Behavioral Assessment of Visual Function via Optomotor Response and Cognitive Function via Y-Maze in Diabetic Rats
Summary
Neural degeneration in both eyes and brain as a result of diabetes can be observed through behavioral tests carried out on rodents. The Y-maze, a measure of spatial cognition, and the optomotor response, a measure of visual function, both provide insight into potential diagnoses and treatments.
Abstract
The optomotor response and the Y-maze are behavioral tests useful for assessing visual and cognitive function, respectively. The optomotor response is a valuable tool to track changes in spatial frequency (SF) and contrast sensitivity (CS) thresholds over time in a number of retinal disease models, including diabetic retinopathy. Similarly, the Y-maze can be used to monitor spatial cognition (as measured by spontaneous alternation) and exploratory behavior (as measured by a number of entries) in a number of disease models that affect the central nervous system. Advantages of the optomotor response and the Y-maze include sensitivity, speed of testing, the use of innate responses (training is not needed), and the ability to be performed on awake (non-anesthetized) animals. Here, protocols are described for both the optomotor response and the Y-maze and examples of their use shown in models of Type I and Type II diabetes. Methods include preparation of rodents and equipment, performance of the optomotor response and the Y-maze, and post-test data analysis.
Introduction
Over 463 million people live with diabetes, making it one of the largest global disease epidemics1. One of the serious complications that arises from diabetes is diabetic retinopathy (DR), a leading cause of blindness for working-age American adults2. In the next 30 years, the percentage of the population at risk for DR is projected to double, so it is crucial to find new ways of diagnosing DR in its earlier stages to prevent and mitigate DR development3. DR has conventionally been thought to be a vascular disease4,5,6. However, now with evidence of neuronal dysfunction and apoptosis in the retina that precedes vascular pathology, DR is defined to have neuronal and vascular components4,5,6,7,8,9. One way to diagnose DR would be to examine neural abnormalities in the retina, a tissue that may be more vulnerable to oxidative stress and metabolic strain from diabetes than other neural tissue10.
Declines in cognitive and motor function also occur with diabetes and are often correlated with retinal changes. Older individuals with Type II diabetes portray worse baseline cognitive performance and show more exacerbated cognitive decline than control participants11. Additionally, the retina has been established as an extension of the central nervous system and pathologies can manifest in the retina12. Clinically, the relationship between retina and brain has been studied in the context of Alzheimer’s and other diseases but is not commonly explored with diabetes12,13,14,15,16. Changes in the brain and retina during the progression of diabetes can be explored using animal models, including the STZ rat (a model of Type I diabetes in which the toxin, streptozotocin or STZ, is used to damage pancreatic beta cells) and the Goto-Kakizaki rat (a polygenic model of Type II diabetes in which animals develop hyperglycemia spontaneously at around 3 weeks of age). In this protocol, a description for the Y-maze and the optomotor response to assess cognitive and visual changes in diabetic rodents, respectively, is provided. The optomotor response (OMR) assesses spatial frequency (similar to visual acuity) and contrast sensitivity by monitoring characteristic reflexive head tracking movements to gauge visual thresholds for each eye17. Spatial frequency refers to the thickness or fineness of the bars, and contrast sensitivity refers to how much contrast there is between the bars and the background (Figure 1E). Meanwhile, the Y-maze tests short-term spatial memory and exploratory function, observed through spontaneous alternations and entries through the arms of the maze.
Both tests can be performed in awake, non-anesthetized animals and have the advantage of capitalizing on innate responses of the animals, meaning that they do not require training. Both are relatively sensitive, in that they can be used to detect deficits early in the progression of diabetes in rodents, and reliable, in that they produce results that correlate with other visual, retinal, or behavioral tests. Additionally, using the OMR and the Y-maze in conjunction with tests such as electroretinogram and optical coherence tomography scans can provide information on when retinal, structural, and cognitive changes develop relative to each other in disease models. These investigations could be useful in identifying neural degenerations that occur due to diabetes. Ultimately, this could lead to new diagnostic methods that effectively identify DR in early stages of progression.
The OMR and the Y-maze systems used to develop this protocol are described in the Table of Materials. Previous research on the OMR, by Prusky et al.18, and the Y-maze, by Maurice et al.19, was used as the starting point to develop this protocol.
Protocol
All procedures were approved by the Atlanta Veterans Affairs Institutional Animal Care and Use Committee and conformed to the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications, 8th edition, updated 2011).
1. The optomotor response (OMR)
- Set up the OMR apparatus (details on apparatus and software in Table of Materials)
- Choose the appropriate-sized platform for the rodent: mouse, rat, or large/impaired rat (Figure 1A).
- Open the OMR software, which should open to a window with several tabs of options and a live video feed of the inside of the OMR/virtual drum (Figure 1B). Zoom in or out with the video camera as needed so that the platform and its surroundings are visible.
- Note the icons along the left-hand side of the live image (Figure 1C). Click on the asterisk icon and the rotating stripes icon so that both the green asterisk and green rotating stripes disappear from the live feed.
- Click on the compass icon so that a green circle and two perpendicular lines appear. Stretch the green circle so that it aligns perfectly with the black circle on the platform, which will ensure that the OMR is perfectly aligned.
- Click on the compass icon because it is not necessary to see the circle during testing. Click on the green asterisk icon and the green rotating stripes icon to make these reappear. Note that the green stripes rotate in the same direction as the stripes in the drum, allowing the researcher to know the direction of the stripes.
- Click on the Testing tab. Under Testing, click on the Psychophysics tab. Under Threshold, select Frequency to measure spatial frequency.
NOTE: The OMR software uses a staircase paradigm to automatically calculate spatial frequency (SF). Contrast will be maintained at 100%. - Under Testing, click on the Presets tab. Select the default settings for Mouse18 or Rat20.
- Under Testing, click on the Blanking tab. Check the Blank on Tracking box, which will pause the stripes/blank out the computer screens in the drum whenever the mouse is right-clicked.
- Click on the Résultats tab, which is where the results of the test will be displayed.
- Evaluate spatial frequency
- Place the rodent on the circular platform in the center of the virtual reality chamber comprising four computer monitors showing vertical sine wave gratings circling the chamber at a velocity of 12˚/s (Figure 1D).
- Note that the video camera positioned at the top of the chamber is projecting the rodent’s behavior live onto the computer monitor.
- Look for the presence or absence of reflexive actions by the rodent’s head as the gratings move in a clockwise or counterclockwise direction. Make sure illustrated bars are visible in the program—these will show the direction of the grating movement.
- Watch for the rodent’s head to move in the same direction as the gratings. Wait until there is a smooth pursuit, not erratic bursts of head motion, to count it as tracking.
- Click on Yes or No as appropriate. Note that SF will start with 0.042 cyc/deg and adjust with each yes and no to become easier or more difficult (Figure 1E). Click on Reset if the test needs to be reset due to accidental or incorrect clicking of yes and no.
- As the rodent is tested, make sure to keep the asterisk positioned over the rodent’s head.
NOTE: This has two effects: 1) It maintains the correct spatial frequency. If the asterisk is positioned between the shoulders, for example, the spatial frequency will be lower and the bars will be easier to see, resulting in a falsely high score. 2) For rodents with slight head movements, the asterisk makes it easier to gauge whether the head is actually moving. - Watch for the system to say “Done” when the rodent’s spatial frequency is reached. Note that the Yes and No buttons will no longer be clickable.
- Click on the Résultats tab, which will display the spatial frequency for the left eye, right eye, and combined eyes.
NOTE: Sometimes the software is set such that the results are flipped, i.e., the right eye is reported as the left eye and the left eye is reported as the right eye. This was discovered when assessing rodents that had only one eye lesioned in a glaucoma model.
- Evaluate contrast sensitivity
NOTE: Contrast Sensitivity testing can be performed immediately after the spatial frequency measurement step or on its own on the same day or a different day if the rodent appears fatigued after spatial frequency testing (follow steps 1–2.2 if only testing contrast sensitivity).- Click on the Testing tab and then on the Psychophysics tab. Under Threshold, select Contrast (single) to measure contrast sensitivity.
- Also using a staircase paradigm, start gratings with SF constant at the peak of the Contrast Sensitivity (CS) curve. To do this, click on the Stimulus tab and then on the Gratings tab. In the Spatial Frequency box, type 0.064 for rats and 0.103 for mice.
- Begin the contrast at 100% and look for the same reflexive head movements as seen during spatial frequency testing. Note that the contrast will decrease as the testing progresses until the rodent no longer has reflexive head movements in response to the stimulus (Figure 1E).
- Watch for the system to say “Done” and the Yes and No buttons to no longer be clickable once the rodent no longer responds to the visual stimulus and the contrast sensitivity threshold has been reached. Click on the Résultats tab, where the contrast sensitivity for the left eye, right eye, and combined eyes will be listed.
- Perform post-testing analysis
- For diabetic retinopathy studies, where both eyes are expected to have similar deficits, use the combined score (average of right and left eyes) for analysis. For models that cause differential damage to eyes (i.e., blast injury or glaucoma), keep the left and right eye data separate.
- For Spatial Frequency, use raw scores (the data from the Résultats tab) for analysis and average these scores together by group (i.e., diabetic, control, etc.).
- For Contrast Sensitivity, use the raw value to calculate the reported contrast sensitivity by the Michelson contrast from a previous measurement of the screen’s luminance.
2. The Y-maze
- Prepare rodents for testing
- Adapt rodents to the room for 30 min prior to testing.
NOTE: The researcher can remain in the room with the lights on but should remain silent during this time. - Clean the Y-maze with sanitizing solution safe for animals and wipe away all sanitizing solution with paper towels. Ensure that the maze is dry.
- Adapt rodents to the room for 30 min prior to testing.
- Conduct the Y-maze
- Label the initial arm of the Y-maze as B and the other 2 arms as A and C (Figure 2A). Place one rodent in the arm closest to the researcher (arm B) near the center of the Y-maze. Once the rodent has been placed, start the timer (details on maze and timer in Table of Materials).
- Allow each rodent to explore the Y-maze for 8 min. Take recordings during this time and note any observations. Sit several feet away from the maze while keeping it in sight and avoid making any noise.
- Record the starting location as A, and each time the rodent makes an entry into a new arm, record the new location of the rodent (Figure 2B). Define an entry as all four limbs of the rodent being in one of the arms.
- Watch for rodents to hide and remain stationary in one arm of the maze. If the rodent remains in the same spot for more than 60 s and does not appear to show exploratory behavior, move the rodent toward the center of the Y-maze, and continue the trial.
- After each rodent, remove any feces and clean the maze with sanitizing solution.
- Ensure that all sanitizing solution is wiped away with paper towels and the maze is completely dry before placing the next rodent in the maze.
- Label the initial arm of the Y-maze as B and the other 2 arms as A and C (Figure 2A). Place one rodent in the arm closest to the researcher (arm B) near the center of the Y-maze. Once the rodent has been placed, start the timer (details on maze and timer in Table of Materials).
- Calculate spontaneous alternation and exploratory behavior
- Calculate exploratory behavior as the total number of entries made during 8 min.
- Calculate spatial cognition as measured by spontaneous alternation:
the number of successful alternations/(the total number of entries – 2)- Define a successful alternation as the rodent moves into three different locations sequentially (Example: ABC, CAB, BCA, etc.). Note each successful alternation (Figure 2B).
- If the movements were recorded as ACABCABABCABC, disregard the two initial starting locations when calculating spontaneous alternation (such that there are 11 movements in the denominator). Count the number of accurate movements (accurate movements = 8). Calculate the percent accuracy as: 8/(13 – 2) = 72.7%.
Representative Results
The OMR is considered successful if spatial frequency and contrast sensitivity thresholds can be obtained from a rodent. Here, the use of the OMR to assess spatial frequency is illustrated in naïve control Brown-Norway and Long-Evans rats, both young (3–6 months) and aged (9–12 months). Brown-Norway rats typically show a higher baseline spatial frequency than Long-Evans rats. Additionally, an aging effect on spatial frequency was observed in the Long-Evans rats (Figure 3A). Data were analyzed using a one-way ANOVA followed by Holms-Sidak post-hoc comparisons as the young and aged results came from different cohorts.
The use of the OMR to assess contrast sensitivity is illustrated in the STZ model of Type I diabetes that received exercise intervention treatment. Long-Evans rats were assigned to one of the four groups: control, control + active, diabetic, and diabetic + active. Diabetic rats were given intravenous injections of the toxin STZ to damage the pancreatic beta cells and induce hyperglycemia. Active rats received 30 min of treadmill exercise, 5 days a week. Inactive rats had a locked treadmill. Significant deficits in contrast sensitivity (Figure 3B) were observed in diabetic rats. Exercise treatment reduced these deficits (Figure 3B). These results demonstrate that the OMR is useful both for detecting and tracking retinal deficits over time and for assessing the effects of treatments and interventions on retinal disease22. Data were analyzed using a two-way repeated measures ANOVA followed by Holms-Sidak post-hoc comparisons. Note that results may be presented as data normalized to control (Figure 3B) or as raw values (Figure 3A; for spatial frequency: in cycles/degree or c/d; for contrast sensitivity: arbitrary units or a.u.). Typically, 6–10 animals, depending on severity of injury, are needed to find a significant difference with the OMR.
The Y-maze is considered successful if the rodent enters at least 5 arms of the maze within 8 minutes. Here, the ability of the Y-maze to assess cognitive function and exploratory behavior is illustrated in the Goto-Kakizaki rat, a polygenic, non-obese model of Type II diabetes that develops moderate hyperglycemia beginning at 2–3 weeks of age and does not require insulin supplementation. Significant deficits in spatial cognition, as measured by spontaneous alternation (Figure 4A), and exploratory behavior, as measured by number of entries (Figure 4B), were observed in Goto-Kakizaki rats compared with Wistar controls beginning at 7 weeks of age. Control rats seem to show a decrease in exploratory behavior from 4 to 8 weeks. This trend is also observed in long term studies (8+ months of age). The decrease in movement could be due to lack of novelty with repeated maze exposure or a general decreased movement with age. Control rats appear to show an increase in spatial cognition from 4 to 8 weeks. This trend is not observed in long-term studies in which the animals are run monthly instead of weekly (in fact, a decline with aging is often observed), and thus, this increase in spatial cognition may be due to a learning effect of running the maze once a week. Data were analyzed using a two-way repeated measures ANOVA followed by Holms-Sidak post-hoc comparisons. A minimum of 10 animals, depending on severity of injury, are typically needed to find a significant difference with the Y-maze.
This protocol generated visual function and cognitive function data in models of Type I and Type II diabetes. Scores for individual animals were averaged together and used to detect significant differences between treatment groups early in the progression of diabetes. Performing both retinal and cognitive assessments over time in models of systemic diseases such as diabetes allows for the monitoring of the temporal appearance of deficits over time. For example, in the Goto-Kakizaki model, retinal function deficits were shown to precede cognitive and exploratory behavior deficits23 (Figure 5).
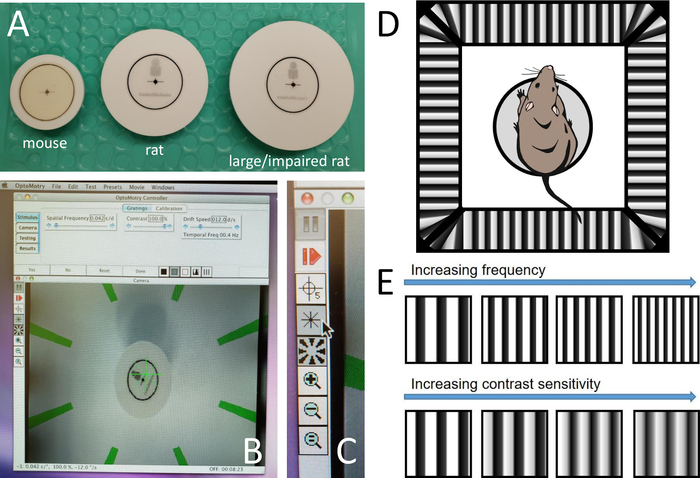
Figure 1: Setup of the OMR equipment. (A) Picture of mouse, rat, and large or impaired rat platforms. (B) Picture of computer screen during testing. (C) Panel of buttons during testing. (D) Schematic of rat on platform in chamber. (E) Example gradients showing increasing spatial frequency and contrast sensitivity. Please click here to view a larger version of this figure.

Figure 2: Setup of the Y-maze equipment. (A) Picture of the Y-maze with arms labeled. (B) Picture of a lab notebook with example of the Y-maze recording. Please click here to view a larger version of this figure.
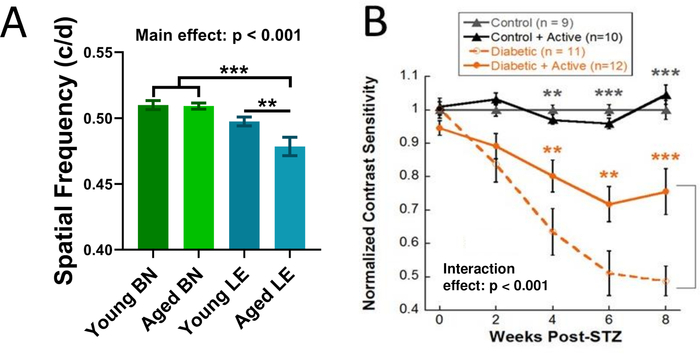
Figure 3: Using the OMR to track visual function. (A) Spatial frequency thresholds for young (n = 11) and aged (n = 15) Brown-Norway (BN) and young (n = 20) and aged (n = 13) Long-Evans (LE) rats. This figure presents Brown-Norway data from Feola et al., 201921. (B) Using the OMR to track reduced retinal function over time and protective effects of exercise in an STZ rat model of Type I diabetes. Contrast sensitivity thresholds for inactive diabetic rats vs. active diabetic rats and control rats. Dark gray asterisks represent differences between both control groups and both diabetic groups. Orange asterisks represent differences between inactive diabetic rats and active diabetic rats. This figure presents data from a subset of rats from Allen et al., 201822. Mean ± SEM. ** p < 0.01, *** p < 0.001. Please click here to view a larger version of this figure.
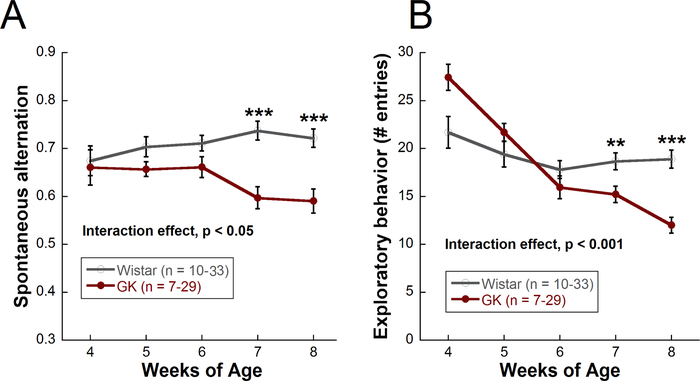
Figure 4: Using the Y-maze to track cognitive function and exploratory behavior over time in the Goto-Kakizaki model of Type II diabetes compared with Wistar controls. (A) Cognitive function (spontaneous alternation) for Goto-Kakizaki (diabetic) and Wistar (control) rats from 4 to 8 weeks of age. (B) Exploratory behavior (number of entries) from 4 to 8 weeks of age. Mean ± SEM. ** p < 0.01, *** p < 0.001. Asterisks represent differences between Goto-Kakizaki and Wistar rats at each timepoint. Only one cohort of rats was run from 4 weeks to 8 weeks (GK: n = 7; Wistar: n = 10). All other cohorts were run from 5 weeks to 8 weeks (GK: n = 22; Wistar: n = 23) for a total n of 29 (GK) and 33 (Wistar) at weeks 5 through 8. This figure has been modified from Allen et al., 201923. Please click here to view a larger version of this figure.
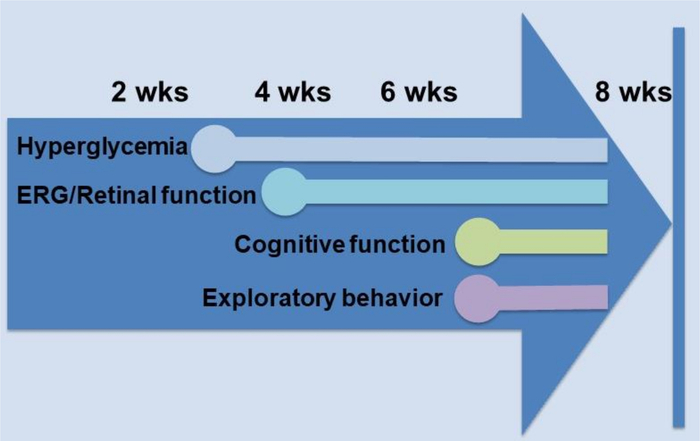
Figure 5: Timeline of functional changes in the Goto-Kakizaki model of Type II diabetes. After the appearance of hyperglycemia, the first changes observed in the Goto-Kakizaki rat were in retinal function, as measured by electroretinogram (ERG), appearing at 4 weeks of age. Cognitive and exploratory behavior changes appeared after 6 weeks of age. This figure has been modified from Allen et al., 201923. Please click here to view a larger version of this figure.
Discussion
The OMR and the Y-maze allow for the non-invasive assessment of visual function and cognitive function deficits in rodents over time. In this protocol, the OMR and the Y-maze were demonstrated to track visual and cognitive deficits in rodent models of diabetes.
Critical steps in the protocol
The OMR
Some important points to consider when performing the OMR to assess visual function are the testing parameters used, experimental design and timing of testing, and experience of the researcher performing the measurements. One of the more critical steps in the protocol is to make sure that the parameters are correctly set. Additionally, as part of the setup, the OMR chamber should be cleaned with sanitizing solution or another approved disinfectant prior to and after each rodent. It is also important that the researcher performing the measures has been trained and is experienced in performing the measures. Best results are seen when the rodents are calm and acclimated to the room by leaving them in their cages for 30 min before starting the experiment. It is also important to determine baseline spatial frequency and contrast sensitivity whenever beginning work with a new strain and to note that not all strains will exhibit the same baseline levels. Brown-Norway rats have greater baseline spatial frequency than Long-Evans rats. Meanwhile, some strains of albino rats seem to have compromised spatial frequency24, while other strains of albino rats do not exhibit tracking behavior at all. Many factors may contribute to the limited response of albino animals on the OMR: disturbed binocularity due to differential decussation of optic nerve fibers, lack of melanin in the back of the eye, and large proportion of dual opsin cones. Regardless, albino rats may not be appropriate subjects for the OMR testing since their performance could be too close to the limit of detection.
The Y-maze
A critical component of performing the Y-maze involves minimizing disturbances during the recording period. The initial placement of the rodent in the maze should only be done after allowing the rodent to acclimate to the room for 30 min. This allows the rodent to become adjusted to the new environment and prevents any confounding factors from impacting the rodent’s normal behavior. Minimizing disturbances during each trial is very important. This includes avoiding loud noises and making sure the researcher is out of sight of the rodent. These distractions may cause stress to the rodent. It is also important to note that the walls of the room should remain as bare as possible with a neutral color. Any bright colors on the walls or posters may distract the rodent and can impact their exploratory behavior pattern.
Limitations of the method and modifications and troubleshooting of the method
The OMR
A potential limitation of the OMR is that it can be affected by experimenter bias, and different experimenters can have slightly different results since the OMR scoring is subjective. It can be easy to miss a head movement that is too subtle or classify exploratory behavior as a head movement. Because bias can affect the OMR outcomes, it is best if the experimenter is masked to treatment group and study design when possible. The development of an automatic OMR or comparing the results of two testers could also help to decrease experimenter bias.
One common issue that can occur during the OMR testing is when the rodent repeatedly jumps off the platform, making it difficult to obtain a visual threshold. If this happens, take note of it and gently place the rat back on the platform; it may also be necessary to measure the rat again the following day. Additionally, rats that have never been measured before might engage in exploratory behaviors when placed in the OMR. If this is an issue, having an additional baseline measure a week or so after the first measurement may help improve accuracy. Tests with excessive amounts of these behaviors should be discarded.
Other factors such as age or olfactory cues could also contribute to unwanted activity. Therefore, it is important to design experiments in accordance with the timeline of visual system development in rats and to thoroughly clean the platform and chamber before and after testing each rodent. The time of day the OMR measurements are performed should also be considered, as previous studies have shown that there are circadian rhythms in spatial frequency25. Running the rats before noon appears to be best for their focus (Rachael Allen’s lab—personal observations). If rats become too distracted, it can help to gently tap on the outside of the OMR.
The speed with which the testing is performed can also affect results. Measures may become less accurate after 30 min or so if the rodents lose interest in the stimulus. Therefore, more accurate results may be obtained when measurements are taken in approximately ≤20 min. Duration of a single trial (for either SF or CS) is 5–10 min for an expert and 30 min for a beginner. If a rodent is showing little movement, spending most of its time grooming, or otherwise not looking in the direction of the bars, it may be fatigued. The rodent may be run again on a different day. Additionally, SF and CS testing can be performed on different days, particularly for newer testers who may be slower. The frequency with which the test is performed can also affect results—performing it weekly or every other week helps the animals stay acclimated to the test, but performing it every day or every other day can cause hyperacuity26. We do not run more than one trial per day, though we often run both SF and CS on the same day or even in the same sitting. Cumulative daily time for running a cohort of rats (n = 10) is 2 h for an expert.
The OMR measures each eye independently, resulting in separate visual scores for each eye. In the Morrison and microbead models of glaucoma and in an optic nerve crush model, our lab has not observed any impact of the damaged eye on the undamaged eye27. In a blast model, with the blast directed at one eye, the contralateral eye did show damage, but this could also be due to a partial blast effect28. In control rats, there should be no difference in results between clockwise or counterclockwise directions, but some rodents could have a bias; thus, it would be best to alternate the directions29, if the OMR system does not alternate automatically.
Depending on the disease model, treatment group differences in visual function can vary based on the parameters used. For example, when testing contrast sensitivity, if the spatial frequency is set to a level that is above the normal spatial frequency threshold and difficult to resolve, the differences in contrast sensitivity between groups will be small. However, if the spatial frequency is set to a level that is normally easy for rats to see, the differences in contrast sensitivity between groups will be larger30. Therefore, it is important to consider study design and normal spatial frequency thresholds of rodents when setting parameters for performing the OMR.
The Y-maze
If an animal is scared, it may freeze in one corner of the maze. Additionally, if a loud noise happens outside the room, an animal may become scared and not move in the maze. To account for these issues, researchers can acclimate rats to the room first, move a frozen animal to a choice point, run an animal again on a different day, or run the animals in red light, which is thought to make them less nervous as they are normally active in darkness (Rachael Allen’s Lab—personal communication). It is also recommended to run the Y-maze at the same time each day to account for changes in activity levels throughout the day due to circadian rhythms. We typically run the rats before noon (Rachael Allen’s Lab—personal observations). Duration of a single trial is 8 min (10 min, with clean up). We never run more than one trial per day. If an additional trial is needed, the trial is performed on another day. Cumulative daily time for running a cohort of rats (n = 10) is 2–3 h. Age-related decreases in spatial alternation were observed in rats at 9–12 months of age and in exploratory behavior at 12 months of age28.
While both exploratory behavior and spatial cognition decrease in diabetic rodents, the two do not appear to be tightly correlated, and thus, we do not independently evaluate locomotor activity prior to the Y-maze testing.
The significance of the method with respect to existing/alternative methods
The OMR
Other methods of visual function testing, such as optokinetic tracking, rely on fixing the animal’s head in place and tracking eye movements. Unrestrained optomotor response (OMR) testing allows for longitudinal, non-invasive, and reliable measurement of visual function in rodents. In this protocol, it was described how the OMR can be used to quantify both spatial frequency and contrast sensitivity thresholds for each eye. This method can be very useful for detecting early stage neuronal dysfunction in diseases such as diabetes. Other tests such as the visual water task can also be used to measure spatial frequency31, but as this involves training rodents to swim toward a gradient in a modified Y-maze, the task is time consuming and involves a lot of training. Further, the OMR measures values for each eye independently, which is useful in models where injury is directed at one eye and the other eye serves as a contralateral control (for example, many glaucoma models). Additionally, OMR is a sensitive assessment, able to detect changes as early as 3–4 weeks post-diabetes, which is sooner than other visual assessments. Electrophysiological assays are an alternative to behavioral visual tests. Electroretinography (ERG) is more available than the OMR and can determine deficits in precise cell types using different components of the ERG wave32 (a- waves represent photoreceptor cell function, b- waves represent bipolar cell function). Meanwhile, the OMR can be used to determine a deficit in visual function, without revealing the precise point of breakdown along the pathway. However, the OMR is a more sensitive measure of DR than ERG, with the OMR deficits typically observed between 2–4 weeks post hyperglycemia and ERG deficits typically observed 4–8 weeks post hyperglycemia in rodents. Severe diabetic cataracts can affect the OMR. However, diabetic cataracts in rodents appear and/or worsen under anesthesia, and thus, tests such as ERG and optical coherence tomography that require anesthesia are affected much more often that the OMR, which is performed in awake animals.
The Y-maze
The Y-maze relies on spatial cognition like the Morris water maze but does not use a strong negative stimulus (i.e., water) to motivate the animal to perform the task. Thus, the Y-maze is less stressful for the animals and is also easier to perform. However, it is possible that the Y-maze may not be as sensitive as the Morris water maze or the Barnes maze. Unlike the Morris water maze, the Y-maze is an automatic behavior and does not require training. Thus, the time burden involved in performing the Y-maze is much lower.
Conclusions and future applications or directions of the method
The OMR
The OMR is useful for taking measurements of visual function in rodents by tracking head movements. It is an effective method, but there are updates and additions that are continuously made to improve the protocol. Some novel methods utilize the rodent pausing its head as a negative OMR indicator combined with head tracking as a positive indicator33. This enables quicker and more accurate measures of visual function34. Another way this process has been modified is to develop a system that will automatically track the head without artificial markers to reduce inconsistencies that could result from human testers35. As of 2016, an automated or quantitative OMR system called qOMR has been well developed and is commercially available. In the above protocol, the OMR was able to detect deficits in spatial frequency and contrast sensitivity in diabetic rats, as well as protection against deficits with a treatment (exercise).
The Y-maze
The Y-maze reveals information about exploratory behavior and spatial cognition and was used here to detect behavioral deficits in diabetic rodents at 7 weeks. Other tests to observe cognitive function exist (i.e., Morris water maze, Barnes maze, novel object recognition), and it is possible that these tests may be able to reveal cognitive decline earlier or provide information about different aspects of cognition. Future directions for the Y-maze include placing a novel object or food stimulus in one of the arms and observing the exploratory pattern of the rodent36. A variation of this involves blocking one of the arms of the Y-maze, allowing the rodent to explore the two remaining arms, and then reopening access to the third arm and evaluating how long the rodent spends in the third novel arm. Another valuable improvement that could be made regarding the Y-maze is developing automatic tracking of the rodents in order to record their movements. This would eliminate the need for manual recording of the rodents’ movements and would make calculations of the spontaneous alternations more accurate and efficient.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Department of Veterans Affairs Rehab R&D Service Career Development Awards (CDA-1, RX002111; CDA-2; RX002928) to RSA and (CDA-2, RX002342) to AJF and the National Institutes of Health (NIH-NICHD F31 HD097918 to DACT and NIH-NIEHS T32 ES012870 to DACT) and NEI Core Grant P30EY006360.
Materials
| OptoMotry HD | CerebralMechanics Inc. | OMR apparatus & software | |
| Timer | Thomas Scientific | 810029AR | |
| Y-Maze apparatus | San Diego Instruments | 7001-043 | Available specifically for rats |
References
- . International Diabetes Federation Diabetes Atlas, 9th edn Available from: https://diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAfinal-web.pdf (2019)
- Wang, W., Lo, A. C. Y. Diabetic retinopathy: pathophysiology and treatments. International Journal of Molecular Sciences. 19 (6), (2018).
- Akpek, E. K., Smith, R. A. Overview of age-related ocular conditions. The American Journal of Managed Care. 19 (5), 67-75 (2013).
- Urano, F. Wolfram syndrome: diagnosis, management, and treatment. Current Diabetes Reports. 16 (1), 6 (2016).
- Adeva-Andany, M. M., Funcasta-Calderón, R., Fernández-Fernández, C., Ameneiros-Rodríguez, E., Domínguez-Montero, A. Subclinical vascular disease in patients with diabetes is associated with insulin resistance. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 13 (3), 2198-2206 (2019).
- Chin, J. A., Sumpio, B. E. Diabetes mellitus and peripheral vascular disease: diagnosis and management. Clinics in Podiatric Medicine and Surgery. 31 (1), 11-26 (2014).
- Barber, A. J., Gardner, T. W., Abcouwer, S. F. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investigative Ophthalmology & Visual Science. 52 (2), 1156-1163 (2011).
- Pardue, M. T., Allen, R. S. Neuroprotective strategies for retinal disease. Progress in Retinal and Eye Research. 65, 50-76 (2018).
- Aung, M. H., Kim, M. K., Olson, D. E., Thule, P. M., Pardue, M. T. Early visual deficits in streptozotocin-induced diabetic long evans rats. Investigative Ophthalmology & Visual Science. 54 (2), 1370-1377 (2013).
- Antonetti, D. A., et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 55 (9), 2401-2411 (2006).
- Logroscino, G., Kang, J. H., Grodstein, F. Prospective study of type 2 diabetes and cognitive decline in women aged 70-81 years. BMJ. 328 (7439), 548 (2004).
- London, A., Benhar, I., Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nature Reviews Neurology. 9 (1), 44-53 (2013).
- Archibald, N. K., Clarke, M. P., Mosimann, U. P., Burn, D. J. The retina in Parkinson’s disease. Brain. 132 (5), 1128-1145 (2009).
- Sakai, R. E., Feller, D. J., Galetta, K. M., Galetta, S. L., Balcer, L. J. Vision in multiple sclerosis: the story, structure-function correlations, and models for neuroprotection. Journal of Neuroophthalmology. 31 (4), 362-373 (2011).
- Wong, T. Y., et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. The Lancet. 358 (9288), 1134-1140 (2001).
- Marquié, M., et al. Association between retinal thickness and β-amyloid brain accumulation in individuals with subjective cognitive decline: Fundació ACE Healthy Brain Initiative. Alzheimer’s Research & Therapy. 12 (1), 37 (2020).
- Thomas, B. B., Seiler, M. J., Sadda, S. R., Coffey, P. J., Aramant, R. B. Optokinetic test to evaluate visual acuity of each eye independently. Journal of Neuroscience Methods. 138 (1-2), 7-13 (2004).
- Prusky, G. T., Alam, N. M., Beekman, S., Douglas, R. M. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Investigative Ophthalmology & Vision Science. 45 (12), 4611-4616 (2004).
- Maurice, T., et al. Behavioral evidence for a modulating role of σ ligands in memory processes. I. Attenuation of dizocilpine (MK-801)-induced amnesia. Brain Research. 647 (1), 44-56 (1994).
- Douglas, R. M., et al. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Visual Neuroscience. 22 (5), 677-684 (2005).
- Feola, A. J., et al. Menopause exacerbates visual dysfunction in experimental glaucoma. Experimental Eye Research. 186, 107706 (2019).
- Allen, R. S., et al. TrkB signalling pathway mediates the protective effects of exercise in the diabetic rat retina. European Journal of Neuroscience. 47 (10), 1254-1265 (2018).
- Allen, R. S., et al. Retinal deficits precede cognitive and motor deficits in a rat model of type II diabetes. Investigative Ophthalmology & Visual Science. 60 (1), 123-133 (2019).
- Prusky, G. T., Harker, K. T., Douglas, R. M., Whishaw, I. Q. Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behavioural Brain Research. 136 (2), 339-348 (2002).
- Hwang, C. K., et al. Circadian rhythm of contrast sensitivity is regulated by a dopamine-neuronal PAS-domain protein 2-adenylyl cyclase 1 signaling pathway in retinal ganglion cells. Journal of Neuroscience. 33 (38), 14989-14997 (2013).
- Mui, A. M., et al. Daily visual stimulation in the critical period enhances multiple aspects of vision through BDNF-mediated pathways in the mouse retina. PLoS One. 13 (2), 0192435 (2018).
- Feola, A. J., et al. Menopause exacerbates visual dysfunction in experimental glaucoma. Experimental Eye Research. 186, 107706 (2019).
- Allen, R. S., et al. Long-term functional and structural consequences of primary blast overpressure to the eye. Journal of Neurotrauma. 35 (17), 2104-2116 (2018).
- Maaswinkel, H., Li, L. Spatio-temporal frequency characteristics of the optomotor response in zebrafish. Vision Research. 43 (1), 21-30 (2003).
- Benkner, B., Mutter, M., Ecke, G., Münch, T. A. Characterizing visual performance in mice: an objective and automated system based on the optokinetic reflex. Behavioral Neuroscience. 127 (5), 788-796 (2013).
- Lehmann, K., Schmidt, K. F., Löwel, S. Vision and visual plasticity in ageing mice. Restorative Neurology and Neuroscience. 30, 161-178 (2012).
- Leinonen, H., Tanila, H. Vision in laboratory rodents-tools to measure it and implications for behavioral research. Behavioral Brain Research. 352, 172-182 (2018).
- Spielmann, M., Schröger, E., Kotz, S. A., Pechmann, T., Bendixen, A. Using a staircase procedure for the objective measurement of auditory stream integration and segregation thresholds. Frontiers in Psychology. 4, 534 (2013).
- Shi, C., et al. Optimization of optomotor response-based visual function assessment in mice. Scientific Reports. 8 (1), 9708 (2018).
- You, M., Yamane, T., Tomita, H., Sugano, E., Akashi, T. A novel rat head gaze determination system based on optomotor responses. PLoS One. 12 (4), 0176633 (2017).
- Whyte, A. J., et al. Reward-related expectations trigger dendritic spine plasticity in the mouse ventrolateral orbitofrontal cortex. The Journal of Neuroscience. 39 (23), 4595-4605 (2019).

