Summary
This protocol isolates high quality total RNA from fecal samples of animal and human subjects. A commercial miRNA isolation kit is used with significant adaption to isolate pure RNA with optimized quantity and quality. The RNA isolates are good for most downstream RNA assays such as sequencing, micro-array, and RT-PCR.
Abstract
It is becoming clear that RNA exists in the gut lumen and feces in animals and humans. The protocol described below isolates total RNA including microRNAs from fecal samples of animal and human subjects. The aim is to isolate total RNA with high purity and quantity for downstream analyses such as RNA sequencing, RT-PCR, and micro-array. The advantages of this optimized protocol in the miRNA isolation are capabilities of isolating highly purified RNA products with additional washing steps described, increased quantity of RNA obtained with an improved method in the resuspension of sample, and important tips of decontamination. One limitation is the inability to process and purify larger sample of more than 200 mg as these sample sizes would cause a difficulty in the clear formation of the interphase. Consequently, the large sample size may contaminate the aqueous phase to be extracted as described in the protocol with organic matters that affect the quality of RNA isolated in the end. However, RNA isolates from a sample of up to 200 mg are sufficient for most of downstream analyses.
Introduction
Extracellular RNA is getting recognized as a significant factor that mediates many biological processes1. Extracellular RNA in feces was first reported in 2008 as a marker for colon cancer and active ulcerative colitis2, and it was recently revealed as a normal component of the gut lumen and feces and mediates host-microbe communications3,4,5. The purpose of this RNA isolation protocol is to extract high quality extracellular RNA from fecal samples collected from animal and human subjects. The protocol was adapted from a commercial miRNA Isolation Kit. The RNA acquired is utilized for downstream analyses such as RNA sequencing, RT-PCR, and micro-array. The protocol includes several important and useful tips to maximize the quantity and quality of RNA found in the feces of animals and humans. The reason to develop and optimize this method of RNA (including microRNA) isolation is to decrease microbial RNA in the feces, limit the variables in the research studies and analyze the RNA composition in the gut without accounting various confounding factors and sources of contamination. Of note, this RNA isolation minimizes the release of RNA from living cell and living microbes (cellular RNA). It focuses on extracellular RNAs that have been released by gut cells or been acquired via food intake. Principally, this method is not suitable for studies where the microbial transcriptome is investigated.
Protocol
All methods involving research animals described here have been approved by the Institutional Animal Care and Use Committee (IACUC) of Brigham and Women’s Hospital, Harvard Medical School.
All methods involving human research subjects described here are in accordance with the guidelines set by the Partners Human Research Committee.
1. Fecal sample collection
- Autoclave or prepare a sterile and nuclease-free 2 mL microcentrifuge tube with a screw cap for each animal subject in an experiment.
- For human subjects, provide an appropriate, nuclease-free, and sterile stool specimen collection device for each subject.
- Collect 25-100 mg (approximately 1-4 fecal pellets for mouse fecal samples) of fecal samples from each animal subject in a sterile environment.
NOTE: Two or more fecal pellets (~50 mg or heavier) are preferred for obtaining RNA of the highest purity.- Utilize all necessary Personal Protective Equipment (PPE) and materials, for example: a pair of laboratory gloves, a disinfectant spray and a sterile paper towel, to sterilize the working area, where the animal research subject is placed on, to avoid fecal sample contamination.
- For human subjects, instruct each research subject/collector to collect 100-200 mg of stool samples in an environment as sterile as possible. Use standard sterile operation and avoid stool specimen contamination.
- Utilize all necessary Personal Protective Equipment (PPE) and materials, for example: a pair of laboratory gloves, a disinfectant spray and a sterile paper towel, to sterilize the working area, where the animal research subject is placed on, to avoid fecal sample contamination.
- Collect fecal samples from each animal subject directly into a 2 mL microcentrifuge tube with a screw cap without touching any other surfaces to avoid contamination.
- For human subjects, instruct each subject to defecate directly into an applicable collection device provided (e.g., a sterile stool specimen collection kit) to avoid contamination.
NOTE: Instruct subject to avoid contamination by toilet surfaces, water, urine, or any other non-sterile surfaces/objects.
- For human subjects, instruct each subject to defecate directly into an applicable collection device provided (e.g., a sterile stool specimen collection kit) to avoid contamination.
- Freeze fecal samples collected in 2 mL microcentrifuge tubes immediately at -80 °C or place in a bucket of dry ice for the better quantity and quality of RNA before feces resuspension as described in the steps below.
- For human stool specimens, aliquot each fresh specimen of 200 mg into 2 mL microcentrifuge tubes with screw caps and freeze them at -80 °C before feces resuspension as described below.
- For the storage of stool specimens not in 2 mL microcentrifuge tubes with screw caps, weigh and transfer 100-200 mg each of frozen specimens into separate 2 mL microcentrifuge tubes with screw caps before feces resuspension as described below.
NOTE: For human stool specimens, avoid taking more than 200 mg of stool specimens for RNA isolation as it may cause difficulties in the following steps. With an overloaded sample in a 2 mL microcentrifuge tube, the aqueous phase, organic phase and interphase may not be clearly formed and separated. The procedure can be paused here.
- For the storage of stool specimens not in 2 mL microcentrifuge tubes with screw caps, weigh and transfer 100-200 mg each of frozen specimens into separate 2 mL microcentrifuge tubes with screw caps before feces resuspension as described below.
- For human stool specimens, aliquot each fresh specimen of 200 mg into 2 mL microcentrifuge tubes with screw caps and freeze them at -80 °C before feces resuspension as described below.
2. Preparations of wash solutions
- Add 21 mL of American Chemical Society (ACS) grade 100% ethanol to the Wash Solution 1 provided in the miRNA Isolation Kit (see Table of Materials) to reach the final volume of 30 mL, as shown on the bottle. Vortex until everything dissolves in the bottle.
- Add 40 mL of ACS grade 100% ethanol to the Wash Solution 2/3 provided to reach the final volume of 50 mL, as shown on the bottle. Vortex for 5 s or until the final mixture is well blended.
3. Preparations of equipment and materials
- Spray the working area and equipment, for example: the laboratory bench, the working area in the chemical fume hood and the micro-centrifuge tube racks, with a Ribonuclease (RNase) decontamination solution (e.g., commercially available RNase decontamination solution). To avoid contamination, apply with the RNase decontamination solution on the surfaces wherever and whenever deemed necessary.
- Don a clean laboratory coat, put on a facial mask, and wear appropriate laboratory gloves to protect the RNA in the fecal samples from nucleases present on human skin. Spray gloves with a RNase decontamination solution and change gloves frequently to avoid contamination.
- Prepare a bucket of dry ice for fecal samples stored at -80 °C to prevent thawing before feces resuspension and a bucket of ice for materials, for example: Acid-Phenol: Chloroform, to prolong the shelf life.
NOTE: Ensure materials including the media used in the protocol are sterile without contamination of nucleases.
4. Feces resuspension
- Resuspend 25-100 mg of fecal samples in 600 µL of sterile 1x Dulbecco's Phosphate-Buffered Saline (DPBS).
CAUTION: Fecal samples should be processed immediately when thawed from -80 °C without even partial thawing to minimize the release of RNases and cellular RNA as ice crystals rupture both interior and exterior cellular compartments when cells in sample thaw.- Add 600 µL of 1x DPBS to the 2 mL microcentrifuge tube with a screw cap containing fecal samples at room temperature (RT).
- Incubate the mixture of fecal samples submerged in 600 µL of 1x DPBS in the 2 mL microcentrifuge tube capped for 30 min at RT.
- Resuspend the mixture by mashing with 1 mL pipette tip and vortex well with the 2 mL microcentrifuge tube capped. To optimize and increase the quantity and quality of RNA, resuspend the mixture with a homogenizer with the setting for one cycle at S4000 (or 4000 rpm) and 45 s.
5. Organic extraction
CAUTION: Use the hazardous chemical fume hood for the following steps until Step 6 with the use of acid-phenol: chloroform and ACS grade 100% ethanol due to their toxicity and inflammability. Change PPE as needed and follow proper standard precautions when dealing with hazardous material.
- Extract RNA with 600 µL of acid-phenol: chloroform (the volume of acid-phenol: chloroform required equals to the initial volume of added 1x DPBS in Step 4.1).
- Add 600 μL of acid-phenol: chloroform to the suspension from Step 4.1.
NOTE: Withdraw acid-phenol: chloroform from the lower phase in the bottle as the upper phase is mixed with an aqueous buffer. If the interphase between these two phases is disturbed, then wait and withdraw acid-phenol: chloroform only when the interphase re-establishes itself to avoid contamination.
- Add 600 μL of acid-phenol: chloroform to the suspension from Step 4.1.
- Vortex the mixture for 60 s to thoroughly mix. Alternatively, to optimize and increase quantity of RNA in the yield, mix by using a homogenizer with the setting for one cycle at S4000 and 45 s.
- Centrifuge for 15 min at 10,000 x g at RT to separate the aqueous and organic phases with a microcentrifuge. After centrifugation, the interphase should be compact. If not, repeat the centrifugation.
NOTE: If the interphase could not be as compact as desired possibly due to uneven ratio of the initial volume to the volume of added acid-phenol: chloroform after several repeats of centrifugation, proceed to recover the aqueous phase with a greater care to avoid contamination. - Recover the aqueous phase and transfer it to a new 2 mL microcentrifuge tube with a hinge cap (not provided by the miRNA isolation kit).
- Remove the aqueous (or upper) phase carefully without disturbing the lower phase and transfer it to a new 2 mL microcentrifuge tube with a hinge cap. Note the volume transferred (e.g., ~500 µL).
NOTE: When the interphase is compact and the upper phase is clearly separated, there are possibly a few tiny residual particles floating on the top of the aqueous phase. Pipette carefully to avoid these residues and only recover visibly and clearly separated aqueous phase to ensure a quality RNA yield, even if you could only obtain a small volume of the aqueous phase.
- Remove the aqueous (or upper) phase carefully without disturbing the lower phase and transfer it to a new 2 mL microcentrifuge tube with a hinge cap. Note the volume transferred (e.g., ~500 µL).
6. Final RNA isolation
- Add 1.25 volumes of RT ACS grade 100% ethanol to the aqueous phase in the 2 mL microcentrifuge tube (e.g., add 625 μL of 100% ethanol if 500 μL of aqueous phase is recovered from Step 5.4.). Vortex 3 s.
- Load the aqueous phase/ethanol mixture through the filter cartridge provided in the miRNA isolation kit.
- For each sample, place the filter cartridge into one of the collection tubes supplied by the kit.
- Pipette and load 600 µL of the aqueous phase/ethanol mixture into the filter cartridge.
NOTE: Vortex the mixture briefly to thoroughly mix the ethanol with aqueous phase before pipetting. No more than 700 µL of the aqueous phase/ethanol mixture can be loaded at a time.
- Pipette and load 600 µL of the aqueous phase/ethanol mixture into the filter cartridge.
- Centrifuge at 10,000 x g for 90 s to filter through the mixture. Spinning at a higher speed may damage the filter.
- Discard the filtrate and repeat Steps 6.2.1 to 6.2.2 until all the mixture is filtered through the same filter membrane in successive applications. Keep and reuse the same collection tube for washing steps below.
- Wash the filter with 700 µL of miRNA Wash Solution 1.
CAUTION: miRNA Wash Solution 1 contains guanidine thiocyanate that can cause skin irritation and serious eye damage. Wear necessary PPE, for examples: gloves, face shield, protective laboratory coat. Change gloves frequently as necessary.- Apply 700 µL of miRNA Wash Solution 1, the working solution prepared with the ACS grade 100% ethanol, into the filter cartridge.
- Centrifuge for 60 s to filter the miRNA Wash Solution 1 through the filter cartridge.
- Discard the filtrate from the collection tube and place the same filter cartridge into the same collection tube.
- Wash the filter with Wash Solution 2/3 one time each with volumes of 700 µL, 500 µL, and 250 µL consecutively.
- Apply 700 µL of Wash Solution 2/3, the working solution prepared with the ACS grade 100% ethanol, into the filter cartridge.
- Centrifuge at 10,000 x g for 1 min.
- Discard the filtrate from the collection tube and place the same filter cartridge into the same collection tube.
- Apply 500 µL of Wash Solution 2/3 into the filter cartridge.
- Centrifuge at 10,000 x g for 1 min.
- Discard the filtrate from the collection tube and place the same filter cartridge into the same collection tube.
- Apply 250 µL of Wash Solution 2/3 into the filter cartridge.
- Centrifuge at 10,000 x g for 1 min.
- Discard the filtrate from the collection tube.
- Transfer the filter cartridge into a new collection tube and spin the assembly for 5 min to remove residual fluid from the filter.
- Apply 700 µL of Wash Solution 2/3, the working solution prepared with the ACS grade 100% ethanol, into the filter cartridge.
- For each sample, place the filter cartridge into one of the collection tubes supplied by the kit.
7. Elute RNA with 50 µL nuclease-free water
- Transfer the filter cartridge into a new collection tube. Pipette 50 µL of nuclease-free water to the center of the filter and cap the collection tube.
- Incubate at RT for 10 min.
- Spin for 5 min at 8,000 x g to recover RNA into the new collection tube.
- Determine the concentration and purity of recovered fecal RNA using a fluorometer. Recovered fecal RNA can be stored at -80 ˚C.
Representative Results
Representative RNAs were isolated from 50 mg mouse fecal samples (2 mouse fecal pellets) and 100 mg human stool specimens respectively and eluted in 50 µL nuclease-free water. Spectrophotometer analysis of the concentration suggests a total amount of 49 µg and 16 µg RNA were isolated respectively (Table 1). The RNA purity was high as indicated by an A260/A280 ratio of ~2.0 and an A260/A230 ratio of ~1.8 (Table 1). As reported3, the majority of RNAs in the feces are microRNA and those microRNAs can exist in the exosome. Consistent with this, a chip-based electrophoresis assay of RNA suggests that representative RNA isolates from mouse and human feces are low in or lack of 18S and 28S rRNA compositions, and the size of RNA isolates falls in the small RNA region (Figure 1A). A further small RNA electrophoresis with the chip-based electrophoresis reveals that a large portion of the RNAs are of microRNA size (Figure 1B). This is consistent with the observation that the quantification completed by using a small RNA bioanalyzer is comparable to that obtained with previous assays6.
| Sample ID | Elution volume (µL) | RNA concentration (ng/µL) | A260/A280 | A260/A230 | Yield (ng) |
| Mouse | 50 | 978.333 | 2.036 | 1.897 | 48916.65 |
| Human | 50 | 330.759 | 1.981 | 1.849 | 16537.95 |
Table 1: Representative nanodrop analysis of RNA isolated with this protocol. Representative RNAs were isolated from 2 mouse fecal pellets or 100 mg human stool specimens, eluted in 50 µL nuclease-free water. RNA concentration, ratio of A260/A280, and ratio of A260/A230 were measured with nanodrop.
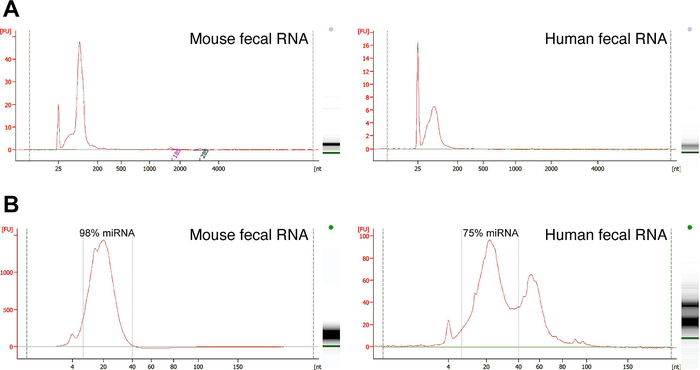
Figure 1: Representative chip-based electrophoresis analyses of size distribution of fecal RNA isolates. (A) Representative RNAs isolated from 2 mouse fecal pellets (left panel) and 100 mg human stool specimens (right panel) using the protocol described here were characterized using the chip-based electrophoresis assay. This assay suggests that the majority of RNA isolates were small RNA. (B) The isolates were then subjected for the small RNA electrophoresis with the chip-based electrophoresis system to analyze the size distribution of the isolates. Please click here to view a larger version of this figure.
Discussion
It is important to use RNase-free technique to prevent RNase contamination during the isolation7. After centrifugation and the formation of a compact interphase, it is key to avoid the interphase, lower phase, and the particle contaminant floating on the top of the aqueous phase when recovering the aqueous phase. Additionally, two washing steps with 500 µL and 250 µL Wash Solution 2/3 are added to eliminate contaminants in the filter membrane for optimized quality. Furthermore, a start sample material of more than 200 mg is not recommended as it may create difficulty in the clear formation of an interphase. Similarly, a sample material of less than 25 mg is not recommended as it may not be sufficient to extract enough RNA samples for downstream analysis.
The incredible growth in microbiome study has driven the measurements of microbial species, genes to metatranscriptional studies of the microbial profile8. MicroRNAs in the stool have been studied as markers for diseases9,10,11. Since the first report of fecal microRNA mediating host-microbe interactions3, increasing studies start to investigate the contributions of host and diet in the gut ecosystem12,13,14. Noteworthily, due to the richness of microbes in the gut lumen and feces, studies focusing on host arm of the host-microbe interaction demands minimal RNA contamination from microbes. Thus, an RNA extraction protocol that includes steps of cell lysing15 is not ideal for the study of RNAs released from host and diet. As such, we adapted this protocol to eliminate lysis steps for minimizing contaminations of RNA from living bacteria and living host cells in feces.
This protocol works for studies where extracellular RNA in the fecal or gut lumen content is an aim of interest. RNA isolated using this protocol is total RNA, including microRNA as major component. This protocol does not distinguish whether the RNA is in exosome, microvesicles, or in a vesicle-free form.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We received technical assistance from the Biopolymers Facility at Harvard Medical School for bioanalyzer. This work was supported by National Multiple Sclerosis Society research grant RG-1707-28516 (H.L.W. and S.L.).
Materials
| Acid-Phenol: Chloroform, pH 4.5 (with IAA, 125:24:1) | Thermo Fischer Scientific | AM9720 | |
| DPBS, no calcium, no magnesium | Thermo Fischer Scientific | 14190-144 | |
| Gloves | |||
| Microcentrifuge | |||
| mirVana miRNA Isolation Kit | Thermo Fischer Scientific | AM1561 | |
| Nuclease-Free microcentrifuge tubes (1.5 mL, 2 mL) | |||
| Nuclease-Free Water (Not DEPC-treated) | Thermo Fischer Scientific | AM9937 | |
| Pipettor and Nuclease-Free Pipette tips (with filter) | |||
| PowerLyzer 24 Homogenizer | QIAGEN | 13155 | |
| RNaseZap RNase Decontamination Solution | Thermo Fischer Scientific | AM9780 | |
| Vortex Shaker |
References
- Das, S., et al. The extracellular RNA communication consortium: Establishing foundational knowledge and technologies for extracellular RNA research. Cell. 177 (2), 231-242 (2019).
- Ahmed, F. E., et al. Diagnostic microRNA markers for screening sporadic human colon cancer and active ulcerative colitis in stool and tissue. Cancer Genomics & Proteomics. 6 (5), 281-295 (2009).
- Liu, S., et al. The host shapes the gut microbiota via fecal microRNA. Cell Host & Microbe. 19 (1), 32-43 (2016).
- Viennois, E., et al. Host-derived fecal microRNAs can indicate gut microbiota healthiness and ability to induce inflammation. Theranostics. 9 (15), 4542-4557 (2019).
- Liu, S., et al. Oral administration of miR-30d from feces of MS patients suppresses MS-like symptoms in mice by expanding Akkermansia muciniphila. Cell Host & Microbe. 26 (6), 779-794 (2019).
- Masotti, A., et al. Quantification of small non-coding RNAs allows an accurate comparison of miRNA expression profiles. Journal of Biomedicine & Biotechnology. 2009, 659028 (2009).
- Green, M. R., Sambrook, J. How to win the battle with RNase. Cold Spring Harbor Protocols. 2019 (2), 101857 (2019).
- Franzosa, E. A., et al. Relating the metatranscriptome and metagenome of the human gut. Proceedings of the National Academy of Sciences. 111 (22), 2329-2338 (2014).
- Wohnhaas, C. T., et al. Fecal microRNAs show promise as noninvasive Crohn’s disease biomarkers. Crohn’s & Colitis. 2 (1), 003 (2020).
- Tomkovich, S., et al. Human colon mucosal biofilms and murine host communicate via altered mRNA and microRNA expression during cancer. mSystems. 5 (1), (2020).
- Tarallo, S., et al. Altered fecal small RNA profiles in colorectal cancer reflect gut microbiome composition in stool samples. mSystems. 4 (5), 70 (2019).
- Xing, S., et al. Breed differences in the expression levels of gga-miR-222a in laying hens influenced H2S production by regulating methionine synthase genes in gut bacteria. Research Square. , (2020).
- Teng, Y., et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host & Microbe. 24 (5), 637-652 (2018).
- Moloney, G. M., Viola, M. F., Hoban, A. E., Dinan, T. G., Cryan, J. F. Faecal microRNAs: indicators of imbalance at the host-microbe interface. Beneficial Microbes. , 1-10 (2017).
- Giannoukos, G., et al. Efficient and robust RNA-seq process for cultured bacteria and complex community transcriptomes. Genome Biology. 13 (3), 23 (2012).

