Coupling Carbon Capture from a Power Plant with Semi-automated Open Raceway Ponds for Microalgae Cultivation
Summary
A protocol is described to utilize the carbon dioxide in natural gas power plant flue gas to cultivate microalgae in open raceway ponds. Flue gas injection is controlled with a pH sensor, and microalgae growth is monitored with real time measurements of optical density.
Abstract
In the United States, 35% of the total carbon dioxide (CO2) emissions come from the electrical power industry, of which 30% represent natural gas electricity generation. Microalgae can biofix CO2 10 to 15 times faster than plants and convert algal biomass to products of interest, such as biofuels. Thus, this study presents a protocol that demonstrates the potential synergies of microalgae cultivation with a natural gas power plant situated in the southwestern United States in a hot semi-arid climate. State-of-the-art technologies are used to enhance carbon capture and utilization via the green algal species Chlorella sorokiniana, which can be further processed into biofuel. We describe a protocol involving a semi-automated open raceway pond and discuss the results of its performance when it was tested at the Tucson Electric Power plant, in Tucson, Arizona. Flue gas was used as the main carbon source to control pH, and Chlorella sorokiniana was cultivated. An optimized medium was used to grow the algae. The amount of CO2 added to the system as a function of time was closely monitored. Additionally, other physicochemical factors affecting algal growth rate, biomass productivity, and carbon fixation were monitored, including optical density, dissolved oxygen (DO), electroconductivity (EC), and air and pond temperatures. The results indicate that a microalgae yield of up to 0.385 g/L ash-free dry weight is attainable, with a lipid content of 24%. Leveraging synergistic opportunities between CO2 emitters and algal farmers can provide the resources required to increase carbon capture while supporting the sustainable production of algal biofuels and bioproducts.
Introduction
Global warming is one of the most important environmental issues that the world faces today1. Studies suggest that the major cause is the increase in greenhouse gas (GHG) emissions, mainly CO2, in the atmosphere due to human activities2,3,4,5,6,7. In the U.S., the largest density of CO2 emissions originates mainly from fossil fuel combustion in the energy sector, specifically electric power generation plants3,7,8,9. Thus, carbon capture and utilization (CCU) technologies have emerged as one of the major strategies to reduce GHG emissions2,7,10. These include biological systems that utilize sunlight to convert CO2 and water via photosynthesis, in the presence of nutrients, to biomass. The use of microalgae has been proposed due to the fast growth rate, high CO2 fixation ability, and high production capacity. Additionally, microalgae have broad bioenergy potential because the biomass can be converted into products of interest, such as biofuels that can replace fossil fuels7,9,10,11,12.
Microalgae can grow and achieve biological conversion in a variety of cultivation systems or reactors, including open raceway ponds and closed photobioreactors13,14,15,16,17,18,19. Researchers have studied the advantages and limitations that determine the success of the bioprocess in both cultivation systems, under either indoor or outdoor conditions5,6,16,20,21,22,23,24,25. Open raceway ponds are the most common cultivation systems for carbon capture and utilization in situations where flue gas can be distributed directly from the stack. This type of cultivation system is relatively inexpensive, is easy to scale up, has low energy costs, and has low energy requirements for mixing. Additionally, these systems can easily be co-located with the power plant to make the CCU process more efficient. However, there are some drawbacks that need to be considered, such as the limitation in CO2 gas/liquid mass transfer. Although there are limitations, open raceway ponds have been proposed as the most suitable system for outdoor microalgal biofuel production5,9,11,16,20.
In this article, we detail a method for microalgae cultivation in open raceway ponds that combines carbon capture from the flue gas of a natural gas power plant. The method consists of a semi-automated system that controls flue gas injection based on the culture pH; the system monitors and records the Chlorella sorokiniana culture status in real time using optical density, dissolved oxygen (DO), electroconductivity (EC), and air and pond temperature sensors. Algal biomass and flue gas injection data are collected by a data logger every 10 min at the Tucson Electric Power facility. Algae strain maintenance, scale up, quality control measurements, and biomass characterization (e.g., correlation between optical density, g/L, and lipid content) are performed in a laboratory setting at the University of Arizona. A previous protocol outlined a method for optimizing flue gas settings to promote microalgae growth in photobioreactors via computer simulation26. The protocol presented here is unique in that it utilizes open raceway ponds and is designed to be implemented on-site at a natural gas power plant in order to make direct use of the flue gas produced. Additionally, real time optical density measurements are part of the protocol. The system as described is optimized for a hot semiarid climate (Köppen BSh), which exhibits low precipitation, significant variability in precipitation from year to year, low relative humidity, high evaporation rates, clear skies, and intense solar radiation27.
Protocol
1. Growth system: outdoor open raceway pond settings
- Set up the open raceway ponds close to the flue gas source (containing 8–10% CO2). Ensure water and electricity are available at the pond reactor location and that the reactor is not in the shade the majority of the day (Figure 1).
- Capture flue gas during the post-combustion process using a 0.95 cm fuel hose, a few meters before the flue gas enters the stack to be discharged into the atmosphere (Figure 2).
- Remove water from the flue gas using a 20 L water trap and a condenser (coil length ~12 m) between the stack and the compressor (Figure 2).
NOTE: Flue gas typically contains approximately 9‒13.8% water28. In addition, the condenser and pipeline cool the flue gas16. - Connect the following sensors to a datalogger to monitor algal growth: (1) a real time optical density sensor29, which measures absorbance at two wavelengths—650 and 750 nm—and can detect a maximum algal cell concentration of 1.05 g/L; (2) a DO sensor; (3) air and pond thermocouples; (4) a pH sensor; and (5) an EC sensor.
NOTE: Additionally, the pH and EC sensors are connected to a transmitter. The data logger unit configuration is shown in Figure 3. - Ensure that all components of the algal growth system are calibrated and properly working before inoculation.
2. pH control system
- Manage flue gas injection by using a compressor, a control valve system, and the data logger program, as shown in Figure 2 and Figure 3 (Supplementary material A).
- Use a tube to direct the flue gas from the control valve to the bottom of the raceway pond through a stone diffuser.
- Inject the flue gas into the growth system based on pH. When the pH value is greater than 8.05, the system will inject flue gas, whereas when the pH is less than 8.00, the system will stop the flue gas injection in periods of no growth. The flow rate is measured in standard liters per minute (SLPM).
NOTE: In the control valve, the inlet flue gas pressure is limited to a maximum of 50 psi.
3. Algae selection and strain maintenance (light and temperature)
NOTE: The green algae Chlorella sorokiniana DOE 1412 was isolated by Juergen Polle (Brooklyn College)30,31 and selected by the National Alliance for Advanced Biofuels and Bioproducts (NAABB); its selection was based on the previous strain characterization studies performed by Huesemann et al.32,33 . Their research regarding algal screening, biomass productivity, and climate-simulated culturing (e.g., temperature and light) in the Southwest region when using outdoor open raceway ponds informed the method used in this project.
- Maintain cultures at room temperature (25 °C) using a 12 h/12 h light/dark cycle.
- Keep light intensity at 200 µM/m2/s for culture maintenance grown on plates and in small liquid cultures (50 mL to 500 mL).
- Keep light intensity for scale up grown in liquid cultures 50 mL to 500 mL at 400 µM/m2/s, and liquid cultures 5 L to 20 L at 600‒800 µM/m2/s.
4. Scale up and quality control
- Prepare the BG11 culture medium using deionized water and the following salts, for macronutrients, in g/L: 1.5 NaNO3, 0.04 K2HPO4, 0.075 MgSO4*H2O, 0.036 CaCl2*H2O, 0.006 (NH4)5Fe(C6H4O7)2, 0.006 Na2EDTA*2H2O, 0.02 Na2CO3; add 1 mL/L of trace element solution, which contains the following micronutrients in g/L: 2.86 H3BO3, 1.81 MnCl2*4H2O, 0.22 ZnSO4*7H2O, 0.39 Na2MoO4*2H2O, 0.079 CuSO4*5H2O, 0.0494 Co(NO3)2*6H2O.
NOTE: For plate inoculation and/or long-term storage, add 7.5 g/L of Bacto agar; for culture inoculation, no addition of agar is needed. Sterilize culture medium in the autoclave for 21 min at 121 °C. - Pour the BG11 medium with agar into Petri dishes in a sterile laminar flow hood or biosafety cabinet. Once plates are firm and cool, pipette 500 µL from a re-suspended frozen algal stock culture and add Ampicillin (100 µg/mL); incubate the algal plates in a shaker table (120 rpm) for 1 to 2 weeks.
- Use a sterile loop to select a single algal colony from a culture plate and inoculate it in a 50 mL tube containing sterile growth medium in a clean biosafety cabinet. Grow the small liquid culture on a shaker table (120 rpm) for one week.
- Transfer 50 mL of algae culture (linear growth phase, OD750nm ≥ 1) into a 1 L flask with 500 mL liquid medium. Fit each flask with a rubber stopper and stainless-steel tubing to provide aeration. Filter the air using 0.2 µm air sterilization filters. Let the culture grow for one to two weeks. Monitor cell density using a spectrophotometer (OD750nm).
- Place the 500 mL liquid culture into a 10 L carboy containing 8 L of non-sterile culture medium and inject a mixture of 5% CO2 and 95% air. Then, cultivate algae under the same conditions as in step 4.4.
- Monitor stock plate and liquid cultures (in steps 4.2‒4.5) once a week. Take an aliquot and observe it under the microscope at 10x and 40x magnification to ensure the growth of the desired strain. Kept cultures until they have been compromised or used for experiments. Discard contaminated cultures.
5. Concentrated medium preparation for open pond cultivation
- To prepare trace elements solution partially fill a 1 L volumetric flask with distilled water (DW). Insert a magnetic stir bar and add the chemicals shown in Table 1 sequentially. Ensure that each ingredient dissolves before the addition of the next constituent. Remove the magnet and fill the flask to the 1 L volume mark.
- Partially fill a 1 L glass bottle with DW and insert the magnetic stir bar. Place the container on the top of a magnetic stirrer plate and add the chemicals for the reactor’s final volume, adding them sequentially, ensuring each fully dissolves. Table 2 lists the chemicals to prepare 1 L of medium, so multiply all the values by the reactor’s final volume. Fill the glass bottle to 1 L.
6. Outdoor open raceway pond inoculation
- Thoroughly clean the reactor using 30% bleach before each inoculation and after harvesting. It is recommended to leave the bleach overnight. Rinse the reactor well to remove all bleach.
- Calibrate all the sensors before algae inoculation according to their corresponding calibration procedure.
- Dilute the concentrated media (in step 5) using the water source by filling the raceway pond up to 80%.
- Inoculate the reactor using a 10 L carboy filled with algae (linear growth phase OD750nm > 2) and bring it to its final volume.
- Acclimate microalgae by partially shading the raceway pond with wooden pallets for ~ 3 days (Figure 4), once the exponential phase has passed, as an adaptation strategy to avoid photoinhibition.
NOTE: This period will also provide time for the microalgae to adapt to the stress caused by the direct injection of flue gas.
7. Batch growth experiment at the generating station
- Inspect and record any day-to-day variations including water evaporation, paddlewheel motor, sensor functionality, and anything out of the ordinary.
- Drain and inspect the compressor and water trap every day to remove any excess water to minimize corrosion since flue gas is highly corrosive34.
- Configure the data logger to scan each sensor measurement every 10 s and to store the average data every 10 min. These include DO, pH, EC, real time optical density as well as air and reactor temperature.
8. Discrete sampling and monitoring
- Make sure water level remains constant at the reactor’s final volume otherwise the optical density measurement will be affected.
- After replenishing water in the reactor, take a 5 mL sample for cell mass measurements by optical density (540, 680, and 750 nm) using an ultraviolet-visible spectrophotometer. Repeat the process daily.
- Take a 500 mL sample three times per week for microscope observations and biomass concentration based on ash-free dry weight (AFDW).
- Perform microscope observations with 10x and 40x objective lenses. Additionally, these microscope magnifications are used as part of the algal quality control described in step 4.6.
- Use 400 mL of the sample in step 8.3 for AFDW
- Set each 0.7 µm pore size glass microfiber filter in an aluminum foil tray and pre-treat each aluminum foil tray/filter using a furnace for 4 h at 540 °C.
- Label each aluminum foil tray using a #2 pencil, record its weight (A), and place it in the vacuum filter apparatus.
- Stir the algae sample vigorously before measuring out a volume to be filtered. Filter enough algae sample to give a pre/post ash weight difference of between 8 and 16 mg. Pick a weight difference to use throughout the course of the experiment and keep this value constant.
- Place each filter containing the algae sample in its foil tray in the oven at 105 °C for at least 12 h.
- Remove the foil tray/filter from the drying oven and place it in a glass desiccator to prevent water uptake. Record each foil tray/filter weight (B).
- Place the foil tray/filter in the 540 °C muffle furnace for 4 h.
- Turn off the muffle furnace, cool down foil trays/filters, place them into the desiccator, and record each foil tray/filter weight (C).
- Calculate AFDW using gravimetrical analysis:
% AFDW= C – A x 100 / B
- Hold 2 L of algae before harvesting for microwave-assisted extraction (MAE) lipid extraction analysis using solvents.
- Centrifuge the algae sample at a relative centrifugal force (RFC) of 4,400 x g for 15 min. Take the algae pellet and dry it using an oven at 80 °C for at least 24 h.
- Grind the algae sample and weigh the algal powder (recommended biomass ranges from 0.3 g to 0.5 g).
- Add the algae powder (dry algal biomass) into the microwave accelerated reaction system (MARS) Xpress vessels, add 10 mL of chloroform:methanol (2:1, v/v) solvent solution under the hood, close the vessels, and let stand overnight.
- Place the vessels into the MARS machine using the solvent sensor for 60 min at 70 °C and 800 W of power.
- Take vessels out of the MARS and let them cool down under the hood.
- Use a funnel and glass wool to separate the liquid part which contains chloroform, methanol, and lipids by transferring each liquid sample to a pre-weighed glass test tube and keep the solids (biomass free of lipids) for other analyses.
- Take the test tubes containing the lipids to the nitrogen evaporator, remove them once the liquid has been evaporated, and then leave the tubes overnight under the hood to ensure complete dryness.
- Calculate lipid content (wt. %) using gravimetric analysis:
Lipid content (wt. %) = Dry biomass of lipids x 100/ Dry Algal mass
9. Algal harvesting and crop rotation
- Harvest 75% of the total algae culture volume when the culture is close to reaching the stationary phase. Take 2‒5 L of culture to perform biomass productivity analyses in the laboratory. Process and convert the rest of the algae into the desired algal products.
- Re-grow the open raceway pond by using the 25% algae remaining as inoculum. Add water up to 80% of the total reactor’s volume, add the concentrated media, and then finish filling up to the reactor’s final volume if necessary.
- Cultivate the appropriate algae strain according to the season, based on temperature and light intensity conditions.
10. Data management
- Record data in the data logger and collect for analysis as in step 7.3.
- Consider saving raw and analyzed data in the Regional Algal Feedstock Testbed (RAFT) share drive. RAFT project collaborators contribute their data to simulate and model algal productivity and validate outdoor cultivation.
Representative Results
Prior experimental results from our lab indicate that microalgae cultivation using a semi-automated open raceway pond can be coupled with carbon capture processes. To better understand the synergy between these two processes (Figure 2), we developed a protocol and tailored it for cultivating the green algal species Chlorella sorokiniana under outdoor conditions in a hot semiarid climate. Natural gas flue gas was obtained from an industrial power generation station. This protocol uses various technologies to assess algal biomass productivity: (1) algae growth using a real time optical density sensor (Figure 5); (2) algae growth with respect to flue gas on-off pulse injections into the culture as a function of pH (Figure 6 and Figure 7); and (3) algae growth correlations with environmental parameters such as temperature, dissolved oxygen, and electroconductivity (Figure 8 and Figure 9).
We test a real time optical density sensor that monitors algae growth and physiological dynamics. This sensor allowed us to establish, via lab correlation, the corresponding ash free dry weight biomass (g/L). Figure 5 shows a comparison between the sensor and laboratory measurements. Both readings show similar trends, increasing as a function of time. However, the in-situ sensor readings can track the day/night algae growth cycle. Said cycle shows that the optical density values increase during the day but decrease at night during respiration, indicating a change in biomass productivity. The integration of the real time optical density sensor makes it possible to make effective management decisions about the overall algal production system.
We deploy a semi-automated on-off flue gas pulse injection system, which is represented in Figure 6 by a 24 h flue gas injection cycle measured during a particularly warm fall season in Tucson, AZ. As shown in Figure 6, flue gas was injected from approximately 8 am to 6 pm (diurnal period) but was not injected between 6 pm and 8 am (nocturnal period). This day/night cycle reflects the daily sunlight exposure and the lack of light during the night, and consequently, the activation of photosynthesis or photorespiration, respectively. Figure 7 presents the cumulative flue gas injected (L) during this algal batch. In this case, 6,564 L of flue gas, corresponding to 538 L CO2, were used to grown 0.29 g of algal biomass. The graph shows that as the algal growth rate increased, more flue gas (CO2) was required (Figure 6). The experimental results have confirmed that the on-off flue gas pulse injection system is effective at facilitating carbon capture and utilization through microalgae cultivation.
We measure and monitor other physicochemical parameters to establish a correlation between them and algal growth and productivity (Figure 8 and Figure 9). The environmental parameters measured were dissolved oxygen, electroconductivity (EC), and both air and pond temperatures. As expected, all the parameters, except EC, displayed similar trends that were highly correlated with solar radiation. The results indicate that these environmental variables had the most significant impact on algal growth and are used for algal biomass modeling35. The EC did not change significantly during the batch process. Thus, it did not provide any relevant information regarding algal growth. For cultivation of Chlorella sorokiniana using non-saline water, EC measurements can be omitted.
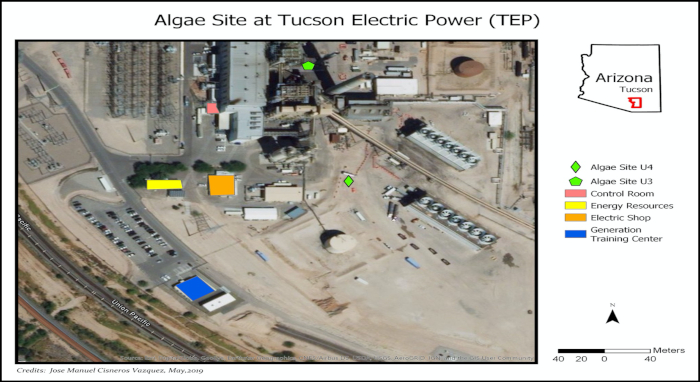
Figure 1: Pilot site location at Tucson Electric Power for coupling carbon capture from power plant and semi-automatized open-pond reactors for microalgae cultivation. The two locations are represented by: 1) Algae Site U3 (unit 3) and 2) Algae Site U4 (unit 4) photo credit: Jose Manuel Cisneros Vazquez. Please click here to view a larger version of this figure.
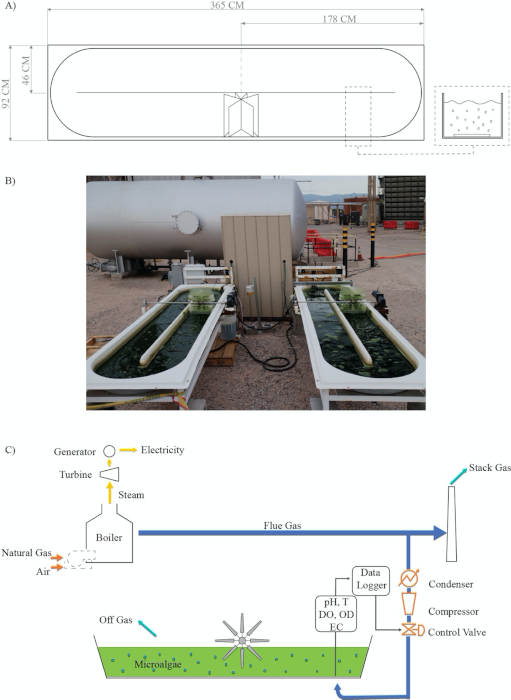
Figure 2: Process flow chart for coupling carbon capture and semi-automatized open raceway ponds for microalgae cultivation in a hot semiarid climate. (A) Open Raceway Paddlewheel design; (B) Real experimental facility; (C) Process: coupling carbon capture and microalgae cultivation modified from Van Den Hende28. Legends: T = Temperature; DO = Dissolved oxygen; OD = Optical density; EC = Electrical conductivity; Data Logger. Please click here to view a larger version of this figure.
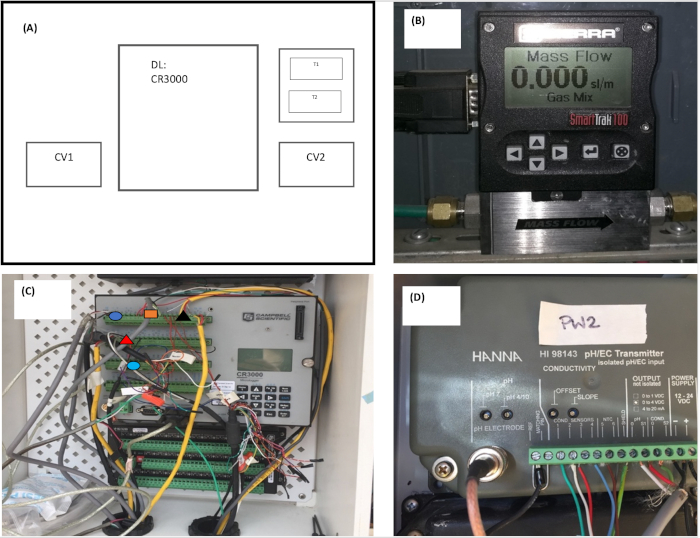
Figure 3: Schematic representation of sensor set up. (A) Representation of the overall outdoor open-pond sensors set up, in which CV1 and CV2 are the control valves, DL is the data logger, and T1 and T2 are the transmitters. (B) Representation of a control valve. (C) Representation of the sensors’ connection to the data logger; dark blue circle: real time optical density, orange triangle: pH and EC, black triangle: thermocouples, red triangle: dissolved oxygen, light blue: control valve. (D) pH and EC transmitter. Please click here to view a larger version of this figure.

Figure 4: Algae under the acclimation process. Microalgae acclimation strategy using wooden pallets during the exponential phase. Please click here to view a larger version of this figure.
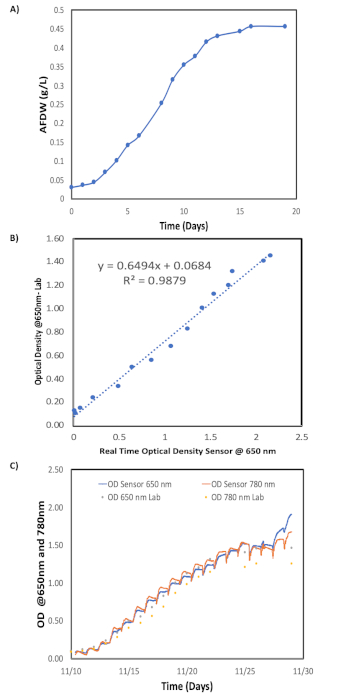
Figure 5: Representation of algae growth monitoring. (A) Graph for AFDW biomass concentration (g/L) vs. time of laboratory measurements; (B) Graph for correlation between optical density sensor and laboratory measurements at 650 nm; and (C) graph for real time optical density sensor vs time for an experimental batch. Please click here to view a larger version of this figure.
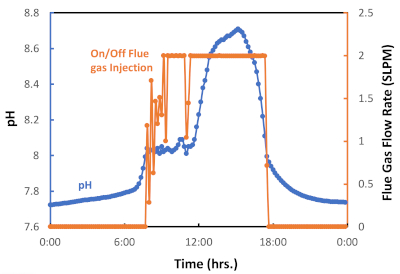
Figure 6: Graph for on/off flue gas pulse injection as a fuction of pH. The data logger was set up to start flue gas injection (controlled valve on) at pH = 8.05 and to end flue gas injection (controlled valve off) at pH = 8.00. Please click here to view a larger version of this figure.
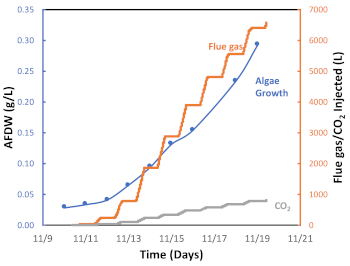
Figure 7: Graph for algal growth (g/L), amount of flue gas injected, and amount of CO2 injected as a function of time. Please click here to view a larger version of this figure.
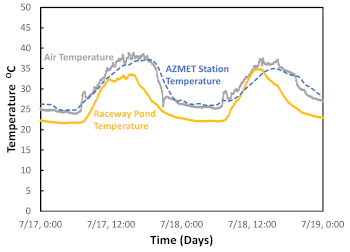
Figure 8: Representation of temperature monitoring. Legends: solid yellow line = raceway pond reactor temperature; solid grey line = air temperature; and dashed blue line = AZMET Station temperature (The Arizona Meteorological Network). Please click here to view a larger version of this figure.
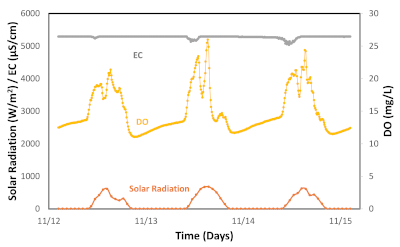
Figure 9: Monitoring of algae growth parameters. Legends: orange solid line = solar radiation; grey solid line = electroconductivy (EC); and yellow solid line = dissolved oxygen (DO). Please click here to view a larger version of this figure.
| Components | Concentration in solution (g/L) |
| H3BO3 | 0.00286 |
| MnCl2·4H2O | 0.00181 |
| ZnSO4·7H2O | 0.0001373 |
| Na2MoO4·2H2O | 0.00039 |
| CuSO4·5H2O | 0.000079 |
| Co(NO3)2·6H2O | 0.00005518 |
| NiCl2·6 H2O | 0.0001 |
Table 1: Trace elements solution recipe.
| Components | Common name | Concentration in solution (g/L) |
| (NH2)2CO | Urea | 0.1 |
| MgSO4·7H2O | Magnesium Sulfate | 0.012 |
| NH4H2PO4 | Ammonium Phosphate | 0.035 |
| KCl | Potash | 0.175 |
| FeCl3 | Ferric Citrate (Citraplex) | 0.005423 |
| Trace Metal Solution | Volume of 1000x Micros (ml) | 1 |
Table 2: Optimized media recipe for 1 L.
Supplemental Coding Files. Please click here to download this file.
Discussion
In this study, we demonstrate that synergistically coupling flue gas carbon capture and microalgae cultivation is possible in a hot semi-arid climate. The experimental protocol for the semi-automated raceway pond system integrates state-of-the-art technology to monitor relevant parameters in real time that correlate to algal growth when using flue gas as a carbon source. The proposed protocol is intended to reduce uncertainty in algal cultivation, which is one of the main drawbacks of raceway ponds20,21,36. In our experience, the protocol’s most critical steps involve the pH control system and an effective method to inoculate the system (Figure 2). The pH control system delivers flue gas/CO2 and represents a strategy to optimize efficiency in CO2 capture and utilization (Figure 3)37. This controlled system has been proven to be more efficient than a continuous injection system for the microalgae cultivation process because it reduces outgassing while delivering enough flue gas to attain the maximum algal growth rate20,37. When the flue gas injection is based on pH, a key factor for algal cultivation is selecting an adequate pH value for the microalgae species before inoculating the raceway pond38,39. Qiu et al.40 found that a pH value of 8 is the best for the freshwater species Chlorella sorokiniania when considering cell growth and lipid production40. Moreover, Molina Grima et al.41 recommend a pH below 8 to reduce nitrogen loss and achieve better nitrogen uptake by the microalgae/biomass41. However, Yuvraj et al.42 suggest that pH is not an appropriate method to evaluate the CO2 content in the water because of the effect of nitrogen fertilization on the medium’s acidity42. Our results show that pH can be effectively used to manage CO2 injection for the system presented here (Figure 6); our flue gas injection management, which kept the culture at pH 8, resulted in high biomass yields and replicability (Figure 7).
After inoculation, the algae must acclimate to the system to avoid photoinhibition and to adjust to the high temperature of the raceway media. In this hot semi-arid climate, we have observed algal photoinhibition due to high solar radiation39,43,44 (Figure 9). This effect can not only delay but also inhibit microalgae inoculation during the exponential phase32,35,45,46,47. To reduce the impact of acclimation on the microalgae, we designed a successful and feasible strategy consisting of partially shading the raceway pond with wooden pallets. This strategy allows the microalgae to be exposed repeatedly but for short periods of time to the solar conditions. Another stress factor is the high temperature of the flue gas and the ambient air33,48 (Figure 8). The flue gas temperature is quite high at the post-combustion stage10,48,49. Utilizing the flue gas by directly injecting it from the dispatched pipeline into the raceway pond can contribute to further increasing the medium’s temperature. Hence, a condenser followed by a water trap located before the compressor will not only reduce the heat transfer but also the amount of water reaching the compressor (Figure 2). We found that both devices were necessary to reduce the compressor failure rate. Additionally, humidity, flue gas temperature, and the corrosive nature of the flue gas must be considered when estimating the compressor’s life cycle and maintenance. Furthermore, high temperatures cause higher evaporation rates.
This protocol is subject to some limitations. According to Figure 6, the control valve was not able to inject enough flue gas when photosynthesis was at its peak. This effect can be attributed to low mass transfer from the gaseous to the liquid phase due to the reactor design5,16,50,51. Mendoza et al.36,52 and de Godos et al.16 stated that raceway ponds have a poor gas/liquid mass transfer, which represents one of the most severe design constraints16,36,52. Their shallow channel design limits CO2 mass transfer due to the short interface area between the gas and the culture medium, which causes an increase in CO2 off-gassing (Figure 2). Thus, devices and novel configurations have been proposed to increase the gas/liquid contact time, including sumps, mixing columns, permeable silicone, and sparging-diffusion systems36,52,53. All these systems have been used in an attempt to enhance CO2 mass transfer; however, some of these systems also improve nutrient distribution, control pH, and remove excess O25,24,36,52. Finally, outages are other limitations that can arise when capturing and utilizing real flue gas from a power plant. These outages are not always scheduled. Thus, temporary alternative sources of CO2 should be considered, for example, relocation or connecting the CO2 mainline to multiple power units (Figure 1).
The ability to produce microalgae with this protocol is supported by our results on algal productivity (Figure 5), algal responses to the selected parameters (Figure 6, Figure 8, Figure 9), and successful cultivation of the desired algal species when nurtured by direct flue gas injection. Open reactors are cheaper to operate, and thus, this protocol builds upon their strengths to accelerate commercial-scale deployment of this form of carbon capture and utilization16,20,54,55,56. This hot semi-arid region experiences high solar radiation and significant temperature fluctuations year-round (Figure 8 and Figure 9)57; hence, it is a prime location to test this sort of protocol. The optical density sensor provided consistent OD readings for our outdoor open system (Figure 5); this type of data collection would be impractical using other sensors. Also, the sensors responded well to the significant temperature variations from day to night (Figure 8), enabling us to make timely algal productivity decisions29. Furthermore, the proposed optimized medium has the critical advantage of being based on commercial fertilizer and readily available nutrient sources58 (Table 1 and 2); this medium can be easily produced in-house or could be sourced upon request from agricultural liquid fertilizer companies58. Finally, the semi-automated protocol was tested in an additional natural gas power plant. The results of that confirmation study are not presented in this paper. In that confirmation study, the protocol was successful despite the extreme weather conditions in Tucson and the exceptionally hot temperatures at the generation station due to the reactor’s location within the power plant layout. Therefore, protocol replicability has been examined for Tucson’s environment when natural gas is used as fuel to produce electricity.
The following steps are recommended to further develop this protocol and to improve and enhance the automation of the processes involved. The first recommendation is to make the flue gas injection a completely variable-rate process, thus improving CO2 and pH management; the current program fully opens the injection valve when the pH rises above 8 and closes it when the pH reaches 8 again. Improving the way CO2 is injected is also necessary. The aim is to reduce the size of the CO2 bubbles, i.e., to generate microbubbles to enhance CO2 diffusion in the medium without resorting to injecting flue gas at higher pressure. Using improved injectors, thus reducing operational energy costs, is deemed necessary in a commercial application of the protocol. The inclusion of predictive tools based on the weather forecast and current microalgae status for controlling the flue gas and fertilizer, mainly N, to improve N use efficiency, is also recommended. The use of computational fluid dynamic modeling is considered a vital tool in developing the proposed protocol further; modeling can help optimize the design, configuration, and operation of all the hardware involved in the monitoring and management of the microalgae. Another area that could be explored in the future is the application of environmental DNA (eDNA) and real time PCR techniques to monitor the health and composition of the microalgae crop. Water samples could be analyzed, and the results would indicate whether the objective microalgae are the predominant species in the medium or whether it is competing or has been replaced by a different organism.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported through the Regional Algal Feedstock Testbed project, U.S. Department of Energy DE-EE0006269. We also thank Esteban Jimenez, Jessica Peebles, Francisco Acedo, Jose Cisneros, RAFT Team, Mark Mansfield, UA power plant staff, and TEP power plant staff for all their help.
Materials
| Adjustable speed motor (paddle wheel system) | Leeson | 174307 | Lesson 174307.00, type: SCR Voltage; Amps:10 |
| Aluminum weight boats | Fisher Scientific | 08-732-102 | Fisherbrand Aluminum Weighing Dishes |
| Ammonium Iron (III) (NH₄)₅[Fe(C₆H₄O₇)₂] | Fisher Scientific | 1185 – 57 – 5 | Medium preparation. Ammonium iron(III) citrate |
| Ammonium Phosphate | Sigma-Aldrich | 7722-76-1 | This chemical is used for the optimized medium |
| Ampicillin sodium salt | Sigma Aldrich | A9518-5G | This chemical is used for avoiding algae contamination |
| Autoclave | Amerex Instrument Inc | Hirayama HA300MII | |
| Bacto agar | Fisher Scientific | BP1423500 | Fisher BioReagents Granulated Agar |
| Bleach | Clorox | Germicidal Bleach, concentrated clorox | |
| Boric Acid (H3BO3) | Fisher Scientific | 10043-35-3 | Trace Elelements: Boric acid |
| Calcium chloride dihydrate (CaCl2*2H2O) | Sigma-Aldrich | 10035-04-8 | Medium preparation. Calcium chloride dihydrate |
| Carboys (20 L) | Nalgene – Thermo Fisher Scientific | 2250-0050PK | Polypropylene Carboy w/Handles |
| Centrifuge | Beckman Coulter, Inc | J2-21 | |
| Chloroform | Sigma-Aldrich | 67-66-3 | This chemical is used for lipid extraction |
| Citraplex 20% Iron | Loveland Products | SDS No. 1000595582 -17-LPI | https://www.fbn.com/direct/product/Citraplex-20-Iron#product_info |
| Cobalt (II) nitrate hexahydrate (Co(NO3)2*6H2O) | Sigma-Aldrich | 10026-22-9 | Trace Elements: Cobalt (II) nitrate hexahydrate |
| Compressor | Makita | MAC700 | This equipment is used for the injection CO2 system |
| Control Valve | Sierra Instruments | SmartTrak 100 | This item needs to be customized for your application. In our case, it was used a 5% CO2 and 95% air mixture. |
| Copper (II) Sulfate Pentahydrate (CuSO4*5H2O) | Sigma-Aldrich | 7758-99-8 | Trace Elements: Copper (II) Sulfate Pentahydrate |
| Data Logger: Campbell unit CR3000 | Scientific Campbell | CR3000 | This equipment is used for controlling all the system, motoring and recording data |
| Dissolvde Oxygen Solution | Campbell Scientific | 14055 | Dissolved oxygen electrolyte solution DO6002 – Lot No. 211085 |
| Dissolved Oxygen probe | Sensorex | | DO6400/T Dissolved Oxygen Sensor with Digital Communication |
| Electroconductivity calibration solution | Ricca Chemical Company | 2245 – 32 ( R2245000-1A ) | Conductivity Standard, 5000 uS/cm at 25C (2620 ppm TDS as NaCl) |
| Electroconductivity probe sensor | Hanna Instruments | HI3003/D | Flow-thru Conductivity Probe – NTC Sensor, DIN Connector, 3m Cable |
| Ethylenediaminetetraacetic acid disodium salt dihydrate (Na2EDTA*2H2O) | Sigma-Aldrich | 6381-92-6 | Medium Preparation: Ethylenediaminetetraacetic acid disodium salt dihydrate |
| Filters | Fisher Scientific | 09-874-48 | Whatman Binder-Free Glass Microfiber Filters |
| Flasks | Fisher scientific | 09-552-40 | Pyrex Fernbach Flasks |
| Furnace | Hogentogler | Model: F6020C-80 | Thermo Sicentific Thermolyne F6020C – 80 Muffle Furnace |
| Glass dessicator | VWR International LLC | 75871-430 | Type 150, 140 mm of diameter |
| Glass funnel | Fisher Scientific | FB6005865 | Fisherbrand Reusable Glass Long-Stem Funnels |
| Laminar flow hood | Fisher Hamilton Safeair | Fisher Hamilton Stainless Safeair hume hood | |
| Magnesium sulfate heptahydrate (MgSO4*7H2O) | Fisher Scientific | 10034 – 99 – 8 | Medium Preparation: Magnesium sulfate heptahydrate |
| Methanol | Sigma-Aldrich | 67-56-1 | Lipid extraction solvent |
| Micro bubble Diffuser | Pentair Aquatic Eco-Systems | 1PMBD075 | This equipment is used for the injection CO2 system |
| Microalgae: Chlorella Sorokiniana | NAABB | DOE 1412 | |
| Microoscope | Carl Zeiss 4291097 | ||
| Microwave assistant extraction | MARS, CEM Corportation | CEM Mars 5 Xtraction 230/60 Microwave Accelerated Reaction System. Model: 907601 | |
| MnCl2*4H2O | Sigma-Aldrich | 13446-34-9 | Manganese(II) chloride tetrahydrate |
| Mortars | Fisher Scientific | FB961B | Fisherbrand porcelein mortars |
| Nitrogen evaporator | Organomation | N-EVAP 112 Nitrogen Evaporatpr (OA-SYS Heating System) | |
| Oven | VWR International LLC | 89511-410 | Forced Air Oven |
| Paddle Wheel | 8-blade horizontal axis propeller. This usually comes as part of the paddlewheel reactor. | ||
| Paddle wheel motor | Leeson | M1135042.00 | Leeson, Model: CM34025Nz10C; 1/4 HP; Volts 90; FR 34; 62 RPM. |
| Pestles | Fisher Scientific | FB961M | Fisherbrand porcelein pestles |
| pH and EC Transmitter | Hanna Instruments | HI98143 | Hanna Instruments HI98143-04 pH and EC Transmitter with Galvanic isolated 0-4V. |
| pH calibration solutions | Fisher Scientific | 13-643-003 | Thermo Scientific Orion pH Buffer Bottles |
| pH probe sensor | Hanna Instruments | HI1006-2005 | Hanna Instruments HI1006-2005 Teflon pH Electrode with matching pin 5m. |
| Pippete tips | Fisher Scientific | 1111-2821 | 1000 ul TipOne graduated blue tip in racks |
| Pippetter | Fisher Scientific | 13-690-032 | Eppendorf Reserch plus Variable Adjustable Volume Pipettes: Single-channel |
| Plastic cuvettes | Fisher scientific | 14377017 | BrandTech BRAND Plastic Cuvettes |
| Plates | Fisher scientific | 08-757-100D | Corning Falcon Bacteriological Petri Dishes with Lid |
| Potash | This chemical is used for the optimazed medium preparation. It was bought in a fertilizer local company | ||
| Potassium phosphate dibasic (K2HPO4) | Sigma-Aldrich | 7758 -11 – 4 | Medium Preparation: Potassium phosphate dibasic |
| Pyrex reusable Media Storage Bottles | Fisher scientific | 06-414-2A | 1 L and 2 L bottels – PYREX GL45 Screw Caps with Plug Seals |
| Raceway Pond | Similar equipment can be bought at https://microbioengineering.com/products | ||
| Real Time Optical Density Sensor | University of Arizona | This equipment was design and build by a member of the group | |
| RS232 Cable | Sabrent | Sabrent USB 2.0 to Serial (9-Pin) DB-9 RS-232 Converter Cable, Prolific Chipset, Hexnuts, [Windows 10/8.1/8/7/VISTA/XP, Mac OS X 10.6 and Above] 2.5 Feet (CB-DB9P) | |
| Shaker Table | Algae agitation 150 rpm | ||
| Sodium Carbonate (Na2CO3) | Sigma-Aldrich | 497-19-8 | Sodium carbonate |
| Sodium molybdate dihydrate (Na2MoO4*2H2O) | Sigma-Aldrich | 10102-40-6 | Medium Preparation: Sodium molybdate dihydrate |
| Sodium nitrate (NaNO3) | Sigma-Aldrich | 7631-99-4 | Medium Preparation: Sodium nitrate |
| Spectophotometer | Fisher Scientific Company | 14-385-400 | Thermo Fisher Scientific – 10S UV-Vis GENESTYS Spectrophotometer cylindrical Longpath cell holder; internal reference dectector, Xenon flash lamp; dual silicon photodiode; 240V, 50 to 60Hz selected automatically. |
| Test tubes | Fisher Scientific | 14-961-27 | Fisherbrand Disposable Borosilicate Glass Tubes with Plain End (10 ml) |
| Thermocouples type K | Omega | KMQXL-125G-6 | |
| Urea | Sigma-Aldrich | 2067-80-3 | Urea |
| Vacuum filtration system | Fisher Scientific | XX1514700 | MilliporeSigma Glass Vacuum Filter Holder, 47 mm. The system includes: Ground glass flask attachment, coarse-frit glass filter support, and flask |
| Vacuum pump | Grainger | Marathon Electric AC Motor Thermally protected G588DX – MOD 5KH36KNA510X. HP 1/4. RPM 1725/1425 | |
| Zinc sulfate heptahydrate (ZnSO4*7H2O) | Sigma-Aldrich | 7446-20-0 | Zinc sulfate heptahydrate |
References
- . The Intergovernmental Panel on Climate Change Available from: https://www.ipcc.ch/ (2018)
- Songolzadeh, M., Soleimani, M., Ravanchi, M., Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological, Review Emphasizing Reduction in Greenhouse Gas Emissions. The Scientific World Journal. 2014, 1-34 (2014).
- Litynski, J., Klara, S., McIlvried, H., Srivastava, R. The United States Department of Energy’s Regional Carbon Sequestration Partnerships program: A collaborative approach to carbon management. Environ International. 32 (1), 128-144 (2006).
- Cuellar-Bermudez, S., Garcia-Perez, J., Rittmann, B., Parra-Saldivar, R. Photosynthetic Bioenergy Utilizing CO2: an Approach on Flue Gases Utilization for Third Generation Biofuels. Journal of Clean Production. 98, 53-65 (2014).
- Cheah, W., Show, P., Chang, J., Ling, T., Juan, J. Biosequestration of Atmospheric CO2 and Flue Gas-Containing CO2 by Microalgae. Bioresource Technology. 184, 190-201 (2014).
- Kao, C., et al. Utilization of Carbon Dioxide in Industrial Flue Gases for the Cultivation of Microalga Chlorella sp. Bioresource Technology. 166, 485-493 (2014).
- White, C., Strazisar, B., Granite, E., Hoffman, S., Pennline, H. Separation and Capture of CO2 from Large Stationary Sources and Sequestration in Geological Formations. Journal of the Air and Waste Management Association. 53 (10), 1172-1182 (2003).
- Benemann, J. CO2 Mitigation with Microalgae Systems. Pergamon Energy Conversion Management Journal. 38, 475-479 (1997).
- U.S.Department of Energy. The Capture , Utilization and Disposal of Carbon Dioxide from Fossil Fuel-Fired Power Plants. Energy. 2, (1993).
- Granite, E., O’Brien, T. Review of Novel Methods for Carbon Dioxide Separation from Flue and Fuel Gases. Fuel Processesing Technology. 86 (14-15), 1423-1434 (2005).
- Benemann, J. Utilization of Carbon Dioxide from Fossil Fuel-Burning Power Plants with Biological Systems. Energy Conversion and Management. 34 (9-11), 999-1004 (1993).
- Joshi, C., Nookaraju, A. New Avenues of Bioenergy Production from Plants: Green Alternatives to Petroleum. Journal of Petroleum & Environmental Biotechnology. 03 (07), 3 (2012).
- Chisti, Y. Constraints to commercialization of algal fuels. Journal of Biotechnology. 22, 166-186 (2013).
- Han, S., Jin, W., Tu, R., Wu, W. Biofuel production from microalgae as feedstock: current status and potential. Critical Reviews in Biotechnology. 35 (2), 255-268 (2015).
- Lam, M., Lee, K. Potential of using organic fertilizer to cultivate Chlorella vulgaris for biodiesel production. Applied Energy. 94, 303-308 (2012).
- de Godos, I., et al. Evaluation of carbon dioxide mass transfer in raceway reactors for microalgae culture using flue gases. Bioresource Technology. 153, 307-314 (2014).
- Posten, C., Schaub, G. Microalgae and terrestrial biomass as source for fuels a process view. Journal of Biotechnology. 142 (1), 64-69 (2009).
- Demirbas, M. Biofuels from algae for sustainable development. Applied Energy. 88 (10), 3473-3480 (2011).
- Shelef, G., Sukenik, A., Green, M. . Microalgae Harvesting and Processing A Literature Review. , (1984).
- Pawlowski, A., Mendoza, J., Guzmán, J., Berenguel, J., Acién, F., Dormido, S. Effective utilization of flue gases in raceway reactor with event-based pH control for microalgae culture. Bioresource Technology. 170, 1-9 (2014).
- Zhu, B., Sun, F., Yang, M., Lu, L., Yang, G., Pan, K. Large-scale biodiesel production using flue gas from coal-fired power plants with Nannochloropsis microalgal biomass in open raceway ponds. Bioresource Technology. 174, 53-59 (2014).
- Kaštánek, F., et al. In-field experimental verification of cultivation of microalgae Chlorella sp. using the flue gas from a cogeneration unit as a source of carbon dioxide. Waste Management & Research. 28 (11), 961-966 (2010).
- Yadav, G., Karemore, A., Dash, S., Sen, R. Performance evaluation of a green process for microalgal CO2 sequestration in closed photobioreactor using flue gas generated in-situ. Bioresource Technology. 191, 399-406 (2015).
- Zhao, B., Su, Y., Zhang, Y., Cui, G. Carbon dioxide fixation and biomass production from combustion flue gas using energy microalgae. Energy. 89, 347-357 (2015).
- He, L., Chen, A., Yu, Y., Kucera, L., Tang, Y. Optimize Flue Gas Settings to Promote Microalgae Growth in Photobioreactors via Computer Simulations. Journal of Visualized Experiments. (80), e50718 (2013).
- He, L., Subramanian, V., Tang, Y. Experimental analysis and model-based optimization of microalgae growth in photo-bioreactors using flue gas. Biomass and Bioenergy. 41, 131-138 (2012).
- Pidwirny, M. . Fundamentals of Physical Geography, 2nd ed. , (2006).
- Van Den Hende, S., Vervaeren, H., Boon, N. Flue gas compounds and microalgae: (Bio-) chemical interactions leading to biotechnological opportunities. Biotechnology Advances. 30 (2012), 1405-1424 (2012).
- Jia, F., Kacira, M., Ogden, K. Multi-wavelength based optical density sensor for autonomous monitoring of microalgae. Sensors (Switzerland). 15 (9), 22234-22248 (2015).
- Unkefer, C., et al. Review of the algal biology program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Research. 22, 187-215 (2017).
- Neofotis, P., et al. Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Research. 15, 164-178 (2016).
- Huesemann, M., Van Wagenen, J., Miller, T., Chavis, A., Hobbs, S., Crowe, B. A screening model to predict microalgae biomass growth in photobioreactors and raceway ponds. Biotechnology Bioengineering. 110 (6), 1583-1594 (2013).
- Huesemann, M., et al. Estimating the Maximum Achievable Productivity in Outdoor Ponds: Microalgae Biomass Growth Modeling and Climate Simulated Culturing. Microalgal Production for Biomass and High-Value Products. 28 (2016), 113-137 (2016).
- Ramezan, M., Skone, T., Nsakala, N., Lilijedahl, G. . Carbon Dioxide Capture from Existing Coal-Fired Power Plants. , 268 (2007).
- Huesemann, M., et al. A validated model to predict microalgae growth in outdoor pond cultures subjected to fluctuating light intensities and water temperatures. Algal Research. 13, 195-206 (2016).
- Mendoza, J., et al. Fluid-dynamic characterization of real-scale raceway reactors for microalgae production. Biomass and Bioenergy. 54, 267-275 (2013).
- Algae Cultivation for Carbon Capture and Utilization Workshop. . Algae Cultivation for Carbon Capture and Utilization Workshop. , (2017).
- Park, J., Craggs, R., Shilton, A. Wastewater treatment high rate algal ponds for biofuel production. Bioresource Technology. 102 (1), 35-42 (2011).
- Mata, T., Martins, A., Caetano, N. Microalgae for biodiesel production and other applications: A review. Renewewable and Sustainable Energy Reviews. 14 (1), 217-232 (2010).
- Qiu, R., Gao, S., Lopez, P., Ogden, K. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Research. 28, 192-199 (2017).
- Molina Grima, E., Fernández, F., Garcıa Camacho, F., Chisti, Y. Photobioreactors: light regime, mass transfer, and scaleup. Journal of Biotechnology. 70 (1-3), 231-247 (1999).
- Padmanabhan, Y. P. Technical insight on the requirements for CO2-saturated growth of microalgae in photobioreactors. 3 Biotech. 7 (2), 1-7 (2017).
- Vonshak, A., Torzillo, G. Environmental Stress Physiology. Handbook of Microalgal Culture. 4 (2007), 57-82 (2007).
- Morales, M., Sánchez, L., Revah, S. The impact of environmental factors on carbon dioxide fixation by microalgae. Federation of European Microbiological Society Microbiology Letters. 365 (3), 1-11 (2018).
- Cuaresma, M., Janssen, M., Vílchez, C., Wijffels, R. Horizontal or vertical photobioreactors? How to improve microalgae photosynthetic efficiency. Bioresource Technology. 102 (8), 5129-5137 (2011).
- Richmond, A., Zou, N. Efficient utilisation of high photon irradiance for mass production of photoautotrophic micro-organisms. Journal of Applied Phycology. 11 (1), 123-127 (1999).
- Kurpan, D., Silva, A., Araújo, O., Chaloub, R. Impact of temperature and light intensity on triacylglycerol accumulation in marine microalgae. Biomass and Bioenergy. 72, 280-287 (2015).
- Maedal, K., Owadai, M., Kimura, N., Karubd, I. CO2 fixation from the flue gas on coal-fired thermal power plant by microalgae To screen microalgac which arc suitable for direct CO2 fixation , microalgae were sampled from. Energy Conversion Managment. 36 (6-9), 717-720 (1995).
- Sakai, N., Sakamoto, Y., Kishimoto, N., Chihara, M., Karube, I. Strain from Hot Springs Tolerant to High Temperature and high CO2. Energy Conversion Managment. 36 (6-9), 693-696 (1995).
- Lam, M., Lee, K., Mohamed, A. Current status and challenges on microalgae-based carbon capture. International Journal of Greenhouse Gas Control. 10, 456-469 (2012).
- Raeesossadati, M., Ahmadzadeh, H., McHenry, M., Moheimani, N. CO2 Bioremediation by Microalgae in Photobioreactors: Impacts of Biomass and CO2 Concentrations, Light, and Temperature. Algal Research. 6, 78-85 (2014).
- Mendoza, J., et al. Oxygen transfer and evolution in microalgal culture in open raceways. Bioresource Technology. 137, 188-195 (2013).
- Carvalho, A., Malcata, F., Meireles, A. Microalgal Reactors A Review of Enclosed System Designs and Performances. Biotechnology Progress. 22 (6), 1490-1506 (2006).
- Pires, J., Alvim-Ferraz, M., Martins, F., Simões, M. Carbon dioxide capture from flue gases using microalgae: Engineering aspects and biorefinery concept. Renewable and Sustainable Energy Reviews. 16 (5), 3043-3053 (2012).
- Lam, M., Lee, K. Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnology Advances. 30 (3), 673-690 (2012).
- Chisti, Y. Biodiesel from microalgae beats bioethanol. Trends in Biotechnology. 26 (3), 126-131 (2008).
- K̈oppen, W., Volken, E., Brönnimann, S. The Thermal Zones of the Earth According to the duration of Hot, Moderate and Cold Periods and to the Impact of Heat on the Organic. Meteorologische Zeitschrift. 20 (3), 351-360 (2011).
- Lammers, P., et al. Review of the Cultivation Program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Research. 22, 166-186 (2017).

