Preparation of Binary and Ternary Deep Eutectic Systems
Summary
This protocol aims to standardize the preparation of deep eutectic systems throughout the scientific community so that these systems can be reproduced.
Abstract
The preparation of deep eutectic systems (DES) is a priori a simple procedure. By definition, two or more components are mixed together at a given molar ratio to form a DES. However, from our experience in the laboratory, there is a need to standardize the procedure to prepare, characterize and report the methodologies followed by different researchers, so that the results published can be reproduced. In this work, we test different approaches reported in the literature to prepare eutectic systems and evaluated the importance of water in the successful preparation of liquid systems at room temperature. These published eutectic systems were composed of citric acid, glucose, sucrose, malic acid, β-alanine, L-tartaric acid and betaine and not all of preparation methods described could be reproduced. However, in some cases, it was possible to reproduce the systems described, with the inclusion of water as a third component of the eutectic mixture.
Introduction
Deep eutectic solvents have been named the solvents for the 21st century and are considered a new generation of solvents. They are defined as a mixture of two or more chemical compounds at a particular molar ratio to result in a significant decrease in the melting temperature of the individual components, becoming liquid at room temperature1,2,3. In this sense, the preparation of the solvents does not require any chemical reaction and hence the production yield is 100%. In 2011, Choi and co-workers reported the possibility of naturally occurring DES and named them, natural deep eutectic solvents (NADES)3,4,5. NADES can be prepared from different combinations of sugars, amino acids, organic acids and choline derivatives; and these systems prepared from natural components are inherently biocompatible and biodegradable, presenting considerably less toxicity compared to other alternative solvents (e.g., ionic liquids)5,6,7,8. Since 2015, the number of publications in the field has risen exponentially and the possible applications of NADES are very broad3. Even though many manuscripts and reviews have been published, there are fundamental questions that persist, and scientists have not yet found the answer to intriguing questions such as the mechanisms underlying DES formation. Understanding the DES formation mechanism would lead to a consolidated approach towards the development of new systems, rather than the current trial and error approach. Furthermore, the opportunities in the field are growing each day, as consumers become more aware of the sustainability of their products, not only in terms of their end-life but also in terms of processing itself8,9,10. To drive major innovations in the field of deep eutectic solvents, the standardization of the production and characterization methods is first required. The lack of reproducibility of some of the systems reported in the literature was the motivation to develop this work as we faced this issue several times. Herein, we demonstrate the need and crucial importance to accurately describe the materials and methods and show that although the preparation of DES is a simple and straightforward procedure, there are some key aspects (e.g., the presence/amount of water) that must always be discussed.
Protocol
NOTE: The NADES studied were betaine:L-(+)-tartaric acid (2:1), β-alanine:DL-malic acid (3:2), glucose:sucrose (1:1) and citric acid:glucose (2:1). These systems were prepared by different methods: freeze-drying (FD), vacuum evaporation (VE), and heat and stirring (HS) with and without water. As an example, the protocol for the system citric acid:glucose (2:1) is given. The NADES were characterized by differential scanning calorimetry (DSC), polarized optical microscopy (POM), water content and nuclear magnetic resonance (NMR) spectroscopy.
1. NADES preparation
- Freeze drying
- In separate containers, add 2 g of citric acid monohydrate and 0.9530 g of glucose monohydrate. Add 10 mL of deionized water to each and stir until the compounds are completely dissolved.
- Mix the two solutions together and ensure the homogenization of the final solution. Place the solution in a round bottom flask.
- Freeze it using liquid nitrogen. Place the flask in a freeze-dryer for 48 h to ensure that all the water is removed from the sample.
- Vacuum evaporation
- Weigh 2 g of citric acid monohydrate and 0.9530 g of glucose monohydrate in separate containers. Add 10 mL of deionized water to each and stir until the compounds are completely dissolved.
- Mix the two solutions together and ensure the homogenization of the solution. Place the solution in a round bottom flask.
- Using a rotary evaporator, dry the sample until a clear, viscous liquid is formed.
- Heating and stirring
- Weigh 2 g of citric acid monohydrate and 0.9530 g of glucose monohydrate into the same vial. Add 278 μL of water.
- Place the vial with a magnetic stirring bar in a 50 °C water bath.
- Leave the sample until a clear, viscous liquid is formed.
2. NADES characterization
- Polarized optical microscopy (POM)
- Place a droplet of NADES on a microscope glass slide for observation.
- Using the transmission mode of a microscope, perform the optical characterization of the sample at room temperature.
- Karl-Fisher titration
- Collect 100 μL of NADES in a syringe, and then clean the excess liquid on the outside.
- Place the syringe on a scale and tare it.
- Press START on the KF equipment and add a small drop of the sample to the vessel.
- Weigh the syringe, enter the mass on the KF equipment and press ENTER. The result will appear on the screen in ppm of water.
- Differential scanning calorimetry (DSC)
- Place 3-10 mg of each sample in a hermetic aluminum pan with a covering lid. Close the pan with a sample press.
- Analyze the samples using a DSC with a temperature range of -90 °C up to the degradation temperature, with a heating rate of 10 °C/min. Perform two cycles with an isothermal hold of 2 min and analyze under a nitrogen atmosphere (50 mL/min).
- Nuclear magnetic resonance (NMR)
- Prepare a 5 mm NMR tube by dissolving 250 µL of NADES with 250 µL of dimethyl sulfoxide-d6 (DMSO-d6).
- Acquire the 1H and NOESY spectra at 25 °C on a 400 MHz spectrometer.
- Use an appropriate software to analyze the spectra, and use the chemical shift of DMSO-d6 (δ 2.50 ppm) to assign all the signals of each component.
Representative Results
From the preparation of NADES, the results we expect to obtain are shown on Figure 1. A description of each system is made below. Using the freeze-drying method, the result should be a solid or a very dense paste since all the water is removed from the system. Using the evaporation method, the result should be a clear and viscous liquid. Using the heating and stirring method with the addition of small amounts of water, the result should be a clear and very viscous liquid.
The results obtained from POM can be seen on Figure 1. When a NADES is completely formed, we expect to see a black image, indicating that the sample is completely amorphous and that there are no crystals remaining in the system. The results obtained from KF titration are described in Table 2. Besides the amount of water that is added to the systems, the percentage of water of the final mixture also depends on the water content of the reagents.
Concerning the DSC, the goal of this technique is also to confirm that the system is liquid in the temperature range that it will be applied, so the expected result is to have a thermogram that shows no thermal events on the temperature range of interest (Table 2). The NMR technique is used to confirm the existence of hydrogen bond formation, which is the main characteristic of NADES systems. This can be confirmed by observation of the change in chemical shifts of each signal, and by analysis of the NOESY spectra, that shows spatial and intermolecular correlations (Figure 2).
| Component 1 | Component 2 | Preparation Method | Reference |
| Betaine (Bet) | L-(+)-Tartaric Acid (LTA) | Vacuum evaporating (VE) | Dai et al. (2013)5 and Espino et al. (2016)6 |
| β-Alanine (β-A) | DL-Malic Acid (MA) | Vacuum evaporating (VE) | Dai et al. (2013)5 and Espino et al. (2016)6 |
| Glucose (Gluc) | Sucrose (Suc) | Freeze-dried (FD) | Choi et al. (2011)4 and Espino et al. (2016)6 |
| Citric Acid (CA) | Glucose (Gluc) | Freeze-dried (FD) | Choi et al. (2011)4 and Espino et al. (2016)6 |
Table 1: Systems reported in literature and their preparation method.
| NADES | Preparation Method | Water Content (%) |
| Karl Fischer measure | ||
| Bet:LTA (2:1 + 20% water) | Heating and stirring, adding water | 19.94 ± 1.28 |
| Bet:LTA (2:1) | Vacuum evaporating | 11.36 ± 0.78 |
| β-A:MA (3:2 + 11% water) | Heating and stirring, adding water | 11.45 ± 0.25 |
| β-A:MA (3:2) | Vacuum evaporating | 18.84 ± 1.78 |
| Gluc:Suc (1:1 + 21% water) | Heating and stirring, adding water | 20.88 ± 0.13 |
| Gluc:Suc (1:1) | Vacuum evaporating | 22.56 ± 0.48 |
| CA:Gluc (2:1 + 17% water) | Heating and stirring, adding water | 17.33 ± 0.68 |
| CA:Gluc (2:1) | Vacuum evaporating | 20.04 ± 0.26 |
Table 2: Water content (%) of the systems prepared by different methods.
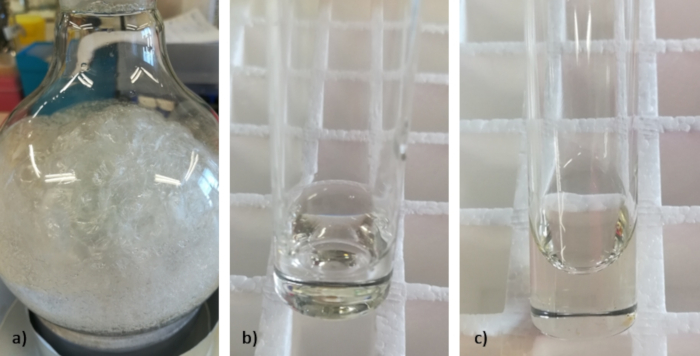
Figure 1: Representative results of the NADES when prepared by a) freeze-drying, b) vacuum evaporation and c) heating and stirring with addition of water. The picture shows that when the system is freeze-dried, the result obtained is a crystal since all the water is removed from the mixture whereas when VE and HS methods are used, the amount of water needed for the NADES to form is present and the obtained result is a homogenous liquid at room temperature. Please click here to view a larger version of this figure.

Figure 2: Polarized optical microscopy of CA:Glu (2:1) prepared by different methods, with cross polarizers (left image) and parallel polarizers (right image) – 100 μm (10x amplification). The black images show that the sample is a liquid at room temperature. The FD sample is completely crystalized since the result obtained from this technique was not a liquid. Please click here to view a larger version of this figure.
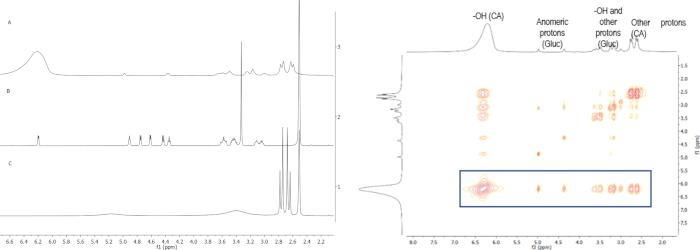
Figure 3: a) Overlay of 1H NMR spectra of the (A) NADES system citric acid:glucose:water (2:1:4), (B) glucose, and (C) citric acid; b) NOESY spectrum of the NADES system citric acid:glucose:water (2:1:4). The overlaid spectra show the difference in chemical shifts of each component upon DES formation, originated by the establishment of hydrogen bonds between them. The NOESY spectrum shows the interaction between the OH proton from citric acid with the remaining protons from both components. Please click here to view a larger version of this figure.
Discussion
The different methodologies reported in the literature for the preparation of NADES are a heating and stirring method (HS), vacuum evaporation (VE), and freeze-drying (FD). The systems we have prepared in this work are described by different authors in the literature4,5,6,10,11. Table 1 lists the components of each mixture, as reported in the original manuscript as well as their preparation method.
Upon our investigations to reproduce the systems described, we realized that in some cases it was not possible to achieve a similar NADES, as a clear, viscous, liquid sample at room temperature. Preparing a NADES relies on many factors. Some can be easily controlled, but others are more difficult to standardize. The most important thing to consider is that the final product cannot rely on external factors such as the equipment used.
The systems prepared by different methods were then characterized. With polarized optical microscopy (POM), it was observed that with the HS method without water, even at different temperatures, the NADES did not form a clear and viscous liquid. However, a homogeneous and clear, viscous liquid was observed as represented in Figure 1 when applying the HS method with small amounts of water and the VE method for the preparation of the NADES.
DSC was used to determine the thermal events of the mixture. The results showed that the system is liquid at room temperature and up to 130 °C, since the thermogram shows no thermal events. The water content of each sample was measured by Karl-Fischer titration, and the results are represented in Table 2. The water content of the systems must be reported, since it is the parameter that most influences the properties of the obtained liquid, such as viscosity and polarity. These changes have great impact on the outcome of the application for which the NADES is designed.
NMR was also used to confirm the formation the mentioned NADES systems, through the formation of hydrogen bonds between the molecules of each system. One example is given in Figure 2 for the NADES system citric acid:glucose (2:1) with 17% water obtained by HS where the proton spectrum of this NADES and the starting materials (citric acid and glucose) are overlaid (Figure 2a). From this, it is possible to observe changes in the chemical shifts of some protons from each molecule. The major change is the shifting of the OH proton from citric acid. Originally, this signal appears at 5.16 ppm, but this signal shifts to 6.22 ppm because of the formation of hydrogen bonds. This is confirmed by the NOESY spectrum (Figure 2b), where the strong interaction between the OH from citric acid and the remaining protons is visible. A similar interaction was observed for the other NADES systems.
In this study we observed that the description of the preparation method for eutectic systems reported in literature sometimes are incomplete, due to the lack of information regarding the water content of most systems. In the VE method, the water is added by preparing solutions of different components and mixing at a temperature that leads to the formation of eutectic systems; however, we cannot be sure of the minimum required water content. The knowledge of percentage of water needed to form the systems is considered hence, a crucial point that should always be reported, for others to be able to reproduce the preparation of the different eutectic mixtures.
The best method to use is the HS method with water added as it takes less time to prepare, for cases where the water content is already described. However, if this information is not available, the easiest method is the VE method, where all the available water is removed and only the water interacting with the NADES components remains in the system. In any case, researchers should let the systems evaporate for enough time to ensure that free water is removed from the system. This timing is dependent on the equipment and therefore it is not enough to describe in the materials section the duration of the VE method, but the water content has always to be reported.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program, under grant agreement No ERC-2016-CoG 725034. This work was also supported by the Associate Laboratory for Green Chemistry-LAQV which is financed by national funds from FCT/MCTES (UID/QUI/50006/2019) and by FCT/MCTES through the project CryoDES (PTDC/EQU-EQU/29851/2017).
Materials
| 5 mm NMR tube | Norell | ||
| Acid citric monohydrate | Sigma-Aldrich | ||
| Advance III spectrometer | Bruker | ||
| Deionized water | |||
| dimethyl sulfoxide-d6 | Sigma-Aldrich | ||
| DSC Q200 | TA Instruments, USA | ||
| Freeze-dryer CHRIST ALPHA 1-4 | Braun Biotec International | ||
| Glucose monohydrate | Cmd chemicals | ||
| Karl Fisher Coulometer | Metrohm | ||
| Olympus BX-51 polarized optical microscope | Olympus |
References
- Paiva, A., et al. Natural deep eutectic solvents – solvents for the 21st century. ACS Sustainable Chemistry & Engineering. 2, 1063-1071 (2014).
- Abbott, A. P., Capper, G., Davies, D. L., Rasheed, R. K., Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chemical Communications. , 70-71 (2003).
- Liu, Y., et al. Natural deep eutectic solvents: properties, applications, and perspectives. Journal of Natural Products. 81, 679-690 (2018).
- Choi, Y. H., et al. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology. Plant Physiology. 156, 1701-1705 (2011).
- Dai, Y., Spromsen, J. V., Witkamp, G. -. J., Verpoorte, R., Choi, Y. H. Natural deep eutectic solvents as new potential media for green technology. Analytica Chimica Acta. 766, 61-68 (2013).
- Espino, M., Fernández, M. A., Gomez, F. J. V., Silva, M. F. Natural designer solvents for greening analytical chemistry. Trends in Analytical Chemistry. 76, 126-136 (2016).
- Hayyan, M., et al. Natural deep eutectic solvents: cytotoxic profile. Springer Plus. 5, 913 (2016).
- Dai, Y., Witkamp, G. -. J., Verpoorte, R., Choi, Y. H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chemistry. 187, 14-19 (2015).
- Choi, Y. H., Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Current Opinion in Food Science. 26, 87-93 (2019).
- Guitérrez, M. C., Ferrer, M. L., Mateo, C. R., Del Monte, F. Freeze-drying of aqueous solutions of deep eutectic solvents: a suitable approach to deep eutectic suspensions of self-assembled structures. Langmuir. 25, 5509-5515 (2009).
- Gomez, F. J. V., Espino, M., Fernández, M. A., Silva, M. F. A greener approach to prepare natural deep eutectic solvents. Chemistry Select. 3, 6122-6125 (2018).

