In Situ Detection of Ribonucleoprotein Complex Assembly in the C. elegans Germline using Proximity Ligation Assay
Summary
This protocol demonstrates use of the proximity ligation assay to probe for protein-protein interactions in situ in the C. elegans germline.
Abstract
Understanding when and where protein-protein interactions (PPIs) occur is critical to understanding protein function in the cell and how broader processes such as development are affected. The Caenorhabditis elegans germline is a great model system for studying PPIs that are related to the regulation of stem cells, meiosis, and development. There are a variety of well-developed techniques that allow proteins of interest to be tagged for recognition by standard antibodies, making this system advantageous for proximity ligation assay (PLA) reactions. As a result, the PLA is able to show where PPIs occur in a spatial and temporal manner in germlines more effectively than alternative approaches. Described here is a protocol for the application and quantification of this technology to probe PPIs in the C. elegans germline.
Introduction
Over 80% of proteins are estimated to have interactions with other molecules1, which emphasizes how important PPIs are to the execution of specific biological functions in the cell2. Some proteins function as hubs facilitating assembly of larger complexes that are necessary for cell survival1. These hubs mediate multiple PPIs and help organize proteins into a network that facilitates specific functions in a cell3. Formation of protein complexes is also affected by biological context, such as the presence or absence of specific interacting partners4, cell signaling events, and developmental stage of a cell.
C. elegans is commonly used as a model organism for a variety of studies, including development. The simple anatomy of this animal is comprised of several organs, including the gonad, gut, and transparent cuticle, which facilitates the analysis of worm development. The germline residing in the gonad is a great tool to study how germline stem cells mature into gametes5 that develop into embryos and eventually the next generation of progeny. The distal tip region of the germline contains a pool of self-renewing stem cells (Figure 1). As stem cells leave the niche, they progress into the meiotic pachytene and eventually develop into oocytes in the young adult stage (Figure 1). This program of development in the germline is tightly regulated through different mechanisms, including a post-transcriptional regulatory network facilitated by RNA-binding proteins (RBPs)6. PPIs are important for this regulatory activity, as RBPs associate with other cofactors to exert their functions.
There are several approaches that can be used to probe for PPIs in the worm, but each has unique limitations. In vivo immunoprecipitation (IP) can be used to isolate protein-protein complexes from whole worm extracts; however, this approach does not indicate where the PPI occurs in the worm. In addition, protein complexes that are transient and only form during a specific stage of development or in a limited number of cells can be difficult to recover by co-immunoprecipitation. Finally, IP experiments need to address the concerns of protein complex reassortment after lysis and non-specific retention of proteins on the affinity matrix.
Alternative approaches for in situ detection of PPIs are co-immunostaining, Förster resonance energy transfer (FRET), and bimolecular fluorescence complementation (BiFC). Co-immunostaining relies on simultaneous detection of two proteins of interest in fixed worm tissue and measurement of the extent of signal colocalization. Use of super-resolution microscopy, which offers greater detail than standard microscopy7, helps to more stringently test protein colocalization beyond the diffraction-limited barrier of 200-300 nm8. However, co-immunostaining using both conventional and super-resolution microscopy works best for proteins with well-defined localization patterns. By contrast, it becomes much less informative for diffusely distributed interacting partners. Measuring for co-localization of signals based on overlap does not provide accurate information about whether the proteins are in complex with each other9,10.
Furthermore, co-immunoprecipitation and co-immunostaining of protein-protein complexes are not quantitative, making it challenging to determine if such interactions are significant. FRET and BiFC are both fluorescent-based techniques. FRET relies on tagging proteins of interest with fluorescent proteins (FPs) that have spectral overlap at which energy from one FP (donor) is transferred to another FP (acceptor)11. This nonradiative transfer of energy results in fluorescence of the acceptor FP that can be detected at its respective wavelength of emission. BiFC is based on reconstitution of a fluorescent protein in vivo. It entails splitting GFP into two complementary fragments, such as helices 1-10 and helix 1112, which are then fused to two proteins of interest. If these two proteins interact, the complementary fragments of GFP become close enough in proximity to fold and assemble, reconstituting the GFP fluorophore. Reconstituted GFP is then directly observed as fluorescence and indicates where a PPI has occurred.
As such, both FRET and BiFC depend on large fluorescent tags that can disrupt the function of the tagged protein. In addition, FRET and BiFC require abundant and comparable expression of the tagged proteins to obtain accurate data. FRET may not be suitable for experiments where one partner is in excess of the other, which can lead to high background13. Overexpression in BiFC experiments should also be avoided, as this can induce nonspecific assembly14 that results in increased background. Both techniques require optimization of expression and imaging conditions of the tagged proteins, which may prolong the time required to complete experiments.
The proximity ligation assay (PLA) is an alternative approach that can address the limitations of the techniques mentioned above. PLA takes advantage of primary antibodies that recognize the proteins of interest (or their tags). These primary antibodies are then bound by secondary antibodies containing oligonucleotide probes that can hybridize with one another when within a 40 nm (or shorter) distance15. The resulting hybridized DNA is amplified through a PCR reaction, which is detected by probes that complement the DNA. This results in foci that are visualized by a microscope. This technology can detect PPIs in situ in complex tissues (i.e., the worm gonad), which is organized as an assembly line containing cells at various stages of development and differentiation. With PLA, PPIs can be directly visualized in a fixed worm gonad, which is advantageous for investigating whether PPIs occur during a specific stage of development. PLA offers greater resolution of PPIs as opposed to co-localization-based assays, which is ideal for making precise measurements. If used, super-resolution microscopy has the potential to provide finer detail about the location of PLA foci within a cell. Another advantage is that the foci resulting from PLA reactions can be counted by an ImageJ-based analysis workflow, making this technique quantitative.
The LC8 family of dynein light chains was first described as a subunit of the dynein motor complex16 and hypothesized to serve as a cargo adapter. Since its initial discovery, LC8 has been found in multiple protein complexes in addition to the dynein motor complex17,18,19,20. Scanning for protein sequences that contain the LC8 interaction motif19 suggests that LC8 may have many interactions with a wide array of different proteins17,18,19,20,21,22. As a result, LC8 family proteins are now considered hubs that help promote the assembly of larger protein complexes19,22, such as assemblies of intrinsically disordered proteins21.
One C. elegans LC8-family protein, dynein light chain-1 (DLC-1), is widely expressed across many tissues and not enriched in specific subcellular structures23,24. Consequently, identification of biologically relevant in vivo partners of DLC-1 in C. elegans is challenging for a number of reasons: 1) co-immunoprecipitation does not indicate the tissue source where the interaction occurs; 2) limited expression of particular partners or transient interactions may hinder the ability to detect an interaction by co-immunoprecipitation; and 3) diffuse distribution of DLC-1 leads to non-specific overlap with potential partner proteins by co-immunostaining. Based on these challenges, PLA is an ideal approach for testing in vivo interactions with DLC-1.
It has been previously reported that DLC-1 directly interacts with and serves as a cofactor for the RNA-binding proteins (RBPs) FBF-223 and GLD-125. Our work supports the model of DLC-1 serving as a hub protein and suggests that DLC-1 facilitates an interaction network that spans beyond dynein19,22. Using a GST pulldown assay, a new DLC-1-interacting RBP named OMA-1 has been identified26. OMA-1 is important for oocyte growth and meiotic maturation27 and functions in conjunction with a number of translational repressors and activators28. While FBF-2 and GLD-1 are expressed in the stem cells and meiotic pachytene regions, respectively, OMA-1 is diffusely expressed in the germline from the meiotic pachytene through the oocytes27 (Figure 1). This suggests that DLC-1 forms complexes with RBPs in different regions of the gonad. It has also been found that the direct interaction between DLC-1 and OMA-1 observed in vitro is not recovered by an in vivo IP. The PLA has been successfully used as an alternate approach to further study this interaction in the C. elegans germline, and results suggest that PLA can be used to probe many other PPIs in the worm.
Protocol
NOTE: This protocol uses C. elegans strains in which potential interacting partners are both tagged. It is strongly recommended that a negative control strain be used, in which one tagged protein is not expected to interact with another tagged candidate interaction partner. Here, GFP alone was used as a negative control to assess background, as DLC-1 is not expected to interact with GFP in the worm. GFP-tagged OMA-1 was used as the experimental strain, as preliminary data suggest an interaction with DLC-1. Nematode strains co-expressing control and test proteins with 3xFLAG-tagged DLC-1 are referred to in this text as 3xFLAG::DLC-1; GFP and 3xFLAG::DLC-1; OMA-1::GFP (strains available upon request; more information in Table of Materials), respectively. Here, the 3xFLAG and GFP tags are used; however, other tags may be substituted as long as their antibodies are compatible with the PLA kit reagents.
1. Animal care
- Keep worms on nematode growth medium (NGM) plates that are seeded with the OP50 strain of E. coli and maintain at 24 °C for optimal expression of GFP.
- Passage adult worms every 2-3 days to propagate worms and keep them well-fed.
2. Preparation of synchronous culture
- Synchronize worms by bleaching a plate of well-fed, gravid hermaphrodites. A bleaching protocol is described in Porta-de-la-Riva et al.29. Let the embryos hatch overnight in a centrifuge tube at 24 °C while rotating end over end in 10 mL of M9 minimal media (M9) buffer. This will produce a culture of arrested L1 larvae.
- Incubate the tube of arrested L1 stage larvae on ice for 10 min, then top off the tube with ice cold 1x M9.
- Use a centrifuge to pellet the larvae at 600 x g for 5 min at 4 °C. Carefully aspirate the supernatant so that only 1-2 mL of supernatant remains.
- Re-suspend the pellet of larvae and use a micropipette to transfer 2 µL of suspended larvae culture to a glass slide. Count how many larvae are present to determine the density of the larvae culture, which will help guide seeding of the worms in step 2.5.
NOTE: A density of 10-15 L1 larvae/1 µL works well for seeding. - Use a micropipette to transfer the volume of larvae culture needed to seed approximately 100-120 L1 stage larvae on a 60 mm OP50 plate. For example, seed 10 µL of a larvae culture that has a density of 10 L1 larvae/1 µL of culture.
NOTE: Do not exceed a volume of 40 µL to seed the larvae, or excess liquid will disrupt the OP50 lawn. If the culture volume exceeds 40 µL, repeat steps 2.3-2.4 to further reduce the volume and increase the density of larvae culture. - Grow worms at 24 °C. Record the time when L1s are seeded on plate and periodically check the stage of development to identify the ideal time for dissection.
NOTE: At 52 h after seeding of L1s, worms cultured at 24 °C are typically in the young adult stage, which is the ideal stage for dissection for gonad-targeted PLA. However, the actual time at which the synchronized worms reach young adult stage may vary among strains and incubation temperature.
3. Dissection/gonad extrusion
NOTE: Dissection to extrude the gonad is necessary for gonad-targeted PLA to work successfully. This approach can also release embryos, which also work using this protocol for PLA (see Discussion for more information). After dissection, both the negative control and experimental samples are fixed and treated for PLA together in parallel. It is also suggested that an additional set of samples be prepared for the purpose of fluorescent co-immunostaining23 to demonstrate expression patterns of the protein partners of interest.
- Pick 30-40 young adult worms into a watch glass dish containing 500 µL of 1x M9 + levamisole (2.5 mM final concentration). After collecting the worms, carefully remove and discard most of the media to remove bacteria that is transferred along with the worms.
- Add in fresh 500 µL of 1x M9 + levamisole and use the pipette to gently draw up and dispense the media to rinse the worms. Carefully remove and discard most of the media to clear bacteria that is transferred along with the worms.
- Repeat this step 2x-3x until all bacteria are removed. After washes are completed, leave worms in about 100 µL of media to keep hydrated.
NOTE: Do not let the worms sit in media for longer than 7 min, as this will impair the extrusion of gonads during dissection. Perform washes under aid of dissecting microscope to monitor removal of media so that worms are not lost.
- Repeat this step 2x-3x until all bacteria are removed. After washes are completed, leave worms in about 100 µL of media to keep hydrated.
- Using a glass or polyethylene pipette, transfer worms to a 25 mm x 75 mm microscope slide coated with 0.001% poly-L-lysine (slides used in this procedure have an epoxy coated perimeter, leaving three workspaces, 14 mm x 14 mm each). Remove excess media so that approximately 10-15 µL of media remains.
- Under the aid of a dissecting microscope and using two 26½ gauge needles, place one needle over the other so that the ends form a pair of scissors. Using needles oriented in this fashion, cut worms behind the pharynx to release the germlines. Dissect all worms within 5 minutes.
NOTE: More detail on how to perform dissections can be found in a previous publication by Gervaise and Arur30. - After all worms are dissected, gently place a 22 mm x 40 mm coverslip over the slide so that it is perpendicular to the slide. The ends of the coverslip should hang off the slide.
- Freeze the slides on a pre-chilled aluminum block maintained on dry ice for at least 20 min. Gently place a chilled pencil on top of the coverslip to prevent the coverslip from becoming loose due to ice expansion.
4. Fixation/blocking
- When ready for fixation, flick off coverslips with a pencil or other blunt-edged tool and immediately dip the slide into a jar containing fresh, ice-cold methanol (chilled to -20 °C) for 1 min.
- Gently wipe the edges of the slide that surround the sample so that the next reagent is held by surface tension around the sample. Apply 150 µL of fixative (2% formaldehyde in 100 mM KH2PO4, pH = 7.2) for 5 min at RT.
NOTE: We have also tested a methanol/acetone fixation procedure31,32 and found that it is compatible with the PLA reaction. - Touch the slide to a paper towel at a perpendicular 90° angle to let the fixative run off the slide and absorb into the paper towel. Block slides 2x for 15 min at RT in a Coplin jar with 50 mL of 1x PBS/1% Triton X-100/1% bovine serum albumin (PBT/BSA).
NOTE: Coplin jars or other types of staining jars are recommended for this blocking step and the washing steps below in sections 6-9. These provide sufficient volumes for efficient exchange of blocking or washing buffer with the sample. - Block slides with a PBT/BSA solution containing 10% normal goat serum. Gently wipe edges that surround the slide and apply 100 µL of the solution to the slide. Incubate for 1 h at RT in a humid chamber.
NOTE: This step is highly recommended for staining with the αFLAG primary antibody. The humid chamber is constructed by securing glass pipettes with tape in the tray for the slides to lay on as they incubate. Dampened task wipes (Table of Materials) are placed in the tray to raise the internal humidity of the tray to prevent evaporation. The lid and tray are covered in foil to protect the samples from light during the light-sensitive steps. - Place slide on a paper towel to let PBT/BSA/10%NGS solution run off the slide and gently wipe the edges of the slide. Use the blocking reagent (Table of Materials) to block slides. Apply one drop to the 14 mm x 14 mm space. Incubate slides for 1 h at 37 °C in a humid chamber.
5. Primary antibody incubation
NOTE: To obtain the best PLA results and minimal background, the dilution factor of the primary antibodies may require optimization (see Discussion for more details). Additionally, the primary antibodies should be raised in different hosts that match the specificity of the secondary antibodies used for PLA.
- Place slide on a paper towel to let blocking reagent run off the slide and gently wipe the edges. Use the antibody diluent (Table of Materials) to dilute the primary antibodies. Apply 40 µL of primary antibody solution per 14 mm x 14 mm space.
- Incubate slides in a humid chamber overnight at 4 °C.
6. PLA probe (secondary antibody) incubation
NOTE: For steps 6-9, use wash buffers A and B at RT. If the buffers are stored at 4 °C, then let them warm to RT prior to using.
- Wash slides 2x for 5 min with 50 mL of 1x wash buffer A (Table of Materials) at RT in a Coplin jar. Set the Coplin jar on an orbital shaker set to 60 rpm.
- Place slide on a paper towel to let wash buffer run off the slide and gently wipe the edges. Prepare a 40 µL solution containing PLUS and MINUS probes (diluted 1:5 with antibody diluent). Apply the solution to each 14 mm x 14 mm space.
- Incubate slides in a humid chamber for 1 h at 37 °C.
7. Ligation
- Wash slides 2x for 5 min with 50 mL of 1x wash buffer A at RT in a Coplin jar. Set the Coplin jar on an orbital shaker set to 60 rpm.
- Dilute the ligation buffer (Table of Materials) 1:5 with ultrapure water. Use this buffer to dilute the ligase (Table of Materials) 1:40 to prepare a working stock of ligation solution.
- Place the slide on a paper towel to let the wash buffer run off the slide and gently wipe the edges. Apply 40 µL of the ligation solution to each 14 mm x 14 mm space.
- Incubate slides in a humid chamber for 30 min at 37 °C.
8. Amplification
NOTE: Using detection reagents with red fluorophores (Table of Materials) results in the least amount of background in C. elegans tissue.
- Wash slides 2x for 5 min with 50 mL of 1x wash buffer A at RT in a Coplin jar. Set the Coplin jar on an orbital shaker set to 60 rpm.
- Dilute the amplification red buffer (Table of Materials) 1:5 with ultrapure water. Use this buffer to dilute the polymerase (Table of Materials) 1:80 to prepare a working stock of amplification solution and protect from light.
- Place the slide on a paper towel to let the wash buffer run off the slide and gently wipe the edges. Apply 40 µL of the amplification solution to each 14mm x 14 mm space.
- Incubate slides in a humid chamber for 1 h and 40 min at 37 °C. Make sure the humid chamber is covered with foil to protect the samples from light.
9. Final washes
- Wash slides 2x for 10 min with 50 mL of 1x wash buffer B (Table of Materials) at RT in a Coplin jar. Set the Coplin jar on an orbital shaker set to 60 rpm.
- Wash slides 1x for 1 min with 50 mL of 0.01x wash buffer B at RT in a Coplin jar. Set the Coplin jar on an orbital shaker set to 60 rpm. This buffer is prepared by diluting wash buffer B with ultrapure water.
10. Coverslip mounting
- Let the excess wash buffer run off the slide onto a paper towel and wipe off any residual buffer remaining on the epoxy-coated perimeter of the slide.
- Add 10 µL of mounting medium (Table of Materials) to sample and gently lay a coverslip on top, allowing for the mounting medium to spread out.
- Paint around the edge of coverslip with nail polish to seal the coverslip and slide. Be gentle with application of nail polish to avoid moving the coverslip, which will damage the germlines. Let the nail polish harden for at least 20 min at RT, while slides are protected from light, before viewing it under a microscope.
- Store slides in a dark container or slide holder, as PLA-labeled samples are light-sensitive. The manufacturer suggests that slides can be stored at -20 °C for long-term storage or at 4 °C for short-term storage. Slides prepared using this protocol will last at least 2 months when stored at 20 °C.
11. Image acquisition
- For the purpose of quantification, use a confocal microscope to capture images of extruded germlines that are in clear view, undamaged, and unobstructed. Capture a z-stack of the germline that spans the whole germline in the z-plane and generate a maximum projection image to use for quantitation.
NOTE: Confocal microscopy is ideal for obtaining and quantifying PLA images with less background compared to those obtained using an epifluorescent microscope.- If the germline does not fit in one field of view, capture the overlapping fields of view as necessary to image the whole germline. Maximum projections of these images can be stitched together in FIJI.
- Be sure to keep imaging conditions the same between control and experimental samples to set a fair and proper threshold for identification of foci during image analysis.
NOTE: Record at least 8-10 germlines from each sample per replicate to facilitate statistical analysis of PLA quantification. At least three biological replicates are recommended for PLA to obtain reliable and consistent quantitative results.
12. Image analysis and quantification using FIJI/ImageJ
NOTE: The following workflow is based on images acquired using the 40x objective on a confocal microscope, in which images are saved in the .czi format. These .czi images and their accompanying metadata, including dimensions, can be accessed and opened in FIJI/ImageJ for further analysis. It should be checked whether FIJI accepts the format of confocal files from the confocal available to the specific user. If not, images in the .tiff format can be alternatively used for analysis, but the user will need to set the scale of the image manually in FIJI/ImageJ (Analyze | Set scale). It is recommended that all negative control images be analyzed together first to establish the level of background.
- Start the analysis workflow by analyzing all negative control images first, then move on to the experimental samples. Open a maximum projection image in FIJI/ImageJ to analyze (Figure 2A). If using a .czi file, a Bio-Formats Import Options box will be prompted.
- Include the following options to open your image: view stack with Data Browser; color mode = Colorized. A window with the image should now open with a slide bar to toggle between different channels captured by confocal (e.g., DAPI or PLA).
- If images need to be stitched, create duplicates of each channel from each image by right-selecting the image with the mouse and selecting Duplicate to open the Duplicate window. Specify only the channel number (c) that corresponds to the PLA or DAPI channel (e.g., 2) and uncheck the box for Duplicate Hyperstack.
- With both images to be stitched open, select Plugins | Stitching | Deprecated | 2D stitching. A Stitching of 2D Images window will open. Select which images will be used for stitching and use the default parameters that are preset in the window, then select Ok. The resulting image will be an assembled grayscale image.
NOTE: If the sub-images do not perfectly align, adjusting parameters (i.e., increasing the number of peaks to be checked from 5 à 500 or changing the fusion method from Linear Blending à Max. Intensity) may help to obtain the desired image. While other stitching tools are available, this approach retains the dimensionality of the image, which is important for quantification.
- Open the ROI (Region of Interest) manager by pressing T on the keyboard. A new window named ROI Manager will open.
- Select the polygon tool from the FIJI toolset box. Drop points around the germline to outline it and generate a ROI (Figure 2B). Connect the last dot to the first to generate a complete ROI.
NOTE: For darker images, it is helpful to adjust the contrast to improve visibility of the germline (which is reversible) so that it can be outlined more accurately.- Immediately after completing the ROI, go to the ROI manager and select Add [t] to store the ROI. It is imperative that any changes to the ROI including adding/removing points or movement of the ROI and points be updated (select Update from ROI manager) before proceeding to the next step, or they will be lost. Further detail on manipulation of ROIs and their points can be found in the FIJI/ImageJ User Guide.
- Once the ROI orientation is set, it can be saved for later reference by selecting the ROI name in the ROI manager followed by More | Save | (name file and specify destination for where to save). Saved ROIs can be opened in the ROI manager by selecting More | Open | (select the file).
- Measure the area inside the ROI by selecting the ROI name from the ROI manager, then selecting the Measure button on the ROI manager. A Résultats window will open with information about the ROI, including the area that it covers in the image (µM2) (inset of Figure 2B). Record this information in a spreadsheet for subsequent calculations.
NOTE: Make sure that the scale of the image has been set properly so that the correct dimensions of the ROI are collected. The types of measurements reported in the Résultats box can be modified by going to Analyze | Set Measurements…. - Open a duplicate image of only the PLA channel by right-selecting the PLA image and selecting Duplicate (Figure 2C). A Duplicate window will open. Specify only the channel number (c) that corresponds to the PLA channel (e.g., 2) and uncheck the box for Duplicate Hyperstack. Duplication of this image is recommended so that the original image does not get modified.
NOTE: These options will only show up when viewing the images containing multiple channels on FIJI/ImageJ. An alternate approach is to split the channels Image | Color | Split Channels, but this will modify your original image file. - With only the image of PLA channel selected, go to Image | Adjust | Threshold. A Threshold window for the image will open. Select Default as the threshold method, Red as the color, and check the Dark background box. Using the upper track on the window, slide the bar towards the right until all PLA foci are distinctly highlighted in the image.
- Record the value in the box to the right of the upper track and take note of what value was used to set the threshold. Select Apply in the Threshold window to finalize the threshold, and the image will convert to a white background with only the threshold foci visible as black dots (Figure 2D). Test the threshold on several negative control images to ensure that it is appropriate for capturing all PLA foci from image to image before quantitation.
NOTE: To view threshold foci as black dots on white background, go to Process | Binary | Options… and uncheck the Black background box. A threshold value between 30-40 is a good starting point for identifying PLA in the germline; however, the ideal value may vary depending on background.
- Record the value in the box to the right of the upper track and take note of what value was used to set the threshold. Select Apply in the Threshold window to finalize the threshold, and the image will convert to a white background with only the threshold foci visible as black dots (Figure 2D). Test the threshold on several negative control images to ensure that it is appropriate for capturing all PLA foci from image to image before quantitation.
- To quantify the PLA foci, apply the ROI generated from steps 12.3-12.3.1 to the threshold image by selecting the ROI name from the ROI manager window. The outline of the ROI should appear on the image in the same location as it was from the source image (Figure 2E).
- Go to Analyze | Analyze Particles. The Analyze Particles window will open and select the following parameters: size (micron^2) = 0-Infinity, circularity = 0.00-1.00, show = Nothing. Check the Summarize box.
- Select Ok, and a Summary table will appear with information about the ROI (i.e., the total count of PLA foci, total area occupied by the PLA foci inside in the ROI, average size of the PLA foci, and percentage of area occupied by PLA foci relative to the size of the ROI) (inset of Figure 2E). Record these measurements in a spreadsheet.
- Repeat steps 12.1-12.8 for several negative control images using the same threshold.
- Once all negative control images have been analyzed, repeat steps 12.1-12.8 for all experimental samples using the same threshold that was determined by the negative control to identify and quantify PLA foci.
Representative Results
Co-immunostaining of both 3xFLAG::DLC-1; GFP and 3xFLAG::DLC-1; OMA-1::GFP germlines with FLAG and GFP antibodies revealed their patterns of expression in the germline (Figure 3Aii-iii,3Bii-iii). While GFP was expressed throughout the germline (Figure 3Aiii), OMA-1::GFP expression was restricted to the late pachytene and oocytes (Figure 3Biii)27. FLAG immunostaining shows that 3xFLAG::DLC-1 was expressed throughout the germline in both strains (Figure 3Aii,3Bii). By co-immunostaining, the overlap between 3xFLAG::DLC-1 and OMA-1::GFP is indistinguishable from that between 3xFLAG::DLC-1 and GFP (negative control).
Since these experiments tested for interactions between DLC-1 and OMA-1, the region of interest for PLA quantification in the germline encompassed the late pachytene through the oocytes in all germlines examined (Figure 2B), as this is the region of OMA-1 expression (Figure 1, Figure 3Biii). 3xFLAG::DLC-1; OMA-1::GFP germlines appeared to have a greater quantity of PLA foci within this region compared to the 3xFLAG::DLC-1; GFP germlines (Figure 3Ciii-iv,3Diii-iv). Quantification of PLA revealed that the number of PLA foci present in 3xFLAG::DLC-1; OMA-1::GFP germlines was significantly greater than 3xFLAG::DLC-1; GFP (Figure 3Ciii-iv,3Diii-iv; Table 1). Further, even with 10x higher dilution of GFP and FLAG antibodies, the difference between the control and experimental PLA was still significantly different; however, the overall density and average size of foci were reduced (Table 1).
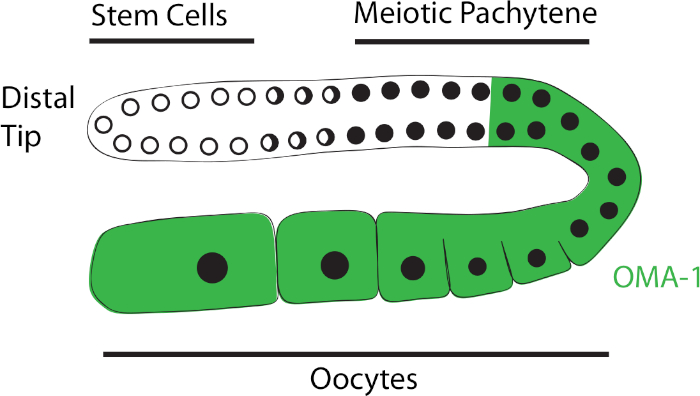
Figure 1: Schematic of C. elegans germline. The distal tip region contains the stem cell pool, which is followed by meiotic pachytene, where cells have switched from mitosis to meiosis. Cells that exit the meiotic pachytene develop into oocytes, with the most mature oocyte at the proximal end. The region shaded in green, which spans from the late meiotic pachytene through all the oocytes, represents the OMA-1 pattern of expression. Please click here to view a larger version of this figure.
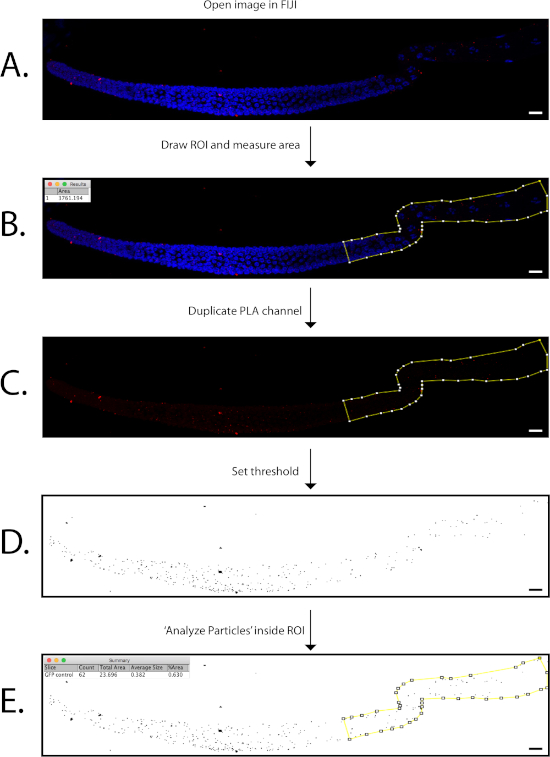
Figure 2: Representative images of workflow for germline PLA quantification. The germline used in this figure is a representative 3xFLAG::DLC-1; GFP germline from Figure 3C. (A) Image of merged PLA and DAPI channels opened in FIJI/ImageJ. (B) The polygon tool in FIJI is used to outline and define the region of interest (ROI) in the germline (yellow line with white boxes) that is quantified, and the area of the ROI (µM2) is measured (inset of B). (C) A single image of the PLA channel is obtained by duplicating or splitting the original image in (A,B). (D) The threshold is carefully set to distinctly highlight all PLA foci in the PLA image. The same threshold must be applied to all experimental and control images that will be analyzed together. (E) With the ROI selected in the threshold image, the Analyze Particles function will return a table of results that includes the total count of foci included inside the ROI (inset of E). Images are snapshots from FIJI/Image J: Plugins | Utilities | Capture Image. Scale bars = 10 µM. Please click here to view a larger version of this figure.
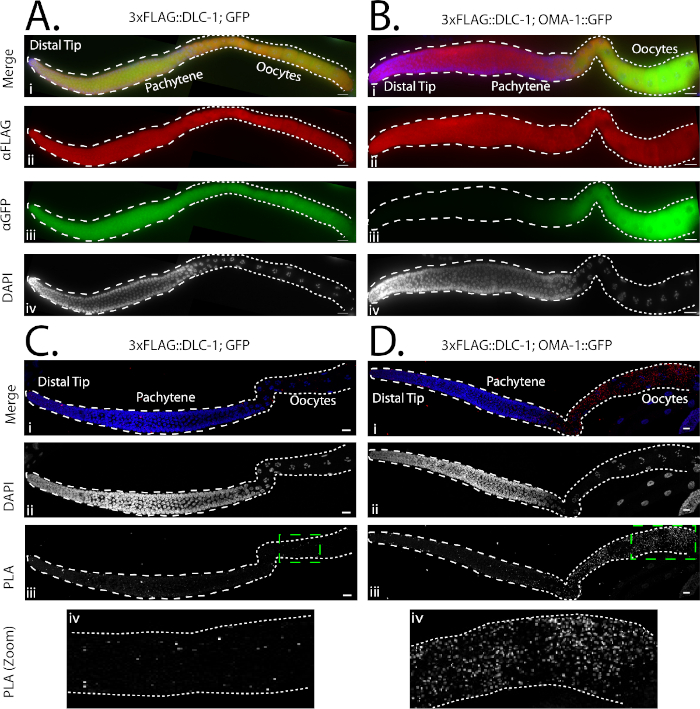
Figure 3: Representative images of germlines following co-immunostaining or PLA. (A,B) The expression patterns of tagged proteins in 3xFLAG::DLC-1; GFP (Ai-iv) and 3xFLAG::DLC-1; OMA-1::GFP (Bi-iv) were evaluated in dissected, fixed, and immunostained gonads. Anti-FLAG antibody was used at a 1:1000 dilution, while anti-GFP antibody was used at a 1:200 dilution, which is optimal for immunofluorescence images. DNA was stained by DAPI, and the individual channel is shown in grayscale for better contrast (Aiv,Biv). In each image, the stem cells and meiotic pachytene are outlined with dashed lines, while the oocytes are outlined with dotted lines. Images were acquired with an epifluorescent microscope. Scale bars = 10 µM. (C,D) PLA in extruded 3xFLAG::DLC-1; GFP (C) and 3xFLAG::DLC-1; OMA-1::GFP (D) gonads. Anti-FLAG antibody was used at a 1:1000 dilution, while anti-GFP antibody was used at a 1:4000 dilution. DNA was stained by DAPI, and both the individual DAPI (Cii,Dii) and PLA channels (Ciii,iv, Diii,iv) are shown in grayscale for better contrast. The green, dashed box (Ciii,Diii) denotes the location of the zoomed-in PLA images (Civ,Div). In each image, the stem cells and meiotic pachytene are outlined with dashed lines, while the oocytes are outlined with dotted lines. Images were acquired with a confocal microscope. Scale bars = 10 µM. (A,B,C,D) were all assembled with image processing software (see Table of Materials). Please click here to view a larger version of this figure.
| Antibody Dilution | Strain Tested | Average PLA Density (foci/µM2) X 10-2 | T test | Average Size of PLA Foci (µM2) | T test |
| αFLAG (1:1000), αGFP (1:4000) | 3xFLAG::DLC-1; GFP | 3.9 ± 1.4 | P = 1.917E-05 | 0.52 ± 0.127 | P = 0.057 |
| 3xFLAG::DLC-1; OMA-1::GFP | 9.1 ± 2.7 | 1.8 ± 2.08 | |||
| αFLAG (1:10,000), αGFP (1:40,000) | 3xFLAG::DLC-1; GFP | 3.2 ± 2.4 | P = 3.395E-04 | 0.51 ± 0.1 | P = 0.019 |
| 3xFLAG::DLC-1; OMA-1::GFP | 7.7 ± 3 | 0.7 ± 0.24 |
Table 1: Summary of PLA results. Table reporting a summary of PLA quantification at two dilutions of primary antibody. The differences in average PLA density or average size of PLA foci for OMA-1::GFP between both antibody titrations were not significant (p-value not shown). The same comparison was also applied to GFP, which also resulted in no significant difference (p-value not shown). The p-values were determined using a two-tailed/equal variance t-test.
Discussion
When studying PPIs in the C. elegans germline, the higher resolution offered by PLA compared to co-immunostaining allows visualization and quantification of locations where interactions occur in the germline. It was previously reported that DLC-1 directly interacts with OMA-1 using an in vitro GST pulldown assay26; however, this interaction was not recovered by an in vivo pulldown. The fluorescent co-immunostaining of 3xFLAG::DLC-1; OMA-1::GFP germlines shows an overlap in the expression patterns for DLC-1 and OMA-1; however, there is no indication of where their interactions occur in the germline, and the overlap itself is not greater than that between 3xFLAG::DLC-1 and GFP that is not fused to any protein (negative control). Using in situ PLA, it was found that DLC-1 does interact with OMA-1 in the germline, which suggests that PLA may be more sensitive for detection of PPIs compared to other approaches. Through this approach we continue to expand upon the emerging role of DLC-1 as an RBP cofactor. This work demonstrates the capability of PLA to detect PPIs in the germline and establishes a reference for future users exploring the interactions between proteins of their own interest.
PLA offers users the ability to test for PPIs with comparable sensitivity without the drawbacks associated with other techniques such as FRET and BiFC. Biologically relevant levels of protein expression may not be optimal for FRET and BiFC. Also, the function of potential interaction partners may be affected by the large tags used in both approaches. Furthermore, FRET assays require a specialized microscopy set-up that may not be readily available. PLA may also be a cost-effective approach to study PPIs compared to other techniques. Users only need to obtain PLA reagents and access to a confocal microscope for imaging in addition to the reagents needed for immunostaining. Image analysis is performed using the open-source program FIJI/ImageJ, which is available to any user at no cost. Users that have no experience with FRET or BiFC may find PLA to be a suitable alternative. The protocol presented here only contains several additional steps beyond a typical immunostaining procedure, making this technique virtually accessible to any user with immunostaining experience.
Extrusion of the gonad by dissection is important for PLA to work successfully. Tissues that are retained inside of the worm cuticle are not labeled by PLA using this protocol. It has been further found that extruded embryos are effectively labeled by this PLA protocol. This suggests that other tissues that are released during dissection, such as the gut, are also likely to be compatible with PLA. It has been found that PLA produces robust signals on gonad as well as embryo samples prepared with two fixation protocols that are often used for immunostaining. This suggests that additional fixation procedures used in the field may be compatible with PLA but will need to be individually evaluated by the user.
Determining the optimal dilution of primary antibodies is critical for successful PLA. It is best to start with the dilution that has been optimized for immunofluorescence. This is typically achieved by titrating the primary antibody in an immunofluorescence experiment to find the optimal dilution where there is low background and a high, specific signal. Once the optimal dilutions for immunofluorescence have been established, these same dilutions can be tested in a PLA assay that compares the signal produced by a pair of potential interactors to the signal produced by a control pair of non-interacting proteins.
In the case in which abundant signal is observed in the control sample, further dilution of primary antibodies is required. It has been found that the optimal primary antibody dilutions for PLA are at least the same or even more dilute than what is used for immunofluorescence. For example, immunofluorescence images in Figure 3A,B are representative of a 1:1000 dilution of anti-FLAG and a 1:200 dilution of anti-GFP. However, the antibody dilutions in PLA images in Figure 3C,D were 1:1000 of anti-FLAG and 1:4000 of anti-GFP. The dilution of anti-GFP antibody used in PLA is greater than what was used for immunofluorescence, suggesting that PLA is much more sensitive. It was found that diluting antibodies 10-fold further resulted in a reduction of PLA density as well as the size of DLC-1/OMA-1 foci (Table 1). Despite this reduction, the difference in PLA density between the negative control and DLC-1/OMA-1 was still significantly different. This suggests that PLA is still very sensitive with higher dilutions of primary antibody; however, the prevalence of detectable interactions will be underestimated.
By contrast, too low of an antibody dilution might have two kinds of detrimental consequences. First, it may produce significant background signal in the negative control. Second, PLA foci produced by the interacting partner proteins might merge and overlap, making them difficult to resolve in a max projection image. This leads to an underestimation of PLA foci number and density during image analysis. PLA signal is a balance of detecting spurious proximity between non-interacting partners and detecting every instance of real PPIs that occur in the sample. As a result, incorporation of a negative control where two proteins do not interact is essential for determining the level of background in PLA experiments. Omission of a primary antibody in a PLA experiment has been used as a negative control in other reports9,10; however, this approach cannot account for nonspecific interactions or nonspecific antibody binding that may impact the result in the experimental PLA. GFP was used here as a negative control, since no direct interaction between DLC-1 and GFP was expected. It was found that the negative control did have some background signal. This further supports the importance of a negative control for a PLA assay when evaluating the experimental data.
Once PLA-optimized dilutions are established, these dilutions can be used to test across an array of different worm strains that contain different pairs of interaction partners tagged with the same affinity tags. It is important to use the same pair of primary antibodies to ensure a fair comparison of resulting PLA signals, as variation in antibody affinity can affect the outcome of a PLA experiment. Another report on PLA suggests optimizing dilution of the PLA secondary antibodies10; however, this is not recommended. Higher dilutions of secondary antibodies may reduce the efficacy of the other downstream PLA steps that depend on recognition of PLUS and MINUS probes that are conjugated to the secondary antibodies.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Some nematode strains used in this study were provided by the Caenorhabditis Genetics Center funded by the NIH (P40OD010440). Confocal microscopy was performed in the University of Montana BioSpectroscopy Core Research Laboratory operated with support from NIH Awards P20GM103546 and S10OD021806. This work was supported in part by the NIH grant GM109053 to E.V., American Heart Association Fellowship 18PRE34070028 to X.W., and Montana Academy of Sciences award to X.W. The funders were not involved in study design or writing the report. We thank M. Ellenbecker for discussion.
Materials
| 16% paraformaldehyde solution | Electron Microscopy services | 15710 | used to make 4% working solution |
| 1M KH2PO4 | Sigma | P0662 | Prepare a 1M working stock |
| 1x M9 | various | various | prepared as 10x stock used at 1x; see wormbook.org for protocol |
| 1x PBS | various | various | see wormbook.org for protocol |
| 26.5 Gauge Needle | Exel International | 26402 | Needles used for dissection |
| BSA | Lampire | 7500802 | |
| Centrifuge Tubes | Thermo Scientific | 05-529C | 50ml Oak ridge centrifuge tube used for synchronization |
| Confocal Microscope | Zeiss | 880 | |
| Coplin Jar | PolyLab | 62101 | |
| Coverslip to Freeze Sample | Globe Scientific | 1411-10 | 22x40mm, No. 1 |
| Coverslip to Seal Slide | Globe Scientific | 1404-15 | 22x22mm, No. 1.5 |
| DAPI Mounting Medium for Immunofluorescence | Vector | H-1200 | |
| Ligase | Sigma-Aldrich | DUO82029 | Duolink 1x Ligase, Comes as part of the Duolink In Situ Detection Reagents Red kit DUO92008 |
| Amplification red buffer | Sigma-Aldrich | DUO82011 | Duolink 5x Amplification Red buffer, Comes as part of the Duolink In Situ Detection Reagents Red kit DUO92008 |
| Ligation Buffer | Sigma-Aldrich | DUO82009 | Duolink 5x Ligation buffer, Comes as part of the Duolink In Situ Detection Reagents Red kit DUO92008 |
| Antibody Diluent | Sigma-Aldrich | DUO82008 | Duolink antibody diluent,Comes with DUO92004 and DUO92002, Note: A 1x PBS/1% BSA solution can also be used as a substitute to dilute the antibody. |
| Blocking Solution | Sigma-Aldrich | DUO82007 | Duolink blocking solution, Comes with DUO92004 and DUO92002 |
| Mounting Medium for PLA | Sigma-Aldrich | DUO82040 | Duolink In Situ mounting medium with DAPI |
| MINUS Probe | Sigma-Aldrich | DUO92004 | Duolink In Situ Probe Anti-Mouse MINUS |
| PLUS Probe | Sigma-Aldrich | DUO92002 | Duolink In Situ Probe Anti-Rabbit PLUS |
| Wash Buffer A | Sigma-Aldrich | DUO82046 | Duolink In Situ wash Buffer A |
| Wash Buffer B | Sigma-Aldrich | DUO82048 | Duolink In Situ wash Buffer B |
| Polymerase | Sigma-Aldrich | DUO82030 | Duolink Polymerase, Comes as part of the Duolink In Situ Detection Reagents Red kit DUO92008 |
| Epifluorescent Microscope | Leica | DFC300G camera, DM5500B microscope | |
| Goat anti-mouse Alexa 594 | JacksonImmuno | 115-585-146 | Use at 1:500 |
| Goat anti-rabbit Alexa 488 | JacksonImmuno | 111-545-144 | Use at 1:200 |
| Image Processing Software | Adobe | Adobe Photoshop + Illsutrator CS3 | |
| Glass Pipette | Corning | 7095B-5X | |
| Levamisole | ACROS Organics | 187870100 | Prepare a 250mM working stock |
| Methanol | Fisher Scientific | A454 | |
| Mouse anti-FLAG | Sigma | F1804 | Use at 1:1000 for immunofluorescence and PLA, pre-block with normal goat serum recommended |
| Nailpolish | L.A. colors | CNP195 | |
| Nematode Growth Medium (NGM) | various | See wormbook.org for protocol | |
| Normal Goat Serum | JacksonImmuno | 005-000-121 | |
| Polyethylene Pasteur Pipette | Globe Scientific | 135030 | |
| Poly-L-Lysine | Sigma-Aldrich | P1524 | Prepared as 0.1% stock solution in water, stored at -20C, and diluted 1:100 in water to coat slides |
| Petri Dishes | Tritech | PD7060 | 60 mm diameter |
| Rabbit anti-GFP | Thermo Fisher | G10362 | Use at 1:200 for immunofluorescence, 1:4000 for PLA |
| Slides | Thermo Fisher | 30-2066A-Brown | Three-square 14x14mm autoclavable slides with bars are custom-ordered through Fisher Scientific. Poly-L-Lysine added to slides in the lab |
| Sodium Hypochlorite solution | Fisher Scientific | SS290-1 | |
| task wipes | Kimtech | 34120 | 4.4×8.4 inch task wipes |
| Trays (242x241x20mm) | Thermo Fisher | 240845 | Used to make humid chamber |
| Triton X-100 | ACROS Organics | 327372500 | |
| Ultrapure water | Milli-Q | Ultrapure water obtained from Milli-Q Integral Water Purification System | |
| Watchglass | Carolina Biological | 742300 | |
| -20 °C freezer | |||
| -80 °C freezer | |||
| Aluminum Foil | |||
| OP50 strain E. coli | |||
| Orbital Shaker | |||
| Tape | |||
| Nematode strains used in this study (both available upon request) | |||
| mntSi13[pME4.1] II; unc-119(ed3) III; teIs1 [pRL475] | UMT 376 | dlc-1 prom::3xFLAG::dlc-1::dlc-1 3'UTR; oma-1 prom::oma-1::GFP; Reference 24 | |
| mntSi13[pME4.1] II; mntSi21[pXW6.22] unc-119(ed3) III | UMT 422 | dlc-1 prom::3xFLAG::dlc-1::dlc-1 3'UTR; gld-1 prom::ceGFP::fbf-1 3'UTR + unc-119(+); Reference: this study |
References
- Berggård, T., Linse, S., James, P. Methods for the detection and analysis of protein-protein interactions. Proteomics. 7 (16), 2833-2842 (2007).
- Nooren, I. M., Thornton, J. M. Diversity of protein-protein interactions. EMBO Journal. 22 (14), 3486-3492 (2003).
- Patil, A., Kinosselecta, K., Nakamura, H. Hub promiscuity in protein-protein interaction networks. International Journal of Molecular Science. 11 (4), 1930-1943 (2010).
- De Las Rivas, J., Fontanillo, C. Protein-protein interactions essentials: key concepts to building and analyzing interactome networks. PLoS Computational Biology. 6 (6), e1000807 (2010).
- Pazdernik, N., Schedl, T. Introduction to germ cell development in Caenorhabditis elegans. Advances in Experimental Medicine and Biology. 757, 1-16 (2013).
- Nousch, M., Eckmann, C. R. Translational control in the Caenorhabditis elegans germ line. Advances in Experimental Medicine and Biology. 757, 205-247 (2013).
- Vangindertael, J., et al. An introduction to optical super-resolution microscopy for the adventurous biologist. Methods and Applications in Fluorescence. 6 (2), 022003 (2018).
- Veeraraghavan, R., Gourdie, R. G. Stochastic optical reconstruction microscopy-based relative localization analysis (STORM-RLA) for quantitative nanoscale assessment of spatial protein organization. Molecular Biology of the Cell. 27 (22), 3583-3590 (2016).
- Thymiakou, E., Episkopou, V. Detection of signaling effector-complexes downstream of bmp4 using PLA, a proximity ligation assay. Journal of Visualized Experiments. (49), (2011).
- Wang, S., et al. Detection of in situ protein-protein complexes at the Drosophila larval neuromuscular junction using proximity ligation assay. Journal of Visualized Experiments. (95), 52139 (2015).
- Algar, W. R., Hildebrandt, N., Vogel, S. S., Medintz, I. L. FRET as a biomolecular research tool – understanding its potential while avoiding pitfalls. Nature Methods. 16 (9), 815-829 (2019).
- Kodama, Y., Hu, C. D. Bimolecular fluorescence complementation (BiFC): a 5-year update and future perspectives. Biotechniques. 53 (5), 285-298 (2012).
- Piston, D. W., Kremers, G. J. Fluorescent protein FRET: the good, the bad and the ugly. Trends in Biochemical Science. 32 (9), 407-414 (2007).
- Hiatt, S. M., Shyu, Y. J., Duren, H. M., Hu, C. D. Bimolecular fluorescence complementation (BiFC) analysis of protein interactions in Caenorhabditis elegans. Methods. 45 (3), 185-191 (2008).
- Söderberg, O., et al. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods. 45 (3), 227-232 (2008).
- Wilson, M. J., Salata, M. W., Susalka, S. J., Pfister, K. K. Light chains of mammalian cytoplasmic dynein: identification and characterization of a family of LC8 light chains. Cell Motility and Cytoskeleton. 49 (4), 229-240 (2001).
- Erdős, G., et al. Novel linear motif filtering protocol reveals the role of the LC8 dynein light chain in the Hippo pathway. PLoS Computational Biology. 13 (12), e1005885 (2017).
- Navarro-Lérida, I., et al. Proteomic identification of brain proteins that interact with dynein light chain LC8. Proteomics. 4 (2), 339-346 (2004).
- Rapali, P., et al. DYNLL/LC8: a light chain subunit of the dynein motor complex and beyond. FEBS Journal. 278 (17), 2980-2996 (2011).
- Rapali, P., et al. Directed evolution reveals the binding motif preference of the LC8/DYNLL hub protein and predicts large numbers of novel binders in the human proteome. PLoS One. 6 (4), e18818 (2011).
- Clark, S. A., Jespersen, N., Woodward, C., Barbar, E. Multivalent IDP assemblies: Unique properties of LC8-associated, IDP duplex scaffolds. FEBS Letters. 589 (19 Pt A), 2543-2551 (2015).
- Jespersen, N., et al. Systematic identification of recognition motifs for the hub protein LC8. Life Science Alliance. 2 (4), (2019).
- Wang, X., et al. Dynein light chain DLC-1 promotes localization and function of the PUF protein FBF-2 in germline progenitor cells. Development. 143 (24), 4643-4653 (2016).
- Dorsett, M., Schedl, T. A role for dynein in the inhibition of germ cell proliferative fate. Molecular Biology of the Cell. 29 (22), 6128-6139 (2009).
- Ellenbecker, M., et al. Dynein Light Chain DLC-1 Facilitates the Function of the Germline Cell Fate Regulator GLD-1 in Caenorhabditis elegans. Génétique. 211 (2), 665-681 (2019).
- Day, N. J., Ellenbecker, M., Voronina, E. Caenorhabditis elegans DLC-1 associates with ribonucleoprotein complexes to promote mRNA regulation. FEBS Letters. 592 (22), 3683-3695 (2018).
- Detwiler, M. R., Reuben, M., Li, X., Rogers, E., Lin, R. Two zinc finger proteins, OMA-1 and OMA-2, are redundantly required for oocyte maturation in C. elegans. Developmental Cell. 1 (2), 187-199 (2001).
- Spike, C. A., et al. Translational control of the oogenic program by components of OMA ribonucleoprotein particles in Caenorhabditis elegans. Génétique. 198 (4), 1513-1533 (2014).
- Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A., Cerón, J. Basic Caenorhabditis elegans methods: synchronization and observation. Journal of Visualized Experiments. (64), (2012).
- Gervaise, A. L., Arur, S. Spatial and Temporal Analysis of Active ERK in the C. elegans Germline. Journal of Visualized Experiments. (117), (2016).
- Duerr, J. S. Antibody staining in C. elegans using “freeze-cracking”. Journal of Visualized Experiments. (80), (2013).
- Crittenden, S., Kimble, J. Preparation and immunolabeling of Caenorhabditis elegans. Cold Spring Harbor Protocols. (5), (2009).

