Click-Chemistry Based Fluorometric Assay for Apolipoprotein N-acyltransferase from Enzyme Characterization to High-Throughput Screening
Summary
Presented here is a sensitive fluorescence assay to monitor apolipoprotein N-acyltransferase activity using diacylglyceryl peptide and alkyne-phospholipids as substrates with click-chemistry.
Abstract
Lipoproteins from proteobacteria are posttranslationally modified by fatty acids derived from membrane phospholipids by the action of three integral membrane enzymes, resulting in triacylated proteins. The first step in the lipoprotein modification pathway involves the transfer of a diacylglyceryl group from phosphatidylglycerol onto the prolipoprotein, resulting in diacylglyceryl prolipoprotein. In the second step, the signal peptide of prolipoprotein is cleaved, forming an apolipoprotein, which in turn is modified by a third fatty acid derived from a phospholipid. This last step is catalyzed by apolipoprotein N-acyltransferase (Lnt). The lipoprotein modification pathway is essential in most γ-proteobacteria, making it a potential target for the development of novel antibacterial agents. Described here is a sensitive assay for Lnt that is compatible with high-throughput screening of small inhibitory molecules. The enzyme and substrates are membrane-embedded molecules; therefore, the development of an in vitro test is not straightforward. This includes the purification of the active enzyme in the presence of detergent, the availability of alkyne-phospholipids and diacylglyceryl peptide substrates, and the reaction conditions in mixed micelles. Furthermore, in order to use the activity test in a high-throughput screening (HTS) setup, direct readout of the reaction product is preferred over coupled enzymatic reactions. In this fluorometric enzyme assay, the alkyne-triacylated peptide product is rendered fluorescent through a click-chemistry reaction and detected in a multiwell plate format. This method is applicable to other acyltransferases that use fatty acid-containing substrates, including phospholipids and acyl-CoA.
Introduction
Bacterial lipoproteins are characterized by covalently bound fatty acids at their amino-termini through which they are anchored into membranes1,2. The mature part of the protein is highly diverse in structure and function, thereby explaining the role of lipoproteins in various biological processes in the bacterial cell envelope.
Lipoproteins are modified by phospholipid-derived fatty acids after insertion into the cytoplasmic membrane. The prolipoproteins contain a signature motif, the lipobox, which contains an invariant cysteine residue that becomes acylated and the first amino acid in the mature protein. The first step of this pathway is catalyzed by prolipoprotein phosphatidylglycerol::diacylglyceryl transferase (Lgt), which transfers the diacylglyceryl group from phosphatidylglycerol onto the prolipoprotein via a thioether link between diacylglyceryl and cysteine. Signal peptidase II (Lsp) cleaves the signal peptide from diacylglyceryl prolipoprotein, resulting in an apolipoprotein that is anchored into the membrane through its diacylglyceryl moiety. The third and last step is catalyzed by apolipoprotein N-acyltransferase (Lnt), which adds a fatty acid from the sn-1 position of phospholipid onto apolipoprotein, resulting in triacylated mature lipoprotein (Figure 1)3. The Lnt reaction is a two-step ping-pong reaction where a stable thioester acyl enzyme intermediate is formed. The lysophospholipid byproduct is released prior to the acylation of the apolipoprotein substrate in the second step of the reaction.
The phospholipid substrate specificity is determined in a Lnt assay based on the mobility shift of N-acyl diacylglyceryl peptide on a high percentage Tris-Tricine Urea SDS-PAGE4. Phospholipids with small polar headgroups, saturated [sn-1] and nonsaturated [sn-2], were preferred substrates4. The gel shift assay is not appropriate for extensive kinetic studies of apolipoprotein N-acyltransferase nor for HTS to identify inhibitory molecules. Click-chemistry using alkyne fatty acids has been successfully used to study lipoprotein modification in bacteria5 and fatty acid metabolism in eukaryotes6. Recently, an in vitro assay of Ras palmitoylation was reported to identify inhibitors7.
In the method described here, purified active Lnt in detergent is incubated with substrates in mixed micelles to form alkyne-triacylated peptide that is subsequently detected by fluorescence spectrometry.
Protocol
1. Enzyme and substrate preparation
- Purification of enzyme
- Produce and purify Lnt enzyme from detergent solubilized membranes as described previously4,8. Briefly, induce expression of the lnt-strep gene, encoding Lnt with a C-terminal Strep tag, at OD600 of 0.6 with anhydrous tetracycline (200 ng/mL) at 37 °C for 16 h.
- Harvest cells by centrifugation at 4,000 x g for 10 min and discard the supernatant.
- Resuspend the cell pellet in buffer WA (20 mM Tris-HCl pH 8, 150 mM NaCl, 0.5 mM EDTA) to 100 OD600 units per mL.
- Break the cells by two passages through a French pressure cell press at 10,000 psi.
- Remove unbroken cells and debris by centrifugation at 13,000 x g for 20 min. Keep the supernatant and discard the pellet.
- Centrifuge the supernatant at 120,000 x g for 60 min and collect the membrane vesicles (translucent brown colored pellet). Discard the supernatant.
- Solubilize the integral membrane proteins from the membrane pellet with 1% (w/v) n-Dodecyl β-D-maltoside (DDM) in buffer WA for 30 min at room temperature (RT). Centrifuge and remove insolubilized material at 120,000 x g for 30 min. Use the supernatant fraction for purification.
- Purify Lnt-strep on an affinity chromatography column (see Table of Materials) and an S400 gel filtration column (see Table of Materials) as described by Nozeret et al.8.
- Store the purified enzyme in buffer WA containing 0.05% DDM and 10% glycerol at -80 °C.
NOTE: The enzyme is stable for over 5 years.
- Preparation of alkyne-phospholipid and biotinylated Fibroblast Stimulating Ligand (FSL-1-biotin) substrates
- Aliquot 100 µL of custom-synthesized alkyne-POPE (1-hexadec-15-ynoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine, see Table of Materials) solubilized in chloroform into 1.5 mL tubes.
- Pierce the tubes with a syringe and evaporate the chloroform in a speed-vac at RT for 2 h. Store the dry phospholipid samples at -20 °C.
- Dissolve alkyne-POPE in 0.1% Triton X-100 at 500 µM prior to use. Add a 3 min sonication step in an ultrasonic water bath at RT to solubilize alkyne-POPE if necessary.
- Resuspend FSL-1-biotin in water at 445 µM.
NOTE: Both solutions can be stored at -20 °C for at least 2 months.
2. Tube assay
NOTE: On Day 1, set up the Lnt reaction in 1.5 mL tubes (step 2.1) and coat 96 well plates with streptavidin (step 2.2).
- Preparation of reagent mixture and enzymatic reaction
- For a standard Lnt assay, mix alkyne-POPE (final 50 µM from 500 µM stock) and FSL-1-biotin (final concentration of 50 µM from a 445 µM stock) in Lnt reaction buffer (50 mM Tris-HCl pH 7.2, 150 mM NaCl, 0.1% Triton X-100 containing 1% BSA) at a final volume of 18 µL in 1.5 mL tubes. Perform all conditions in triplicate.
- Sonicate the substrate mixture (prepared in step 2.1.1) for 3 min in an ultrasonic water bath and incubate at 37 °C for 5 min prior to the addition of Lnt enzyme (step 2.1.3).
NOTE: MTSES (sodium (2-sulfonatoethyl)methanethiosulfonate), a thiol-specific reagent, inhibits Lnt8. Add 10 mM MTSES (100 mM stock solution in 100% DMSO) to the reaction tubes (step 2.1.1) to be used as negative control samples. Adjust the volume of the Lnt reaction buffer so the total volume is 18 µL. - Add active (1 ng/µL, corresponding to 17.2 nM) or inactive Lnt (C387S) (2 µL of 10 ng/μL stock in buffer WA containing 0.05% DDM) and mix with the substrates (step 2.1.2) by pipetting up and down.
- Incubate the reaction at 37 °C for 16 h in a thermomixer with heated lid.
- Streptavidin coating of 96 well plates
- Prepare a stock solution of streptavidin at 2 mg/mL in H2O.
NOTE: This solution can be stored at -20 °C for up to 6 months. - From the stock solution prepare 10 µg/mL streptavidin in water as a working solution. Add 100 µL of the solution to each well of the 96 well plate. Bind streptavidin to the plate by incubating at 37 °C overnight without a lid to air-dry the wells.
- Prepare a stock solution of streptavidin at 2 mg/mL in H2O.
NOTE: On Day 2 perform click-chemistry (step 2.3), bind Lnt reaction mixture to streptavidin-coated plates (step 2.4), and detect fluorescence and analyze results (step 2.5).
- Click-chemistry reaction
- Prepare stock solutions of Azido-FAM (5 mM in DMSO), TCEP (Tris(2-carboxyethyl)phosphine hydrochloride; 50 mM prepared in water), TBTA (Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine; 2 mM in tert-butanol:DMSO (4:1)), and CuSO4∙5H2O (50 mM freshly prepared in H2O).
- In the 1.5 mL tubes containing the Lnt reaction mixture prepared in step 2.1.4, perform click-chemistry by adding the reagents in the following order: 0.2 µL of stock Azido-FAM (final concentration 50 µM), 0.4 µL of stock TCEP (final concentration 1 mM), 0.2 µL of stock TBTA (final concentration 0.02 mM).
- Vortex the solution for 5 s. Then add 0.4 µL of stock CuSO4 (final concentration 1 mM). Vortex again for 5 s. Incubate the samples in the dark at RT for 1 h.
- Binding of Lnt reaction mixture to streptavidin-coated plates
- Wash the wells of the streptavidin-coated plates after the overnight incubation from step 2.2.2 3x with 200 µL of PBS-T1 (PBS containing 0.05% Tween-20).
NOTE: Streptavidin plates can be used immediately or stored dry at 4 °C. - Transfer 18 µL from the click-chemistry mixture from step 2.3.3 to a well in a 96 well streptavidin-coated plate and add 100 µL of PBS-T1 to bind N-acyl-FSL-1-biotin-FAM product to streptavidin plates.
- Use biotin-fluorescein (0.26 µM from a 3.1 mM stock in DMSO) as positive control for streptavidin binding and fluorescence readout.
- Incubate the plates at RT for 1 h in the dark in a thermomixer, shaking at 300 rpm.
- Perform manual wash steps of the 96 well plates with a multichannel electronic pipette as follows: six washes with 200 µL of PBS-T2 (PBS containing 1% Tween-20), three flushes with a pipette, three washes with 200 µL of PBS, three flushes with a pipette.
- Wash the wells of the streptavidin-coated plates after the overnight incubation from step 2.2.2 3x with 200 µL of PBS-T1 (PBS containing 0.05% Tween-20).
- Fluorescence detection and analysis
- Add 200 µL of PBS and record the fluorescence at 520 nm in a fluorescence microplate reader. Save results in spreadsheet software.
NOTE: Biotin-fluorescein is used as a positive control for streptavidin binding and fluorescence detection at 520 nm. A reproducible readout of 30,000–40,000 A.U. with 0.26 µM biotin-fluorescein is expected. All samples are analyzed in triplicate, including controls. - Calculate the standard deviation for each reaction and the calculate P-values for negative controls and positive samples using unpaired t-test using a statistical software.
- Add 200 µL of PBS and record the fluorescence at 520 nm in a fluorescence microplate reader. Save results in spreadsheet software.
3. Multiwell plate assay
NOTE: On Day 1 set up the Lnt reaction in 384 well plates (step 3.1) and coat 384 well plates with streptavidin (step 3.2).
- Preparation of reagent mixture and enzymatic reaction
NOTE: The quantity of the reagents and enzyme is reduced compared to the tube assay and the Lnt reaction is performed directly in 384 well plates. This allows the use of less material and a reduction of steps that can be further automated.- Mix the substrates as follows: alkyne-POPE (final concentration 50 µM from 500 µM stock) and FSL-1-biotin (final 50 µM from 500 µM stock) in Lnt reaction buffer (50 mM Tris-HCl pH 7.2, 150 mM NaCl, 0.1% Triton X-100 containing 1% BSA) at a volume of 13.5 µL per reaction per well in a 384 well plate format. Perform all conditions in triplicate. Calculate the total amount of reagents required for the number of reactions to be performed (i.e., 5,184 µL for one 384 well plate).
- Sonicate the substrate mixture for 3 min in an ultrasonic water bath.
- Aliquot 13.5 µL of the substrate mixture per well in a 384 well plate.
NOTE: As a negative control, 10 mM of MTSES (625 mM stock solution in 100% DMSO) can be added per well. Adjust the volume of Lnt buffer (step 3.1.1) to reach a final reaction volume of 13.5 µL. - Incubate at 37 °C for 5 min prior to the addition of Lnt enzyme (step 3.1.5).
- Add 0.5 ng/µL (corresponding to 8.6 nM) active Lnt enzyme, or inactive variant (C387S) (1.5 µL from 5 ng/µL stock in buffer WA containing 0.05% DDM) in Lnt reaction buffer (50 mM Tris-HCl pH 7.2, 150 mM NaCl, 0.1% Triton X-100, 1% BSA) to each well containing the reaction mixture from step 3.1.3. Mix reagents by pipetting up and down. The total reaction volume is 15 µL.
- Seal the plate with plastic foil for multiwell plates.
- Incubate at 37 °C for 16 h in a thermomixer with heated lid.
- Streptavidin coating 384 well plates
- Use 75 µL of streptavidin (10 µg/mL in H2O) from step 2.2.2 to coat wells of a 384 well plate. Let streptavidin bind to the plate by incubating at 37 °C overnight without a lid to air-dry the wells.
NOTE: On Day 2 perform click-chemistry (step 3.3), bind Lnt reaction mixture to streptavidin-coated plates (step 3.4), detect fluorescence, and analyze results (step 3.5).
- Click-chemistry reaction
- Prepare stock solutions of Azido-FAM (1.2 mM in DMSO), TCEP (tris(2-carboxyethyl)phosphine hydrochloride; 24 mM prepared in H2O), TBTA (Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine; 0.48 mM in tert-butanol:DMSO (4:1)) and CuSO4∙5H2O (24 mM freshly prepared in H2O).
- Combine the three click-chemistry reagents: a mixture of Azido-FAM (final concentration 50 µM), TCEP (1 mM final), and TBTA (0.02 mM final), each at 0.75 µL in final volume of 2.25 µL per well. Calculate the required volume per 384 well plate (i.e., use 864 µL when all wells of the 384 well plate are used). Mix vigorously.
- Add 2.25 µL of the reagent solution per well directly to the completed Lnt reaction in the 384 well plate from step 3.1.7.
- Mix by pipetting up and down with a multichannel electronic pipette.
- Add CuSO4 (0.75 µL from 24 mM stock for a 1 mM final concentration) and mix by pipetting up and down with a multichannel electronic pipette.
- Incubate at RT for 1 h in the dark.
- Binding of Lnt reaction mixture to streptavidin-coated 384 well plates
- Wash the wells of the streptavidin-coated plates after the overnight incubation from step 3.2.1 1x with 85 µL PBS-T1.
- Transfer 11 µL of the click-chemistry reaction from each well of the incubation plate from step 3.3.6 to the streptavidin-coated plate from step 3.4.1 to bind the N-acyl-FSL-1-biotin-FAM product to the streptavidin plates.
- Add 64 µL of buffer PBS-T1.
- Include biotin-fluorescein (0.19 µM from a 3.1 mM stock in DMSO) as a control for binding to streptavidin-coated wells.
- Incubate the 384 well plates at RT for 1 h in the dark in a thermomixer, shaking at 300 rpm.
- Wash the plates using an automated plate washer as follows: 10 washes with 85 µL PBS-T2, plates are shaken between washes; 5 washes with 85 µL PBS, plates are shaken between washes.
- Fluorescence detection and analysis
- Add PBS (85 µL) and record fluorescence in a fluorescence microplate reader at 535 nm.
- Analyze data as described (step 2.5.2).
Representative Results
In the Lnt reaction the sn-1 fatty acid from phospholipids is transferred onto a diacylglyceryl peptide, resulting in mature triacylated peptide8. The in vitro Lnt assay described here is designed to use phospholipids containing an alkyne fatty acid (alkyne-POPE) and FSL-1-biotin as substrates, resulting in the formation of alkyne-FSL-1-biotin. Upon a click-chemistry reaction with azido-FAM, this product should become fluorescently labeled and detected by fluorescence spectrometry (Figure 2).
The reaction conditions were optimized for maximum fluorescence readout at 520 nm in a plate reader. At 1 ng/µL enzyme, complete conversion of FSL-1-biotin was observed8 (Figure 3). Negative controls included reactions without enzyme, with an inactive variant of the enzyme (active site mutant C387S), or with thiol-specific inhibitor MTSES that result in low fluorescence detection. Biotin-fluorescein bound efficiently to streptavidin-coated plates and was used as an internal control for maximum fluorescence signal. All experiments were performed in triplicate and standard deviation calculations and statistical analysis showed that the assay was sensitive and reproducible.
In order to develop an HTS assay to screen for small molecule inhibitors of Lnt, the quantity of reagents was reduced, and the reactions were performed directly in 384 well plates. Furthermore, wash steps were automated using a plate washer (see Table of Materials). As for the tube assay, the reaction was sensitive and reproducible, with a significant difference between the negative control (C387S) and active enzyme (Lnt) (Figure 4). In HTS the Z’ factor determines whether a response in an assay is large enough for screening purposes9 and is calculated using the following equation:

Where S is the average signal, B is background signal, and σ is standard deviation.
The average Z’ factor was >0.6 for the Lnt assay performed in HTS format using 384 well plates, suggesting that the assay for screening of small molecule for Lnt inhibition was outstanding.

Figure 1: Enzymatic reactions in posttranslational modification of lipoprotein in proteobacteria. The sequential steps were catalyzed by Lgt10, Lsp11, and Lnt12,13,14 in the cytoplasmic membrane. PGN: peptidoglycan, SP: signal peptide, LB: lipobox, conserved motif containing Cys+1 and modified with fatty acids and the first amino acid in mature lipoprotein, PG: phosphatidylglycerol, G-1-P: glycerol-1-phosphate, PE: phosphatidylethanolamine, lysoPE: lyso- phosphatidylethanolamine. Please click here to view a larger version of this figure.

Figure 2: Schematic of the fluorometric enzyme assay for apolipoprotein N-acyltransferase (Lnt). The substrates FSL-1-biotin (yellow) and alkyne-POPE (blue) were mixed with purified Lnt enzyme solubilized in detergent. The reaction was performed in mixed micelles at 37 °C (step 1). The alkyne-FSL-1-biotin product was labeled with fluorescein (orange) by click-chemistry (step 2) and detected in a fluorescence plate reader upon binding to streptavidin-coated plates (step 3). The figure is a modified version of Figure 1B published by Nozeret et al.8 according to the Creative Commons license (http://creativecommons.org/licenses/by/4.0/). Please click here to view a larger version of this figure.

Figure 3: Fluorescence detection of Lnt activity in 96 well format. Lnt reactions were performed in tubes at 37 °C for 16 h. After fluorescent labeling by click-chemistry and binding to streptavidin-coated plates, fluorescence was measured by fluorescence spectrometry. Biotin-fluorescein (biotin-fluo 0.26 µM) was used as control for fluorescence. Buffer was reaction buffer only. Samples indicating alkyne-POPE (50 µM), FSL-1-biotin (50 µM), and substrates (mix of alkyne-POPE and FSL-1-biotin, 50 µM each) did not contain enzyme. Negative controls included inhibition with MTSES (10 mM) and an inactive variant of Lnt (C387S). Lnt enzyme was added at 1 ng/μL. Standard deviations were calculated for n = 3 experiments. ** P-value < 0.005. Excitation at 494 nm, bandwidth 5 nm and emission at 520 nm, bandwidth 5 nm. Please click here to view a larger version of this figure.
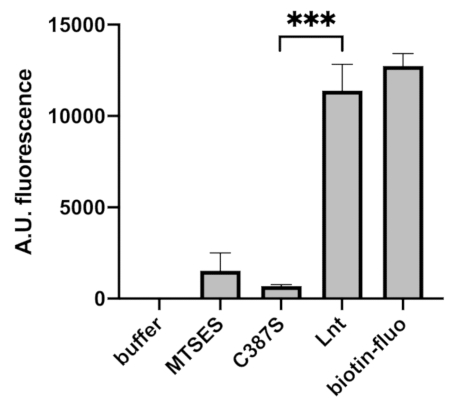
Figure 4: Fluorescence readout of the Lnt reaction in 384 well plate compatible with HTS. Enzymatic Lnt reactions were performed in 384 well plates at 37 °C. Biotin-fluorescein (biotin-fluo 0.19 µM) was used as control for fluorescence. Buffer was reaction buffer containing DMSO. Negative controls included inhibition with MTSES (10 mM) and an inactive variant of Lnt (C387S). Lnt enzyme was added at 0.5 ng/µL. Standard deviations were calculated for n = 3 experiments. *** P-value < 0.0005. Excitation at 485 nm, bandwidth 20 nm and emission at 535 nm, bandwidth 25 nm. Please click here to view a larger version of this figure.
Discussion
The protocol for the Lnt assay described here, based on fluorescence detection of the triacylated product, is sensitive and reproducible. The specific and efficient binding of biotin to streptavidin is a key element in the assay. Alkyne-POPE substrate left after completion of the Lnt reaction is also fluorescently labeled with FAM but is efficiently removed after binding onto the streptavidin plates by multiple wash steps. Furthermore, addition of DMSO does not affect Lnt activity and has no impact on the assay. Both substrate and enzyme are highly lipophilic and require reaction conditions in mixed micelles. Techniques that depend on soluble enzymes and substrates for product detection, including fluorescence polarization, are not applicable8. The only limitation of the assay is the compatibility of the enzyme with alkyne substrate, because the click-chemistry reaction is dependent on this chemical group.
Gel shift assays have been reported in previous studies to analyze Lnt activity and to determine substrate specificity4. With the current protocol, and in parallel with fluorescence spectrometry, in-gel fluorescence detection can be used to monitor Lnt activity8, although this format is not suitable for HTS. The tube assay is particularly interesting for kinetic and comparative studies of Lnt mutants. Phospholipid substrate specificity can be addressed using alkyne-phospholipids obtained by metabolic labeling of bacteria with alkyne fatty acids composed of various chain lengths and degree of saturation as described8.
The 384 well plate format is compatible with HTS studies because a significant difference is observed between active enzyme and a substrate only control. An average Z’ factor of >0.6 was calculated with the optimized conditions presented here. The high reproducibility of the assay contributes to the high Z’ factor. For successful HTS a Z’ factor above 0.6 is recommended9. Wash steps are efficient with the use of an automated plate washer. Other steps can be further optimized, including pipetting of reagents, which would allow screening of large libraries of small molecules.
The protocol is applicable to other acyltransferases that use fatty acid-containing substrates if alkyne groups are compatible with substrate recognition by the enzyme. A recent study on the identification of specific inhibitors of eukaryotic Palmitoyl Acyl Transferase (PAT) describes using membrane-bound enzyme and alkyne-acyl-CoA as substrate7. Palmitoylation of a small biotinylated Ras peptide was observed by click-chemistry fluorogenic detection. A pilot screen in 384 well plate format of this assay identified specific inhibitors of PAT, suggesting that substrates composed of alkyne fatty acid group combined with click-chemistry and sensitive fluorescence detection is a promising method for target-based HTS.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Fabrice Agou and Alix Boucharlat from the Chemogenomic and Biological Screening Platform, Center for Technological Resources and Research (C2RT) at Institut Pasteur Paris for helpful suggestions on the protocol, all members of the BGPB Unit for support and scientific discussions, and Simon Legood for critical reading of the manuscript. Work was funded by Global Care Initiatives of the Institute Carnot Infectious diseases and the Institute Carnot Microbes and Health (15 CARN 0017-01 and 16 CARN 0023-01).
Materials
| Äkta Purifier FPLC system | GE Healthcare | NA | Purity: NA Lnt purification |
| alkyne-POPE | Avanti Polar Lipids | 900414P | Purity: >99% Lnt substrate |
| Azido-FAM | Lumiprobe | A4130 | Purity: 100% (pure) Click reagent |
| BioPhotometer Plus | Eppendorf | N/A | Purity: NA OD 600 nm |
| BioTek ELX 405 Select plate washer | BioTek | N° serie 115800, n° materiel 405 Select | Purity: NA Wash steps |
| biotin-fluorescein | Sigma | 53608 | Purity: ≥90% Fluorescence control |
| Copper(II) sulfate pentahydrate | Sigma | C3036 | Purity: ≥98% Click reagent |
| DDM | Anatrace | D310A | Purity: ≥ 99% β+α; < 15% α Detergent for Lnt purification |
| Dimethylsulfoxide (DMSO) | Invitrogen | D12435 | Purity: anhydrous Solbilization Click reagent |
| Electronic pipet Voyager 8 channels 0.5-12.5 uL | INTEGRA | 4721 | Purity: NA Handling reagents |
| French Pressure Cell | N/A | N/A | Purity: NA Cell disruption |
| FSL-1-biotin | EMC microcollections | L7030 | Purity: NA Lnt substrate |
| Greiner Bio-One 384-well standard CELLSTAR polystyrene microplate | Greiner | 781091 | Purity: NA Black with transparent bottom (up or bottom reading) |
| Greiner Bio-One 96-well sterile polystyrene plate, high binding | Greiner | 655097 | Purity: NA Black with transparent bottom (up or bottom reading) |
| Microplate reader Infinite M1000 pro | Tecan | N/A | Purity: NA Fluorescence detection |
| MTSES (sodium (2-sulfonatoethyl)methanethiosulfonate) | Anatrace | S110MT | Purity: ~100% Thiol specific inhibitor |
| Optically Clear Adhesive Seal Sheets | Thermo Scientific | AB-1170 | Purity: NA Foil to seal multi-well plate |
| Sephacryl S400 HR 16/60 gel filtration column | GE Healthcare | GE28-9356-04 | Purity: NA Lnt purification |
| StrepTactin Sepharose 50 % | IBA Biotechnology | 2-1201-010 | Purity: NA Lnt purification |
| streptavidin | Sigma | S4762-1MG | Purity: ≥13 units/mg protein Biotin binding |
| TBTA (tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine | Sigma | 678937 | Purity: 97% Click reagent |
| TCEP (tris(2-carboxyethyl)phosphine hydrochloride | Sigma | 75259 | Purity: ≥98% Click reagent |
| TECAN Infinite F500 | Tecan | N/A | Purity: NA Fluorescence detection |
| TECAN Infinite M1000 pro | Tecan | N/A | Purity: NA Fluorescence detection |
| Thermomixer C | Eppendorf | 5382000015 | Purity: NA Heated lid |
| Triton X-100 | Sigma | 93443 | Purity: 10% in H2O Lnt reaction buffer |
| Ultra centrifuge | Beckman LC | N/A | Purity: NA Cell fractionation |
References
- Buddelmeijer, N. The molecular mechanism of bacterial lipoprotein modification–how, when and why. FEMS Microbiology Reviews. 39 (2), 246-261 (2015).
- Kovacs-Simon, A., Titball, R. W., Michell, S. L. Lipoproteins of bacterial pathogens. Infections and Immunity. 79 (2), 548-561 (2010).
- Jackowski, S., Rock, C. O. Transfer of fatty acids from the 1-position of phosphatidylethanolamine to the major outer membrane lipoprotein of Escherichia coli. Journal of Biological Chemistry. 261, 11328-11333 (1986).
- Hillmann, F., Argentini, M., Buddelmeijer, N. Kinetics and phospholipid specificity of apolipoprotein N-acyltransferase. Journal of Biological Chemistry. 286 (32), 27936-27946 (2011).
- Rangan, K. J., Yang, Y. Y., Charron, G., Hang, H. C. Rapid Visualization and Large-Scale Profiling of Bacterial Lipoproteins with Chemical Reporters. Journal of American Chemical Society. 132 (31), 10628-10629 (2010).
- Thiele, C., et al. Tracing fatty acid metabolism by click-chemistry. ACS Chemical Biology. 7 (12), 2004-2011 (2012).
- Ganesan, L., Shieh, P., Bertozzi, C. R., Levental, I. Click-Chemistry Based High Throughput Screening Platform for Modulators of Ras Palmitoylation. Science Reports. 7, 41147 (2017).
- Nozeret, K., Boucharlat, A., Agou, F., Buddelmeijer, N. A sensitive fluorescence-based assay to monitor enzymatic activity of the essential integral membrane protein Apolipoprotein N-acyltransferase (Lnt). Science Reports. 9 (1), 15978 (2019).
- Zhang, J. H., Chung, T. D., Oldenburg, K. R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Journal of Biomolecules Screening. 4 (2), 67-73 (1999).
- Mao, G., et al. Crystal structure of E. coli lipoprotein diacylglyceryl transferase. Nature Communication. 7, 10198 (2016).
- Vogeley, L., et al. Structural basis of lipoprotein signal peptidase II action and inhibition by the antibiotic globomycin. Science. 351 (6275), 876-880 (2016).
- Wiktor, M., et al. Structural insights into the mechanism of the membrane integral N-acyltransferase step in bacterial lipoprotein synthesis. Nature Communication. 8, 15952 (2017).
- Noland, C. L., et al. Structural insights into lipoprotein N-acylation by Escherichia coli apolipoprotein N-acyltransferase. Proceedings of the National Academy of Sciences USA. 114 (30), 6044-6053 (2017).
- Lu, G., et al. Crystal structure of E. coli apolipoprotein N-acyl transferase. Nature Communication. 8, 15948 (2017).

