Design and Development of a Three-Dimensionally Printed Microscope Mask Alignment Adapter for the Fabrication of Multilayer Microfluidic Devices
Summary
This project allows small laboratories to develop an easy-to-use platform for the fabrication of precise multilayer microfluidic devices. The platform consists of a three-dimensionally printed microscope mask alignment adapter using which multilayer microfluidic devices with alignment errors of <10 µm were achieved.
Abstract
This project aims to develop an easy-to-use and cost-effective platform for the fabrication of precise, multilayer microfluidic devices, which typically can only be achieved using costly equipment in a clean room setting. The key part of the platform is a three dimensionally (3D) printed microscope mask alignment adapter (MMAA) compatible with regular optical microscopes and ultraviolet (UV) light exposure systems. The overall process of creating the device has been vastly simplified because of the work done to optimize the device design. The process entails finding the proper dimensions for the equipment available in the laboratory and 3D-printing the MMAA with the optimized specifications. Experimental results show that the optimized MMAA designed and manufactured by 3D printing performs well with a common microscope and light exposure system. Using a master mold prepared by the 3D-printed MMAA, the resulting microfluidic devices with multilayered structures contain alignment errors of <10 µm, which is sufficient for common microchips. Although human error through transportation of the device to the UV light exposure system can cause larger fabrication errors, the minimal errors achieved in this study are attainable with practice and care. Furthermore, the MMAA can be customized to fit any microscope and UV exposure system by making changes to the modeling file in the 3D printing system. This project provides smaller laboratories with a useful research tool as it only requires the use of equipment that is typically already available to laboratories that produce and use microfluidic devices. The following detailed protocol outlines the design and 3D printing process for the MMAA. In addition, the steps for procuring a multilayer master mold using the MMAA and producing poly(dimethylsiloxane) (PDMS) microfluidic chips is also described herein.
Introduction
A well-developed and promising field in engineering research is microfabrication because of the vast expanse of applications employing microfluidic platforms. Microfabrication is a process wherein structures are produced with µm- or smaller-sized features using different chemical compounds. As microfluidic research has developed over the last 30 years, soft lithography has become the most popular microfabrication technique with which to produce microchips made from poly(dimethylsiloxane) (PDMS) or similar substances. These microchips have been widely used for the miniaturization of common laboratory practices1,2,3,4 and have become powerful research tools for engineers to mimic reaction processes5,6,7, study reaction mechanisms, and mimic organs found in the human body in vitro (e.g., organ-on-a-chip)8,9,10. However, as the complexity of the application increases, it is typical that a more complex microfluidic device design allows for better replication of the real-life system it is intended to imitate.
The basic soft lithography procedure involves coating a substrate with a photoresist substance and placing a photomask over the coated substrate before subjecting the substrate to UV light11. The photomask has transparent regions that mimic the desired pattern of the microfluidic device channels. When subjecting the coated substrate to UV light, the transparent regions allow the UV light to penetrate through the photomask, causing the photoresist to be crosslinked. After the exposure step, the un-crosslinked photoresist is washed away using a developer, leaving solid structures with the intended pattern. As the complexity of the microfluidic devices becomes greater, they require multiple-layer construction with extremely precise dimensions. The process of multilayer microfabrication is much more difficult compared to single-layer microfabrication.
Multilayer microfabrication requires precise alignment of the first layer features with the designs on the second mask. Normally, this process is performed using a commercial mask aligner, which is expensive and requires training to operate the machinery. Thus, the process of multilayer microfabrication is typically unattainable for smaller laboratories that lack the funds or time for such endeavors. While several other custom-built mask aligners have been developed, these systems often require the purchase and assembly of many different parts and can still be quite complex12,13,14. This is not only expensive for smaller laboratories, but also requires time and training to build, understand, and use the system. The mask aligner detailed in this paper sought to alleviate these issues as there is no need for the purchase of additional equipment, only requiring equipment that is typically already present in laboratories that produce and use microfluidic devices. In addition, the mask aligner is fabricated by 3D printing, which with the recent advancement of 3D printing technology, has become readily available to most laboratories and universities at an affordable cost.
The protocol detailed in this paper aims to create a cost-effective and easy-operation alternative mask aligner. The mask aligner detailed herein can make multilayer microfabrication feasible for research laboratories without conventional fabrication facilities. Using the microscope mask alignment adapter (MMAA), functional microchips with complex features can be achieved using a regular UV light source, optical microscope, and common laboratory equipment. The results show that the MMAA performs well with an example system using an upright microscope and a UV light-exposure box. The MMAA produced using the 3D printing process was used to acquire a bilayer master mold of a herringbone microfluidic device with minimal alignment errors. Using the master mold fabricated with a 3D-printed MMAA, microfluidic devices were prepared with multilayered structures containing alignment errors of <10 µm. The alignment error of <10 µm is minimal enough to not hinder the application of the microfluidic device.
In addition, the successful alignment of a four-layer master mold produced using the MMAA was confirmed, and alignment errors were determined to be <10 µm. The functionality of the microfluidic device and minimal alignment errors validate the successful application of the MMAA in creating multilayer microfluidic devices. The MMAA can be customized to fit any microscope and UV exposure system by making minor changes to the file in the 3D printer. The following protocol outlines the steps necessary to fine-tune the MMAA to fit the equipment available in each laboratory and 3D-print the MMAA with the required specifications. In addition, the protocol details how to develop a multilayer master mold using the system and subsequently produce PDMS microfluidic devices using the master mold. Generation of the master mold and microfluidic chips then allows the user to test the effectiveness of the system.
Protocol
1. Designing the MMAA
- Obtain the dimensions of the tray of the available UV light emission system to be the upper bound for the dimensions of the wafer holder (or UV exposure unit) shown in Figure 1. As shown in Figure 2A, measure the diameter (d) of the inner circular rim, the inner height (h) of the UV light emission system's tray, the total width (w), and length (l) of the tray.
NOTE: As an example, the available UV light exposure system had inner tray dimensions of 5 inch (") x 5" x 0.25" with a 4" circular cut-out. The dimensions of the MMAA were then designed to be no greater than the inner tray dimensions to fit properly and sit flat within the tray of the system as shown in Figure 2B. See Figure 3 for the 3D-printed pieces of the MMAA: photoresist-coated silicon wafer and a fastener to fix the setup to the microscope. - Measure the length between the screws on the available upright microscope stage that hold the slide holder in place. Additionally, measure the width of the screws. Apply these dimensions to customize the magnetic holder (Figure 1) to fit the available microscope to allow for easy and precise fixation of the MMAA to the microscope (Figure 4A).
- Using an available computer design application, customize the wafer holder and magnetic microscope fastener to fit within the measured dimensions. Design the height, width, and length of the wafer holder to be no greater than the height (h), width (w), and length (l) of the UV light emission system's tray. In addition, include the circular cut-out at the bottom of the wafer holder with the same diameter (d) as the UV light emission system's tray. Generate STL or CAD files for the two pieces of the MMAA to be used for 3D printing of the device (see Supplemental Material).
2. 3D Printing the MMAA
- Upload the generated STL or CAD files to the available 3D-printing software. 3D-Print the two pieces of the MMAA by following the appropriate procedure for the 3D process and printer used. Complete the pieces by following any required post-printing steps (e.g., removal of support material, removal of uncured resin, additional washing or curing steps). Alternatively, use an available 3D printing facility to have the designed pieces printed and completed elsewhere.
- Ensure the wafer holder fits well and sits flat inside the tray of the available UV light exposure system (Figure 2B). Additionally, ensure that the microscope fastener is attached to the microscope stage and can be moved easily using the knobs that control the x- and y- positions of the microscope stage (Figure 4A).
- Once the pieces have been finalized, insert and fix the magnets into the wafer holder and microscope fastener (Figure 3A), using super glue or any other fixing substance. Allow for the glue to dry before testing the system.
NOTE: If desired, a protype piece can first be printed using a Fused Deposition Modeling (FDM) 3D printer to save resources and money15. This protype can then be assessed for accurate fit to the available equipment, and the design can then be modified, if needed. The final device can then be printed using a more accurate process (e.g., Stereolithography) for better precision. The final device can also be printed with a translucent finish for optimal use under the microscope.
3. Experimental testing of the MMAA
- Design and printing of the microfluidic device photomasks with alignment markers
- Use a computer design application to design photomasks for the desired bilayer microfluidic device.
- Include additional structures on the side of the microfluidic device channel structures that will act as alignment markers (closer towards the edge of the photomask/master mold) as shown in Figure 5A,B. Ensure there is one alignment marker on each side of the microfluidic device (for a total of at least four). In addition, ensure the photomask contains a straight edge that can align perfectly with the straight edge of the silicon wafer.
NOTE: The higher intricacy of the alignment marker structure will allow for greater alignment accuracy of the additional layers. At the least, a simple cross structure with measurements of 1 mm x 1 mm should be used (Figure 6A). An example of the alignment markers can be seen in the corners and bottom middle edge of Figure 5A,B, which depict the first- and second-layer photomasks used to generate a double-layer master mold. - Print the photomasks either through a commercial vendor or through other accessible facilities
- Creation of the bilayer master mold using the MMAA (photolithography)
- Using standard photolithography techniques and the photoresist manufacturer's instructions, create the first layer of the master mold using the first layer photomask16. Use a 4" silicon wafer with the appropriate photoresist (i.e., SU-8) to create the desired layer thickness. Ensure the first layer thickness is greater than the subsequent layers for easy identification of the alignment markers.
- Use a light-colored marker pen (e.g., gold) to color the first layer's alignment markers on all four sides.
- Using the photoresist manufacturer's instructions, initiate the second layer of the master mold by spin-coating the photoresist onto the wafer and performing the soft bake16. Insert the coated wafer into the wafer holder of the MMAA (Figure 3B) and fix the coated wafer to the MMAA using tape.
- Attach the wafer holder to the available upright microscope using the magnetic microscope fastener (Figure 4A). Move the position of the MMAA using the x- and y-direction knobs of the microscope stage until one of the colored alignment markers on the wafer is in view through the microscope lens.
- Insert the second-layer photomask into the wafer holder, on top of the coated wafer (Figure 3C). Ensure that the first-layer's colored alignment markers can be partially seen through the alignment markers on the photomask.
- Attach the photomask to a scissor lift (also known as a support jack) through one of the side cut-outs (Figure 4B) with tape. Use the scissor lift to adjust the z-direction position of the photomask until it lies right above the coated wafer (Figure 3C).
NOTE: The scissor lift allows for fine adjustment of the z-position of the photomask, as the scissor lift can be used to move the position of the attached photomask in the z-direction. - While keeping the photomask still, look through the microscope lens and identify the first-layer’s colored alignment markers beneath the alignment markers of the photomask. Use the x- and y-direction knobs of the microscope stage to move the position of the MMAA (Figure 4D). Adjust the position of the MMAA until the alignment marker on the photomask is superimposed with the colored alignment marker on the first layer (Figure 6A,B) by observing the position of the alignment markers through the microscope lens.
- Carefully apply a slight force to the photomask and use tape to secure the photomask in place on top of the coated wafer. Detach the photomask from the scissor lift. Ensure all four alignment markers on the photomask are in alignment with the four alignment markers on the first layer.
- Once the alignment is achieved, carefully detach the wafer holder from the microscope stage. Insert the glass top plate on top of the wafer and photomask to decrease the gap between the two pieces (Figure 1). Place the entire wafer holder into the available UV light exposure system as shown in Figure 4E. Expose the second layer for the appropriate time and light intensity as described in the photoresist manufacturer's instructions16.
- Remove the wafer holder from the UV light exposure system. Remove the coated wafer from the wafer holder and detach the photomask from the wafer. Complete the processing of the second layer (e.g., post-bake, developing, and rinse and dry) as per the photoresist manufacturer's instructions16.
NOTE: The exact spin-coating, soft baking, exposing, post-baking, and developing conditions (time, temperature) will vary based on the photoresist being used and the desired layer thickness. The actual conditions and exact photolithography procedure should be based on the photoresist manufacturer's instructions.
- Preparation of a microfluidic device using the master mold (soft lithography)
- Retrieve the master mold and secure it in the middle of a 150 mm x 15 mm plastic Petri dish with tape.
- Prepare ~15-20 g of PDMS based on the manufacturer's instructions. Place the PDMS in a vacuum chamber or let it rest until free of any bubbles. Pour the PDMS into the Petri dish containing the master mold.
- Let the Petri dish with the master mold rest on the countertop until the PDMS is free of any bubbles. Place the Petri dish in an oven at 65 °C until the PDMS is fully cured (at least 3 h).
- Cut out the PDMS to reveal the microchannel structures. Cut the PDMS around the microchannel structures into separate microchips and create the inlet and outlet holes for the microfluidic device. Use tape to gently remove any small particulates that may lie on the PDMS surface.
- Complete the microchip fabrication by bonding the PDMS chip to the PDMS or a microscope slide by plasma-treating the PDMS chip and the additional substrate.
- Determination of the alignment error
- Retrieve the master mold and use the upright microscope to determine the gap distance (alignment error) between the first layer and second layer. Do this by simply measuring the distance by which the second layer is shifted and misaligned from the first layer on the microchannel structures (see Figure 5D for an example of a measured gap distance).
- Use the upright microscope to determine whether the PDMS chip contains channel walls that are straight with clear device edges. Additionally, check the PDMS chip for any possible defects that may hinder device functionality.
NOTE: The master mold fabrication (sections 3.2 and 3.3) may need to be repeated to achieve a lower alignment error. Repeated practice using the MMAA is shown to enhance the user's ability to create a well-aligned master mold. In addition, images can be obtained by scanning electron microscopy (SEM) (Figure 7) to confirm the alignment error.
Representative Results
Through the optimization and use of the MMAA (Figure 1), multilayer master molds with minimal alignment error were fabricated. The final MMAA was fabricated using the fused filament fabrication (FFF) 3D-printing process (Figure 2). The FFF process confers increased accuracy for the desired device dimensions. The MMAA consists of two main pieces (Figure 3): the base piece and the custom fastener. The base piece consists of the UV exposure unit, which acts as the wafer holder. The UV exposure unit allows proper alignment of the photomask and the coated silicon wafer. The second piece is the custom fastener that fixes the wafer holder to the platform of the microscope with magnets. The entire setup used to assist in the alignment of the top and bottom layers of the double-layer master mold is depicted in Figure 4. This system and the described protocol were used for the alignment of the markers on the photomask with the markers on the initial layer of the master mold (Figure 6). The double-layer SU-8 master mold for a microfluidic device with a herringbone pattern was then fabricated and was shown to have a gap distance of <5 µm between the two layers (Figure 5).
The two-layer master mold (Figure 7A) was then used to fabricate PDMS microchips that can be seen in Figure 7D. The SEM images seen in Figure 7B,C show that the microfluidic device with the herringbone pattern contains clear edges, straight-channel walls, and well-aligned layers, which are essential for proper device functionality. In addition, a four-layer master mold with simple circular features (Figure 8A) was created using the MMAA to show successful alignment of a multilayer master mold. Profilometer data (Figure 8B) confirms the four distinct layers of the master mold. Measurements taken of the alignment error obtained for multiple four-layer features of differing geometry confirm that the alignment error is no greater than 5% of the designed distance between the layers. From the images of the final device, it is clear that human error during fixation of the mask onto the MMAA before the UV exposure of the second layer increased the gap distance between the two device layers and caused misalignment. However, as the user becomes more familiar with the procedure, the final device can be produced with a resulting alignment error of <10 µm, as confirmed by the depicted results.
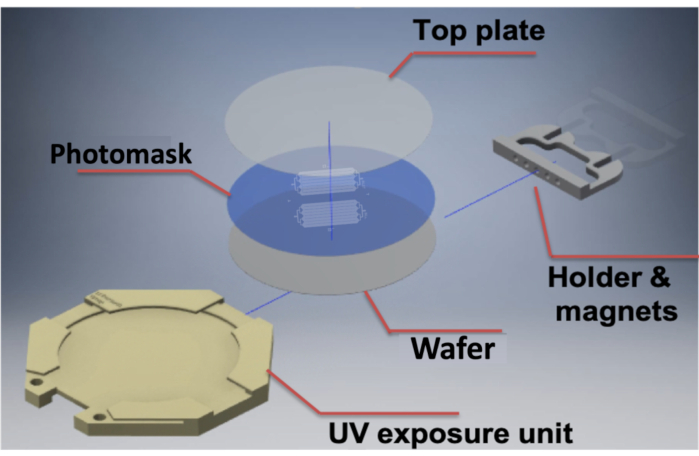
Figure 1: Design of a 3D-printable MMAA for multilayer microfabrication. The illustration depicts the two pieces of the MMAA: the UV exposure unit and the custom microscope fastener. The UV exposure unit houses, in descending order, the glass top plate, which holds the photomask against the wafer; the photomask; and the photoresist-coated wafer. The UV exposure unit is then magnetically attached to the custom microscope fastener, which is attached to the microscope stage, and then allows for proper alignment of the photomask and wafer. Abbreviations: MMAA = microscope mask alignment adapter; UV = ultraviolet. Please click here to view a larger version of this figure.
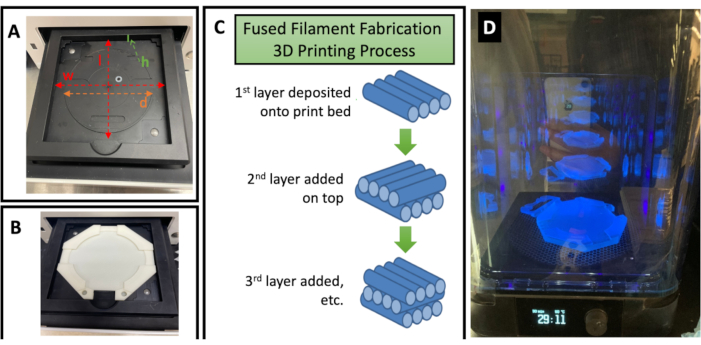
Figure 2: Customization and 3D printing of an MMAA and post-processing for a fully cured device. (A) Photo of the tray of the available UV light emission system showing the necessary measurements needed to customize the MMAA. The user should measure the diameter (d) of the inner circular rim, the inner height (h), the total width (w), and the length (l) of the tray. (B) After customization, the MMAA should then sit flat inside the tray as shown here. (C) Illustration of the FFF 3D printing process. The FFF process produces structures by layering the 3D-printed filament. The filament is deposited in thin layers, one on top of the next, until the final 3D-printed piece is produced. (D) The curing of the final 3D-printed MMAA in the UV curing chamber as part of the post-printing process. Abbreviations: MMAA = microscope mask alignment adapter; UV = ultraviolet; FFF = fused filament fabrication. Please click here to view a larger version of this figure.
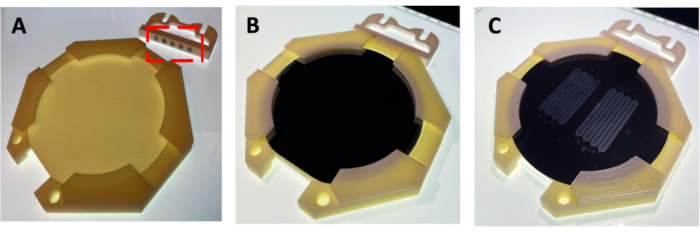
Figure 3: 3D-Printed pieces of an MMAA. (A) Two pieces were connected by magnets (indicated by red dashed rectangle). (B) MMAA containing a silicon wafer coated with a thin layer of photoresist (SU-8). (C) MMAA with a photomask over the coated silicon wafer in preparation for the alignment process. Abbreviation: MMAA = microscope mask alignment adapter. Please click here to view a larger version of this figure.
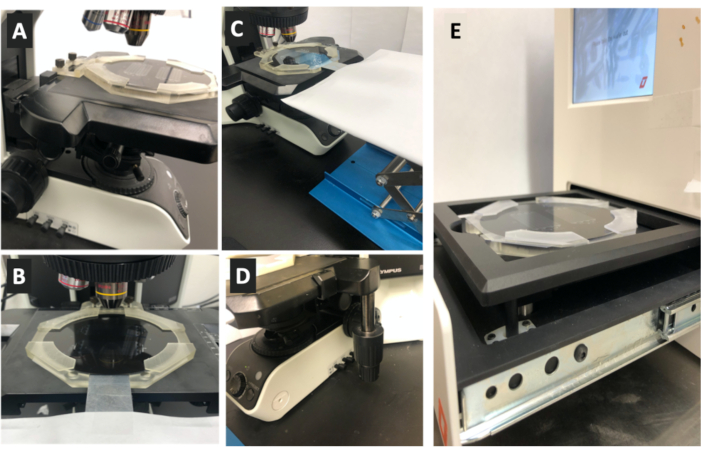
Figure 4: Procedure to use a 3D-printed MMAA for alignment of the photomask. (A) After the MMAA has been loaded with the photoresist-coated silicon wafer, the MMAA is then placed on the stage of an upright microscope system and fixed to the stage using the magnetic microscope fastener as shown in the image. (B) The photomask is then inserted into the MMAA and attached to the z-direction-adjusting platform, otherwise known as a scissor lift, through one of the sides of the MMAA as shown in the image. (C) The scissor lift platform height is then adjusted until the photomask lies right above the coated silicon wafer as shown in the image. From this point onwards, the photomask is not moved until alignment is completed. (D) To achieve perfect alignment, the position of the MMAA and hence, of the silicon wafer, on the microscope stage is then adjusted in the x- and y-directions using the microscope's knobs as shown in the image. The x- and y-positions of the silicon wafer are finely adjusted, while the user observes through the microscope lens until the alignment markers on the silicon wafer and the photomask are superimposed. Once this is achieved, the photomask can then be secured to the wafer. (E) After alignment is achieved, the MMAA is carefully detached from the microscope stage and placed in the tray of the UV light exposure system. The tray can be closed so that the wafer can be exposed to UV irradiation to cure the photoresist. Abbreviation: MMAA = microscope mask alignment adapter. Please click here to view a larger version of this figure.
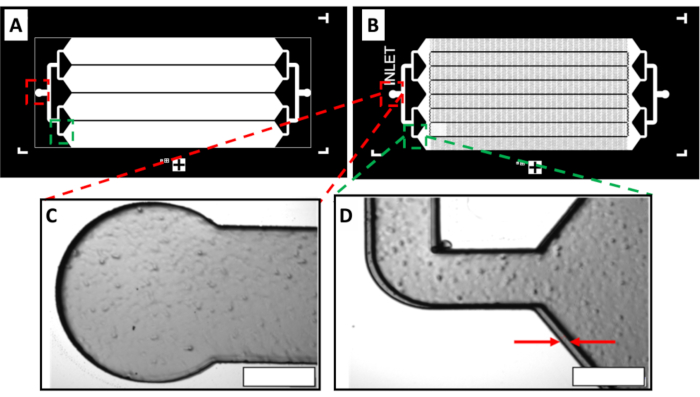
Figure 5: Double-layer channel structure created using the MMAA. The double-layer master mold is designed for the production of herringbone microfluidic devices with four parallel channels. (A) Image of the first-layer photomask design, which includes the outline for the channels and generates the hollow floor of the microfluidic device. (B) Image of the second-layer photomask design, which incorporates the herringbone pattern inside the channels that line the roof of the microfluidic device. (C) The inlet structure of the double-layer master mold indicated by red dashed rectangles in (A) and (B). The image shows minimal gap distance between the two layers.(D) A section of the double-layer master mold showing a bend in the channel indicated by green dashed rectangles in (A) and (B). The gap distance between the two arrows is 5 µm. Scale bars = 100 µm. Abbreviation: MMAA = microscope mask alignment adapter. Please click here to view a larger version of this figure.
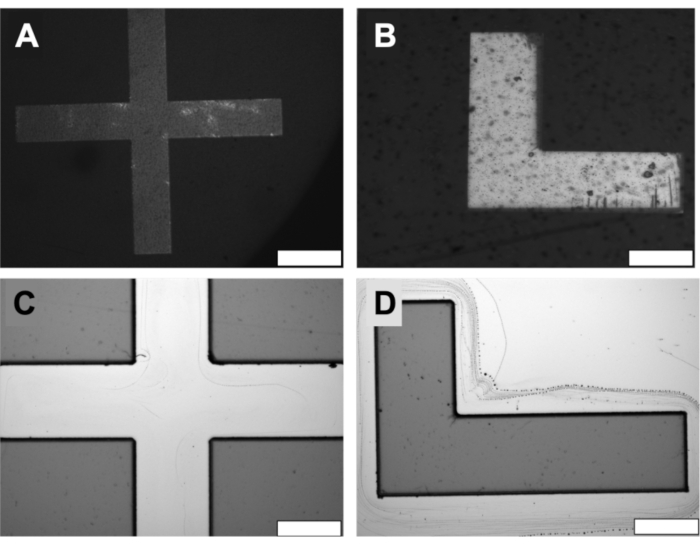
Figure 6: Microfabrication results with the MMAA. (A) and (B) show the alignment of the markers on the photomask. Scale bars = 200 µm. (C) and (D) are the corresponding images of the markers on the wafer after exposure. Scale bars = 100 µm. Abbreviation: MMAA = microscope mask alignment adapter. Please click here to view a larger version of this figure.
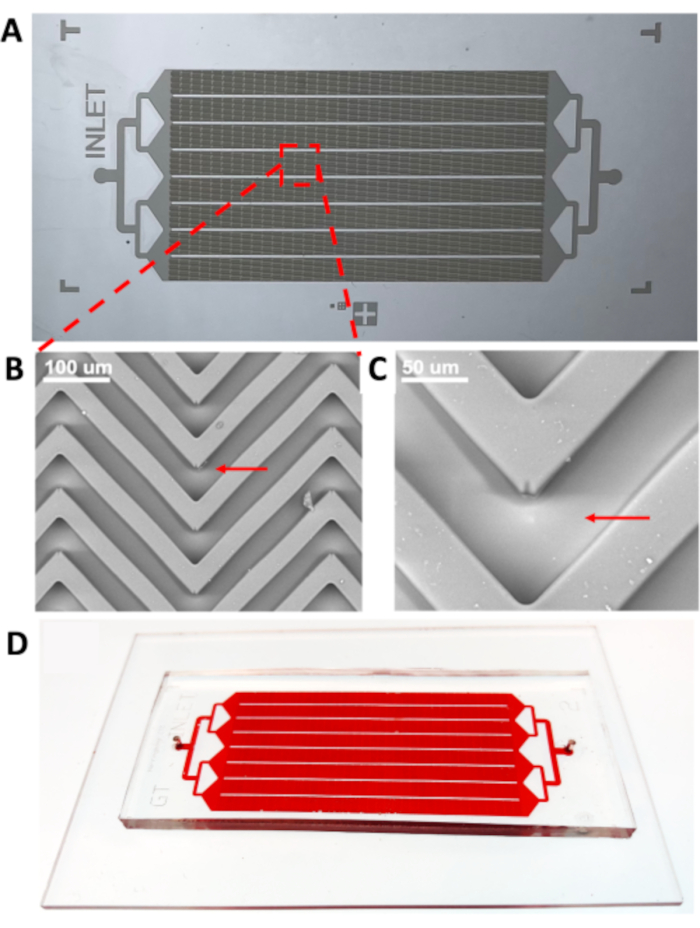
Figure 7: The master mold prepared using the MMAA and the resulting PDMS device made from the master mold. (A) Double-layer master mold of herringbone microfluidic device prepared using the MMAA to achieve alignment of layers. (B) and (C) are SEM images of the herringbone device in different scales with the red arrows pointing at the lower layer. (D) PDMS microfluidic device with herringbone pattern made using the double-layer master mold in (A). Abbreviations: MMAA = microscope mask alignment adapter; PDMS = poly(dimethylsiloxane); SEM = scanning electron microscopy. Please click here to view a larger version of this figure.
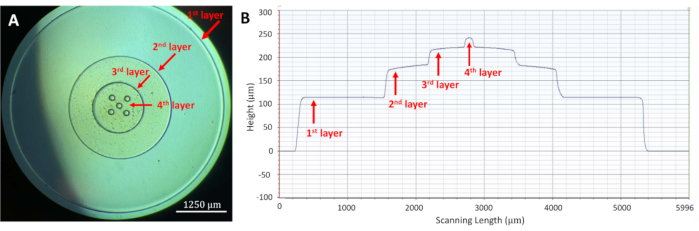
Figure 8: Image and profilometer data of a four-layer master mold created using the MMAA. (A) Image of a four-layer master mold created using the MMAA showing successful alignment of the layers. Simple circular features in descending size were chosen to demonstrate the alignment capability of the MMAA. Scale bar = 1,250 µm. (B) Profilometer data of the same circular four-layer master mold confirming the presence of the four distinct layers. Abbreviations: MMAA = microscope mask alignment adapter. Please click here to view a larger version of this figure.
Supplemantal Material. Please click here to view a larger version of this figure.
Discussion
The aforementioned protocol outlines the procedure for 3D-printing an MMAA and using the system to create a precise, multilayer, microfluidic device master mold. Although the device is easy to use, there are critical steps within the protocol that require practice and care to ensure proper alignment of the master mold layers. The first critical step is the design of the MMAA. It is essential when designing the MMAA to determine the exact measurements for the device that will allow for a proper fit inside the UV light exposure system. A misalignment of the device can cause uneven UV light exposure, which can create deformities of the master mold features. The second critical step is to take care when aligning the first and second layers of the master mold when using the MMAA. It is imperative after aligning the second-layer photomask with the first-layer alignment markers that the user takes great care when fixing the photomask to the wafer and MMAA. The micron-sized features mean that any small misalignment due to movement of the photomask during fixation can create alignment errors that can render the final PDMS device unusable. Therefore, this step requires accuracy that can be developed with practice using the MMAA. The last critical step is to ensure there is no gap between the photomask and the coated wafer to ensure even UV light exposure. This technique in using the MMAA to create multilayer master molds is limited by the attention to detail and care of the user when following the given protocol as the critical steps above must be followed to ensure well-aligned layers.
Multilayer microfluidic devices are typically difficult to produce with little error unless traditional alignment equipment is available. This equipment is expensive and because of its sensitivity, requires special training and typically a clean room environment that is not always available to smaller laboratories. In addition, previously published custom-built mask aligners typically require the purchase and assembly of many different pieces, which can still render the platforms expensive to produce and difficult to use12,13,14. The significance of the MMAA is that it is an easy-to-fabricate and cost-effective alternative to standard equipment used for multilayer microfluidic device fabrication. Additionally, the MMAA requires no special training for its use, as its application is fairly simple and uses standard laboratory equipment already present in laboratories that regularly produce and use microfluidic devices. This allows small and resource-limited laboratories to produce multilayer microfluidic devices with improved functionality.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the Center for Transformative Undergraduate Experiences from Texas Tech University for providing funding for this project. The authors would also like to acknowledge support from the Chemical Engineering Department at Texas Tech University.
Materials
| Acrylonitrile Butadiene Styrene (ABS), 3D Printing Filament | Provided by the Texas Tech University 3D printing facility | ||
| BX53, Upright Microscope | Olympus | ||
| Form 2, Stereolithography 3D printer | Formlabs | ||
| Advanced Hot Plate Stirrer | VWR | 97042-642 | |
| Isoproyl Alcohol, 70% (v/v) | VWR | BDH7999-4 | |
| Light Colored Marker | Sharpie | ||
| Magnets, 3 mm x 3 mm | WOTOY | ASIN #: B075PLVW8W | |
| SYLGARD 184 Silicone Elastomer Kit | DOW | 4019862 | |
| Petri Dish, 150 mm x 15 mm | VWR | 25384-326 | |
| Printed Photomasks | CAD/Art Services, Inc. | ||
| Aluminum Support Jack – 8" x 8", Scissor Lift | VWR | 12620-904 | |
| Silicon Wafer | University Wafer | 452 | |
| Sodium Hydroxide | VWR | ||
| Sonication Bath | Branson | CPX3800H | |
| Spin Coater | Laurell Technologies Corporation | Model WS-650MZ-23NPPB | |
| STRATASYS SR-30 | MakerBot Industries, LLC | SR-30 | Dissolvable support material for 3D printing |
| Stratasys uPrint SE 3D Printer | Computer Aided Technology, LLC | ||
| SU-8 50 | Kayaku | Y131269 0500L1GL | |
| SU-8 100 | Kayaku | Y131273 0500L1GL | |
| SU-8 Developer | Kayaku | Y020100 4000L1PE | |
| Super glue | Gorilla Glue | ||
| Trichloro(1H,1H,2H,2H-perfluorooctyl)silane | Sigma-Aldrich | 448931-10G | |
| Tape | Scotch | ||
| Form Cure, UV Curing Chamber | Formlabs | FH-CU-01 | |
| UV-KUB2, UV Light-Exposure Box | Kloe | UV-KUB2 |
References
- Betancourt, T., Brannon-Peppas, L. Micro- and nanofabrication methods in nanotechnological medical and pharmaceutical devices. International Journal of Nanomedicine. 1 (4), 483-495 (2006).
- Wheeler, A. R., et al. Microfluidic device for single-cell analysis. Analytical Chemistry. 75 (14), 3581-3586 (2003).
- Kong, D. S., Carr, P. A., Chen, L., Zhang, S., Jacobson, J. M. Parallel gene synthesis in a microfluidic device. Nucleic Acids Research. 35 (8), 61 (2007).
- Yang, M., Li, C. -. W., Yang, J. Cell docking and on-chip monitoring of cellular reactions with a controlled concentration gradient on a microfluidic device. Analytical Chemistry. 74 (16), 3991-4001 (2002).
- Keles, H., et al. Development of a robust and reusable microreactor employing laser based mid-IR chemical imaging for the automated quantification of reaction kinetics. Organic Process Research & Development. 21 (11), 1761-1768 (2017).
- Losey, M. W., Jackman, R. J., Firebaugh, S. L., Schmidt, M. A., Jensen, K. F. Design and fabrication of microfluidic devices for multiphase mixing and reaction. Journal of Microelectromechanical Systems. 11 (6), 709-717 (2002).
- Kobayashi, J., et al. A microfluidic device for conducting gas-liquid-solid hydrogenation reactions. Science. 304 (5675), 1305-1308 (2004).
- Shuler, M. L. Advances in organ-, body-, and disease-on-a-chip systems. Lab on a Chip. 19 (1), 9-10 (2019).
- Kimura, H., Sakai, Y., Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metabolism and Pharmacokinetics. 33 (1), 43-48 (2018).
- Lee, H., et al. A pumpless Multi-Organ-on-a-Chip (MOC) combined with a Pharmacokinetic-Pharmacodynamic (PK-PD) model. Biotechnology and Bioengineering. 114 (2), 432-443 (2017).
- Kang, S. -. W., Wang, M. Application of soft lithography for nano functional devices. Lithography. , 403-426 (2010).
- Challa, P. K., Kartanas, T., Charmet, J., Knowles, T. P. J. Microfluidic devices fabricated using fast wafer-scale LED-lithography patterning. Biomicrofluidics. 11, 014113 (2017).
- Li, X., et al. Desktop aligner for fabrication of multilayer microfluidic devices. Review of Scientific Instruments. 86 (7), 075008 (2015).
- Pham, Q. L., Tong, N. -. A. N., Mathew, A., Voronov, R. S. A compact low-cost low-maintenance open architecture mask aligner for fabrication of multilayer microfluidics devices. Biomicrofluidics. 12 (4), 044119 (2018).
- Ravi, T., Ranganathan, R., Shunmugam, M. S., Kanthababu, M. Topology and build path optimization for reducing cost in FDM uPrint SE. Advances in Additive Manufacturing and Joining. , 189-198 (2019).
- SU-8 Permanent Negative Epoxy Photoresist. Kayaku Advanced Materials Available from: https://kayakuam.com/wp-content/uploads/2020/09/KAM-SU-8-50-100-Datasheet-9.3.20-Final.pdf (2020)

