectasis (Figure 1 and Figure 2). Ground-glass opacities have a broad etiology and are often non-specific40,41,42. Central predominance or peripheral distribution with subpleural sparing is highly suggestive of NSIP (Figure 3).
Typically, ILD patterns in HRCT images include reticulations with architectural distortion resulting in traction bronchiectasis/bronchiolectasis (consistent with a fibrotic form of NSIP). Indeed traction bronchiectasis and traction bronchiolectasis are often the predominant features of SSc-ILD (Figure 4)43. Additional findings may include honeycombing (Figure 5; more common in limited forms of SSc), interlobular septal thickening and intralobular lines, and micronodules40,44. Honeycombing refers to clustered cystic airspaces of typically consistent diameter (~3–10 mm) with thick, well defined walls31. Honeycombing and traction bronchiectasis are key features of usual interstitial pneumonia (UIP) on HRCT. Although this pattern is most commonly associated with idiopathic pulmonary fibrosis (IPF), the prototype fibrosing ILD with a progressive phenotype, it can sometimes be seen in patients with SSc-ILD10. Recently, several signs have been identified in patients with connective tissue disease-related ILD (including SSc-ILD) and the UIP pattern on HRCT, but not in those with IPF. These are the straight edge sign (i.e., isolation of fibrosis to the lung bases with sharp demarcation in the craniocaudal plane without substantial extension along the lateral margins of the lungs on coronal images), the honeycombing predominant (or exuberant) sign (>70% of fibrotic portions of the lung), and the anterior upper lobe sign (i.e., concentration of fibrosis within the anterior aspect of the upper lobes, with relative sparing of the other aspects of the upper lobes, and concomitant lower lobe involvement)45. The straight edge sign has also been associated with NSIP pathology46, which is the main CT pattern in SSc-ILD10.
Dilated air-filled esophagus is frequently observed in patients with SSc (Figure 6)47,48,49 and in patients with SSc-ILD47,48. While there is no accepted upper age limit where a dilated esophagus may no longer help to differentiate SSc-ILD and IPF, a dilated esophagus may be more difficult to interpret in patients over age 65 due to increasing incidence of esophageal motility disorders. Mediastinal lymphadenopathy (usually reactive), in which the short axis of the lymph node exceeds 10 mm, is also often observed in patients with SSc-ILD47,50. Pulmonary artery size greater than the adjacent ascending aorta suggests coexistent pulmonary hypertension (Figure 6), even in patients without fibrotic lung disease51,52,53. Areas of consolidation suggest superimposed infection, aspiration, organizing pneumonia, hemorrhage or malignancy. Nodules must be monitored due to the increased risk for lung cancer in SSc-ILD7; the most common primary cancer to arise in patients with SSc-ILD is adenocarcinoma7,54.
SSc-ILD shares a number of clinical, mechanistic, and pathological similarities with IPF15,55. However, some radiologic features allow the differentiation of these two ILDs15,45. In SSc-ILD, compared with IPF, there is a greater proportion of ground-glass opacity and fibrosis is less coarse. In cases of UIP in SSc, honeycombing may be observed in more than 70% of the fibrotic-lung tissue ─ the exuberant honeycombing sign56,57. In addition, the four-corners sign (also known as the anterior upper lobe sign) is significantly more common in SSc-ILD than in IPF; this is a pattern of inflammation and/or fibrosis focally or disproportionately involving the bilateral anterolateral upper lobes and posterosuperior lower lobes58.
Chest radiographs may initially detect ILD; however, they do not offer enough contrast resolution for reliable diagnosis. In chest radiographs from patients with SSc-ILD, the most frequent pattern is basal predominant reticulation59. Further features may include visible bronchiectasis, volume loss and honeycombing. As with HRCT, the presence of a dilated air-filled esophagus may be helpful in supporting the diagnosis of SSc-ILD47.
Prognosis
Several different imaging findings have been shown to be associated with prognosis in SSc-ILD. Mortality risk has been reported to be higher in patients with a disease extent of at least 20% on HRCT (10-year survival was 43% versus 67%, respectively, in patients with disease extent above versus below the 20% threshold)60. Similarly, a high fibrosis score on HRCT (based on the extent of reticulation and honeycombing) has been associated with increased mortality61. Large esophageal diameters are associated with increased ILD severity and decreased DLCO48. Lung density and pulmonary artery diameter may potentially be used to predict the risk of pulmonary hypertension62. Computerized, quantitative CT parameters could also be harnessed to identify patients’ risk of lung function decline or mortality. One study suggested that the extent of ILD, quantified from HRCT, could be used to predict the decline in FVC over 12 months63. In another study, quantitative chest CT parameters provided mortality risk results that were consistent with clinical prediction models64. Despite their apparent potential, imaging-based biomarkers are currently best considered at a population level as their clinical utility in individual patients has not been established.
Treatment response
Cyclophosphamide and mycophenolate mofetil provide modest benefit in patients with SSc-ILD. In the landmark Scleroderma Lung Study I, cyclophosphamide treatment led to slower progression of fibrosis compared with placebo65. More recently, the Scleroderma Lung Study II reported similar efficacy and improved tolerability with mycophenolate mofetil in comparison with cyclophosphamide66. Based upon positive SENSCIS® trial results67, nintedanib became the first US Food and Drug Administration-approved treatment to slow the rate of decline in lung function in patients with SSc-ILD. However, there remains a need for improved treatment options for patients with SSc-ILD. Therapies currently being investigated include monoclonal antibodies (e.g. rituximab, abituzumab), antifibrotic agents (e.g., pirfenidone), the direct thrombin inhibitor dabigatran, the proteasome inhibitor bortezomib, and hematopoietic stem cell transplantation19,68.
Serial HRCT scans showing disease progression in a patient with SSc-ILD
HRCT assessments performed at different timepoints may be used to investigate disease progression. Figure 7 shows two sets of axial and coronal chest HRCT images taken 10 years apart in a patient with SSc-ILD. The initial axial and coronal images (Figure 7A and B) from chest HRCT show basilar predominant ground-glass opacity and reticulation with mild traction bronchiectasis and subpleural sparing consistent with NSIP in this patient with SSc. The latter set of images (Figure 7C and D) taken 10 years later, show increased reticulation and traction bronchiolectasis at the lung bases with decrease in ground-glass opacity on axial and coronal (Figure 7C and D) images from chest CT consistent with mild worsening of pulmonary fibrosis. Serial HRCT scans can also be used to monitor treatment response69,70,71; this was demonstrated in the Scleroderma Lung Study II, in which computer-aided diagnosis scores based on HRCT scans were used to compare the efficacy of cyclophosphamide with mycophenolate mofetil in patients with SSc-ILD69.
| Phase | Detector collimation |
Voltage (kVp) |
Current (mAs) | Scan interval |
Pitch | Rotation | Tube current modulation |
| Supine inspiratory | Helical 1.2 mm | 120 (may be lowered) | 230 (may be lowered) | N/A | ~1.0 | 0.5 seconds or faster | On |
| Supine expiratory | Axial 2 x 1.0 mm | 120 | 150 | 20 mm | N/A | 1.0 seconds | On |
| Prone inspiratory | Axial 2 x 1.0 mm | 120 | 150 | 20 mm | N/A | 1.0 seconds | On |
Table 1: Computed tomography acquisition parameters37. kVp = kilovoltage peak; N/A = not applicable.
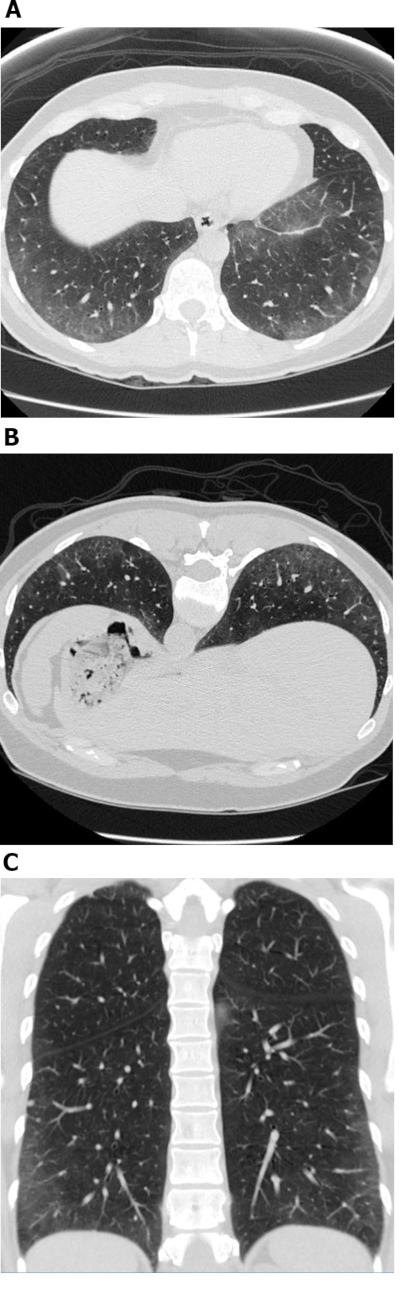
Figure 1: Systemic sclerosis with a cellular NSIP pattern of disease. Axial (A), prone (B) and coronal (C) high-resolution computed tomography images all show extensive peripheral and basal predominant ground-glass opacities; these are typical observations with NSIP. The lack of traction bronchiectasis is suggestive of a cellular NSIP pattern of disease. NSIP = non-specific interstitial pneumonia. Please click here to view a larger version of this figure.
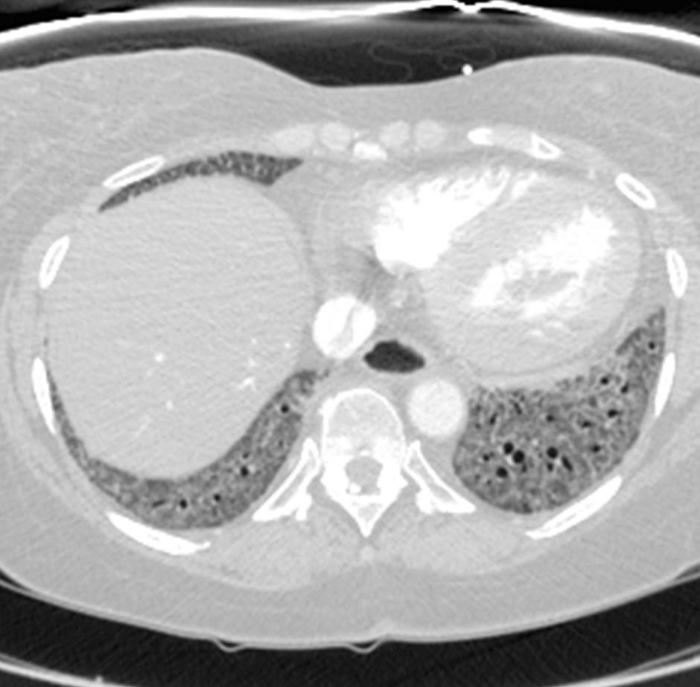
Figure 2: Systemic sclerosis with a fibrotic non-specific interstitial pneumonia pattern of disease. Axial computed tomography image shows extensive, basal-predominant ground-glass opacities with associated traction bronchiectasis. Notably, the esophagus shows marked dilation; this is typical of scleroderma. Please click here to view a larger version of this figure.

Figure 3: Systemic sclerosis with a fibrotic NSIP pattern. Axial high-resolution computed tomography images (A and B) show extensive ground-glass opacities, reticulation, architectural distortion and traction bronchiectasis. Notably, subpleural sparing is apparent; this is typical of NSIP and is seen in about 50% of all cases. NSIP = non-specific interstitial pneumonia. Please click here to view a larger version of this figure.
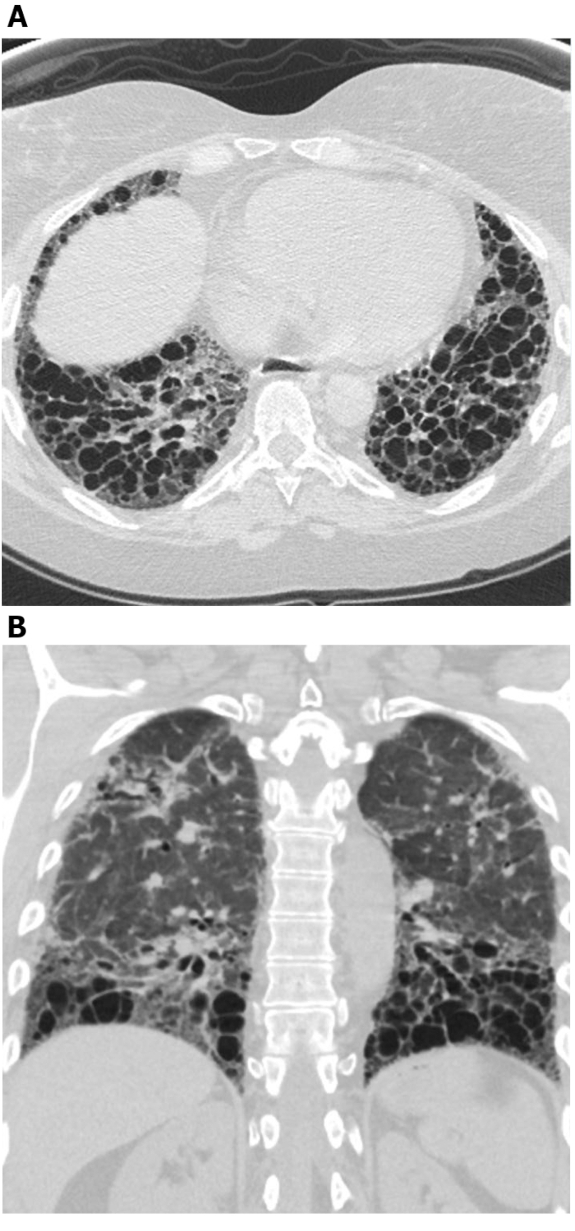
Figure 4: Systemic sclerosis with exuberant traction bronchiectasis. Axial (A) and coronal (B) high-resolution computed tomography images show extensive middle and lower lung zone predominant traction bronchiectasis. While this may be mistaken for honeycombing, the cystic areas connect with each other and spare the immediate subpleural lung; this is typical of bronchiectasis. Please click here to view a larger version of this figure.
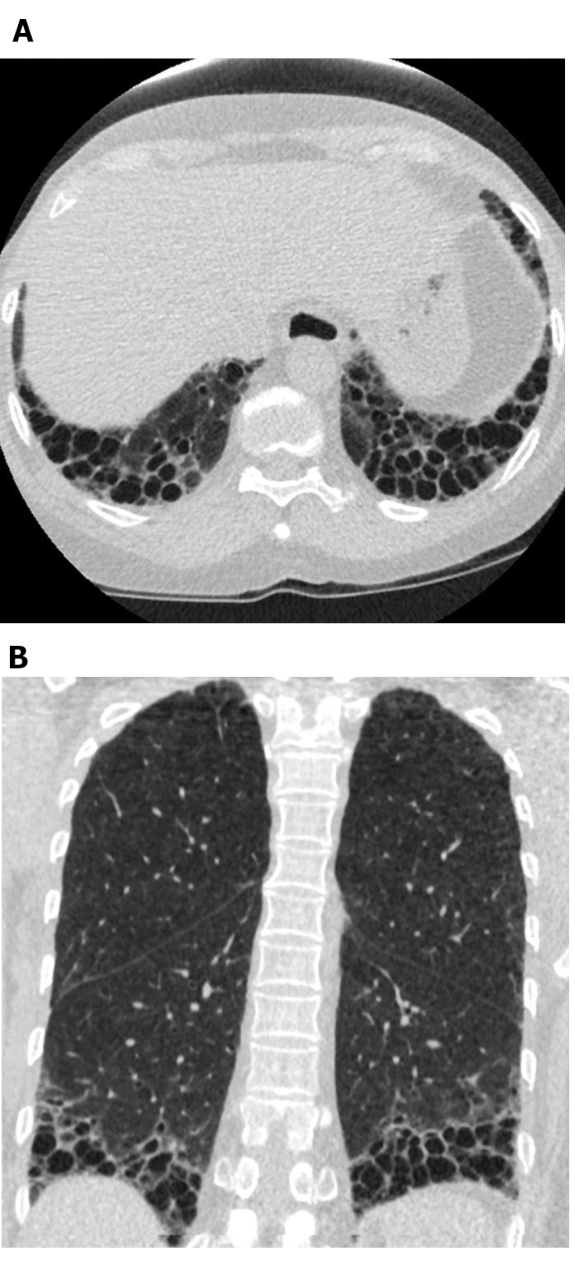
Figure 5: Systemic sclerosis with a UIP pattern of lung fibrosis. Axial (A) and coronal (B) computed tomography images show peripheral and basal predominant honeycombing and traction bronchiectasis in keeping with the typical UIP pattern of lung fibrosis. Note the dilated esophagus (attributable to scleroderma) and the ‘exuberant’ honeycombing (suggestive of ILD related to connective tissue disease rather than idiopathic pulmonary fibrosis). UIP = usual interstitial pneumonia. Please click here to view a larger version of this figure.
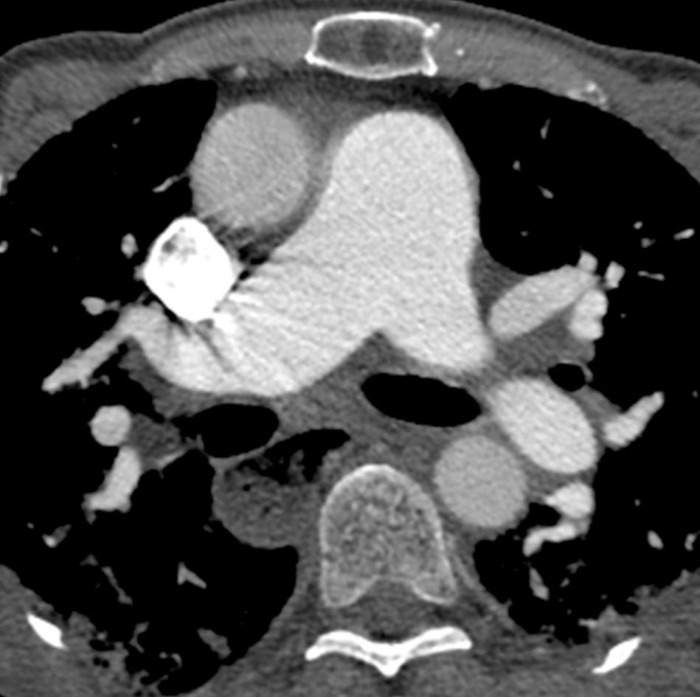
Figure 6: Systemic sclerosis with pulmonary hypertension and dilated esophagus. Contrast-enhanced chest computed tomography shows marked enlargement of the pulmonary trunk, with a larger measurement than the adjacent ascending aorta that suggests underlying pulmonary hypertension. The esophagus is markedly dilated; this is attributable to scleroderma. Please click here to view a larger version of this figure.
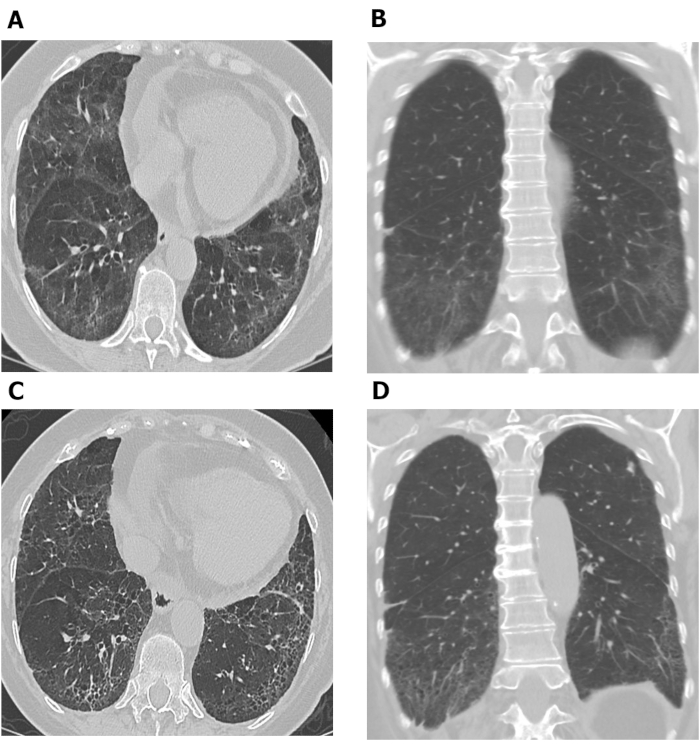
Figure 7: Serial chest HRCT images showing progression of pulmonary fibrosis in patient with SSc-ILD. Axial (A) and coronal (B) images from chest HRCT show basilar predominant ground-glass opacity and reticulation with mild traction bronchiectasis and subpleural sparing consistent with non-specific interstitial pneumonia in this patient with SSc. After 10 years, increased reticulation and traction bronchiolectasis at the lung bases with decrease in ground-glass opacity are observed on axial (C) and coronal (D) chest HRCT images, consistent with mild worsening of pulmonary fibrosis. HRCT = high-resolution computed tomography; SSc-ILD = systemic scleroderma-associated interstitial lung disease. Please click here to view a larger version of this figure.
Discussion
While HRCT is currently the definitive imaging method for diagnosing and assessing SSc-ILD, it uses ionizing radiation and is relatively expensive. Chest radiographs may be undertaken instead, although these do not facilitate differential diagnosis to the same extent as HRCT, and a normal chest radiograph does not eliminate the possibility of ILD. Perhaps the best use of chest radiographs is to monitor for progressive disease between HRCT scans and for the exclusion of complicating disease, such as infectious pneumonia, in the setting of acute worsening of symptoms.
A perceived limitation of HRCT is radiation exposure. As described earlier, new methods of conducting CT scans may enable radiation exposure to be reduced31, and furthermore, current CT scanners provide an array of advanced techniques that offer the possibility in the future to lower radiation exposure to nearly chest radiograph levels. Alternatively, imaging methods such as MRI or lung ultrasound could potentially be used to avoid exposing the patient to radiation in the future32,72,73,74. We believe that, while there are risk-benefit considerations associated with imaging utilization, the advantages of CT in diagnosis and patient management far outweigh the potential risks.
Imaging data, particularly HRCT, provide arguably the most important information to enable diagnosis of SSc-ILD. Detailed consideration of the patterns and features of HRCT scans is usually sufficient to distinguish SSc-ILD from other lung diseases, with the benefit of avoiding the need for an invasive biopsy procedure.
Visual assessment of HRCT scans introduces a degree of subjectivity and the possibility of inter-observer variability. Computer-based methods of HRCT scan interpretation have been investigated as a possible approach to improving accuracy63,75. For example, quantitative approaches to the assessment of lung fibrosis or the extent of disease may be used to assess treatment response69,71,76. However, these methods are not widely used in daily clinical practice at this time.
We hope the information presented in this manuscript will serve as a practical guide to assist physicians in using HRCT scans for diagnosing SSc-ILD and determining prognosis. Improved methods for obtaining images and for interpreting scans have the potential to reduce patients’ exposure to radiation and improve diagnostic/prognostic accuracy.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no direct compensation related to the development of the manuscript. Writing assistance was provided by Ken Sutor, BSc, of GeoMed, an Ashfield company, part of UDG Healthcare plc, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
The authors received no direct compensation related to the development of the video. Medical writing support for the video script was provided by Leon Newman, PhD, of GeoMed, an Ashfield company, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the final video for medical and scientific accuracy as well as intellectual property considerations.
References
- Denton, C. P., Khanna, D. Systemic sclerosis. Lancet. 390 (10103), 1685-1699 (2017).
- Arnett, F. C., et al. Familial occurrence frequencies and relative risks for systemic sclerosis (scleroderma) in three United States cohorts. Arthritis & Rheumatism. 44 (6), 1359-1362 (2001).
- Barnes, J., Mayes, M. D. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Current Opinion in Rheumatology. 24 (2), 165-170 (2012).
- Marie, I., Gehanno, J. F. Environmental risk factors of systemic sclerosis. Seminars in Immunopathology. 37 (5), 463-473 (2015).
- Silman, A. J. Epidemiology of scleroderma. Annals of the Rheumatic Diseases. 50, 846-853 (1991).
- Scholand, M. B., et al. Interstitial lung disease in systemic sclerosis: diagnosis and management. Rheumatology. 1, 008 (2012).
- Solomon, J. J., et al. Scleroderma lung disease. European Respiratory Review. 22 (127), 6-19 (2013).
- Walker, U. A., et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Annals of the Rheumatic Diseases. 66 (6), 754-763 (2007).
- Hinchcliff, M., Fischer, A., Schiopu, E., Steen, V. D., Investigators, P. Pulmonary hypertension assessment and recognition of outcomes in scleroderma (PHAROS): baseline characteristics and description of study population. The Journal of Rheumatology. 38 (10), 2172-2179 (2011).
- Giacomelli, R., et al. Interstitial lung disease in systemic sclerosis: current and future treatment. Rheumatology International. 37 (6), 853-863 (2017).
- Sanchez-Cano, D., et al. Interstitial lung disease in systemic sclerosis: data from the spanish scleroderma study group. Rheumatology International. 38 (3), 363-374 (2018).
- Silver, K. C., Silver, R. M. Management of systemic-sclerosis-associated interstitial lung disease. Rheumatic Diseases Clinics of North America. 41 (3), 439-457 (2015).
- Fischer, A., Kong, A. M., Swigris, J. J., Cole, A. L., Raimundo, K. All-cause healthcare costs and mortality in patients with systemic sclerosis with lung involvement. The Journal of Rheumatology. 45 (2), 235-241 (2018).
- Tyndall, A. J., et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Annals of the Rheumatic Diseases. 69 (10), 1809-1815 (2010).
- Herzog, E. L., et al. Review: interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis: how similar and distinct. Arthritis & Rheumatology. 66 (8), 1967-1978 (2014).
- Rubio-Rivas, M., Royo, C., Simeon, C. P., Corbella, X., Fonollosa, V. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Seminars in Arthritis and Rheumatism. 44 (2), 208-219 (2014).
- Stock, C., et al. Serum KL-6 as a marker of disease progression in SSc-ILD. European Respiratory Journal. 52, (2018).
- Winstone, T. A., et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest. 146 (2), 422-436 (2014).
- Khanna, D., et al. Ongoing clinical trials and treatment options for patients with systemic sclerosis-associated interstitial lung disease. Rheumatology (Oxford). 58 (4), 567-579 (2019).
- Behr, J., Furst, D. E. Pulmonary function tests. Rheumatology (Oxford). 47, 65-67 (2008).
- Hax, V., et al. Clinical algorithms for the diagnosis and prognosis of interstitial lung disease in systemic sclerosis. Seminars in Arthritis and Rheumatism. 47 (2), 228-234 (2017).
- Molberg, O., Hoffmann-Vold, A. M. Interstitial lung disease in systemic sclerosis: progress in screening and early diagnosis. Current Opinion in Rheumatology. 28 (6), 613-618 (2016).
- Raghu, G., Goldman, L., Schafer, A. I. Interstital lung disease. Goldman-Cecil Medicine. , 575-588 (2016).
- Showalter, K., et al. Performance of forced vital capacity and lung diffusion cutpoints for associated radiographic interstitial lung disease in systemic sclerosis. The Journal of Rheumatology. 45 (11), 1572-1576 (2018).
- Suliman, Y. A., et al. Brief report: pulmonary function tests: high rate of false-negative results in the early detection and screening of scleroderma-related interstitial lung disease. Arthritis & Rheumatology. 67 (12), 3256-3261 (2015).
- Roth, M. D., et al. Predicting treatment outcomes and responder subsets in scleroderma-related interstitial lung disease. Arthritis & Rheumatology. 63 (9), 2797-2808 (2011).
- Kalra, M. K., Maher, M. M., Rizzo, S., Kanarek, D., Shepard, J. A. Radiation exposure from chest CT: issues and strategies. Journal of Korean Medical Science. 19 (2), 159-166 (2004).
- Siegel, J. A., Pennington, C. W., Sacks, B., Welsh, J. S. The birth of the illegitimate linear no-threshold model: an invalid paradigm for estimating risk following low-dose radiation exposure. American Journal of Clinical Oncology. 41 (2), 173-177 (2018).
- Frauenfelder, T., et al. Screening for interstitial lung disease in systemic sclerosis: performance of high-resolution CT with limited number of slices: a prospective study. Annals of the Rheumatic Diseases. 73 (12), 2069-2073 (2014).
- Kubo, T., et al. Radiation dose reduction in chest CT: a review. American Journal of Roentgenology. 190 (2), 335-343 (2008).
- Nguyen-Kim, T. D. L., et al. The impact of slice-reduced computed tomography on histogram-based densitometry assessment of lung fibrosis in patients with systemic sclerosis. Journal of Thoracic Disease. 10 (4), 2142-2152 (2018).
- Muller, C. S., Warszawiak, D., Paiva, E. D. S., Escuissato, D. L. Pulmonary magnetic resonance imaging is similar to chest tomography in detecting inflammation in patients with systemic sclerosis. Revista Brasileira de Reumatologia English Edition. 57 (5), 419-424 (2017).
- Pinal-Fernandez, I., et al. Fast 1.5 T chest MRI for the assessment of interstitial lung disease extent secondary to systemic sclerosis. Clinical Rheumatology. 35 (9), 2339-2345 (2016).
- Sverzellati, N. Highlights of HRCT imaging in IPF. Respiratory Research. 14, 3 (2013).
- Lynch, D. A., et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. The Lancet Respiratory Medicine. 6 (2), 138-153 (2018).
- Raghu, G., et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. American Journal of Respiratory and Critical Care Medicine. 198 (5), 44-68 (2018).
- . Exam: CT thorax for interstitial lung disease Available from: https://www.pulmonaryfibrosis.org/docs/default-source/medical-community-documents/ct-chest-for-ild-protocol.pdf?sfvrsn=0 (2019)
- Doyle, T. J., Hunninghake, G. M., Rosas, I. O. Subclinical interstitial lung disease: why you should care. American Journal of Respiratory and Critical Care Medicine. 185 (11), 1147-1153 (2012).
- Peroni, D. G., Boner, A. L. Atelectasis: mechanisms, diagnosis and management. Paediatric Respiratory Reviews. 1 (3), 274-278 (2000).
- Branley, H. M. Pulmonary fibrosis in systemic sclerosis: diagnosis and management. Respiratory Medicine CME. 3, 10-14 (2010).
- Engeler, C. E., Tashjian, J. H., Trenkner, S. W., Walsh, J. W. Ground-glass opacity of the lung parenchyma: a guide to analysis with high-resolution CT. American Journal of Roentgenology. 160 (2), 249-251 (1993).
- Goldin, J. G., et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest. 134 (2), 358-367 (2008).
- Strollo, D., Goldin, J. Imaging lung disease in systemic sclerosis. Current Rheumatology Reports. 12 (2), 156-161 (2010).
- Pandey, A. K., et al. Significance of various pulmonary and extrapulmonary abnormalities on HRCT of the chest in scleroderma lung. Indian Journal of Radiology and Imaging. 23 (4), 304-307 (2013).
- Chung, J. H., et al. CT Features of the usual interstitial pneumonia pattern: differentiating connective tissue disease-associated interstitial lung disease from idiopathic pulmonary fibrosis. American Journal of Roentgenology. 210 (2), 307-313 (2018).
- Zhan, X., et al. Differentiating usual interstitial pneumonia from nonspecific interstitial pneumonia using high-resolution computed tomography: the “Straight-edge Sign”. Journal of Thoracic Imaging. 33 (4), 266-270 (2018).
- Farrokh, D., Abbasi, B., Fallah-Rastegar, Y., Mirfeizi, Z. The extrapulmonary manifestations of systemic sclerosis on chest high resolution computed tomography. Tanaffos. 14 (3), 193-200 (2015).
- Salaffi, F., et al. Relationship between interstitial lung disease and oesophageal dilatation on chest high-resolution computed tomography in patients with systemic sclerosis: a cross-sectional study. La Radiologia Medica. 123 (9), 655-663 (2018).
- Vonk, M. C., et al. Oesophageal dilatation on high-resolution computed tomography scan of the lungs as a sign of scleroderma. Annals of the Rheumatic Diseases. 67 (9), 1317-1321 (2008).
- Chowaniec, M., Skoczynska, M., Sokolik, R., Wiland, P. Interstitial lung disease in systemic sclerosis: challenges in early diagnosis and management. Reumatologia. 56 (4), 249-254 (2018).
- McCall, R. K., Ravenel, J. G., Nietert, P. J., Granath, A., Silver, R. M. Relationship of main pulmonary artery diameter to pulmonary arterial pressure in scleroderma patients with and without interstitial fibrosis. Journal of Computer Assisted Tomography. 38 (2), 163-168 (2014).
- Pandey, A. K., et al. Predictors of pulmonary hypertension on high-resolution computed tomography of the chest in systemic sclerosis: a retrospective analysis. Canadian Association of Radiologists Journal. 61 (5), 291-296 (2010).
- Raymond, T. E., Khabbaza, J. E., Yadav, R., Tonelli, A. R. Significance of main pulmonary artery dilation on imaging studies. Annals of the American Thoracic Society. 11 (10), 1623-1632 (2014).
- Colaci, M., et al. Lung cancer in scleroderma: results from an Italian rheumatologic center and review of the literature. Autoimmunity Reviews. 12 (3), 374-379 (2013).
- Distler, O., et al. Design of a randomised, placebo-controlled clinical trial of nintedanib in patients with systemic sclerosis-associated interstitial lung disease (SENSCIS). Clinical and Experimental Rheumatology. 35 (4), 75-81 (2017).
- Desai, S. R., et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology. 232 (2), 560-567 (2004).
- Mira-Avendano, I., et al. Interstitial lung disease and other pulmonary manifestations in connective tissue diseases. Mayo Clinic Proceedings. 94 (2), 309-325 (2019).
- Walkoff, L., White, D. B., Chung, J. H., Asante, D., Cox, C. W. The four corners sign: a specific imaging feature in differentiating systemic sclerosis-related interstitial lung disease from idiopathic pulmonary fibrosis. Journal of Thoracic Imaging. 33 (3), 197-203 (2018).
- Kotnur, M. R., Suresh, P., Reddy, V. S., Sharma, T., Salim, N. A. Systemic sclerosis with multiple pulmonary manifestations. Journal of Clinical & Diagnostic Research. 10 (6), 16-17 (2016).
- Goh, N. S., et al. Interstitial lung disease in systemic sclerosis: a simple staging system. American Journal of Respiratory and Critical Care Medicine. 177 (11), 1248-1254 (2008).
- Takei, R., et al. Radiographic fibrosis score predicts survival in systemic sclerosis-associated interstitial lung disease. Respirology. 23 (4), 385-391 (2018).
- Bakker, M. E., et al. Lung density and pulmonary artery diameter are predictors of pulmonary hypertension in systemic sclerosis. Journal of Thoracic Imaging. 32 (6), 391-397 (2017).
- Khanna, D., et al. Predictors of lung function decline in scleroderma-related interstitial lung disease based on high-resolution computed tomography: implications for cohort enrichment in systemic sclerosis-associated interstitial lung disease trials. Arthritis Research & Therapy. 17, 372 (2015).
- Ariani, A., et al. Quantitative chest computed tomography is associated with two prediction models of mortality in interstitial lung disease related to systemic sclerosis. Rheumatology (Oxford). 56 (6), 922-927 (2017).
- Goldin, J., et al. Treatment of scleroderma-interstitial lung disease with cyclophosphamide is associated with less progressive fibrosis on serial thoracic high-resolution CT scan than placebo: findings from the scleroderma lung study. Chest. 136 (5), 1333-1340 (2009).
- Tashkin, D. P., et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. The Lancet Respiratory Medicine. 4 (9), 708-719 (2016).
- Distler, O., et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. New England Journal of Medicine. 380, 2518-2528 (2019).
- Cappelli, S., et al. Interstitial lung disease in systemic sclerosis: where do we stand. European Respiratory Review. 24 (137), 411-419 (2015).
- Goldin, J. G., et al. Longitudinal changes in quantitative interstitial lung disease on CT after immunosuppression in the Scleroderma Lung Study II. Annals of the American Thoracic Society. 5 (11), 1286-1295 (2018).
- Wangkaew, S., Euathrongchit, J., Wattanawittawas, P., Kasitanon, N. Correlation of delta high-resolution computed tomography (HRCT) score with delta clinical variables in early systemic sclerosis (SSc) patients. Quantitative Imaging in Medicine and Surgery. 6 (4), 381-390 (2016).
- Kim, H. J., et al. Transitions to different patterns of interstitial lung disease in scleroderma with and without treatment. Annals of the Rheumatic Diseases. 75 (7), 1367-1371 (2016).
- Tardella, M., et al. Ultrasound B-lines in the evaluation of interstitial lung disease in patients with systemic sclerosis: cut-off point definition for the presence of significant pulmonary fibrosis. Medicine (Baltimore). 97 (18), 0566 (2018).
- Hassan, R. I., et al. Lung ultrasound as a screening method for interstitial lung disease in patients with systemic sclerosis. Journal of Clinical Rheumatology. , (2018).
- Wang, Y., Gargani, L., Barskova, T., Furst, D. E., Cerinic, M. M. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: a literature review. Arthritis Research & Therapy. 19 (1), 206 (2017).
- Ariani, A., et al. Quantitative CT indexes are significantly associated with exercise oxygen desaturation in interstitial lung disease related to systemic sclerosis. The Clinical Respiratory Journal. 11 (6), 983-989 (2017).
- Kim, H. J., et al. Quantitative texture-based assessment of one-year changes in fibrotic reticular patterns on HRCT in scleroderma lung disease treated with oral cyclophosphamide. European Radiology. 21 (12), 2455-2465 (2011).

