Embryo Injections for CRISPR-Mediated Mutagenesis in the Ant Harpegnathos saltator
Summary
Many characteristics of insect eusociality rely on within-colony communication and division of labor. Genetic manipulation of key regulatory genes in ant embryos via microinjection and CRISPR-mediated mutagenesis provides insights into the nature of altruistic behavior in eusocial insects.
Abstract
The unique traits of eusocial insects, such as social behavior and reproductive division of labor, are controlled by their genetic system. To address how genes regulate social traits, we have developed mutant ants via delivery of CRISPR complex into young embryos during their syncytial stage. Here, we provide a protocol of CRISPR-mediated mutagenesis in Harpegnathos saltator, a ponerine ant species that displays striking phenotypic plasticity. H. saltator ants are readily reared in a laboratory setting. Embryos are collected for microinjection with Cas9 proteins and in vitro synthesized small guide RNAs (sgRNAs) using home-made quartz needles. Post-injection embryos are reared outside the colony. Following emergence of the first larva, all embryos and larvae are transported to a nest box with a few nursing workers for further development. This protocol is suitable for inducing mutagenesis for analysis of caste-specific physiology and social behavior in ants, but may also be applied to a broader spectrum of hymenopterans and other insects.
Introduction
The evolution of eusociality in insects, namely those of the orders Hymenoptera and Blattodea (formerly Isoptera), has resulted in unique and often sophisticated behavioral traits that manifest on both the individual and the colony levels. Reproductive division of labor, a trait characterizing the most advanced groups of social insects, often involves caste systems composed of several behaviorally and often morphologically distinctive groups. Such behavioral and morphological diversity between castes is controlled not only by their genetic system, but also often by the environment1,2,3,4, making eusocial insects attractive subjects for genetic and epigenetic research.
The ability to manipulate the genetic system of eusocial insects has proven to be challenging as many species do not mate and reproduce in laboratory settings. Most eusocial insects also have very few reproductive individuals in a colony, limiting the number of offspring that can be produced and consequently, limiting the sample size for genetic manipulation5. Additionally, many eusocial insects have long generation times compared to insects commonly used for genetic studies (such as Drosophila), adding to the difficulty of establishing genetic lines5. Some eusocial species, however, can generate a large proportion of reproductively active individuals in a colony, which alleviates the challenges and provides opportunities to establish mutant or transgenic lines.
In the case of the ponerine ant species, Harpegnathos saltator, all female workers can become reproductively active upon the death of a queen or social isolation. These workers are referred to as "gamergates" and can be used to generate new colonies6. Furthermore, there may be more than one gamergate present in a colony, thus increasing offspring production5,7,8. Thus far, mutant and/or transgenic lines have been developed in the European honeybee, Apis mellifera, and in the ant species, H. saltator, Ooceraea biroi, and Solenopsis invicta9,10,11,12,13,14,15. Genetic analyses in social bees and ants have paved the way toward a better understanding of eusociality, providing an array of opportunities to study genes and their impacts on eusocial insect behavior and caste-specific physiology.
Here, we provide a protocol for genetic modification via the CRISPR/Cas9 system in H. saltator. Specifically, this technique was used to generate a germline mutation in orco, the gene encoding the obligate co-receptor of all odorant receptors (ORs)10. OR genes have been remarkably expanded in hymenopteran eusocial insects16, and orco plays an essential role in insect olfaction; in its absence, ORs do not assemble or function normally. Mutations of the orco gene therefore disrupt olfactory sensation, neural development, and associated social behaviors9,10.
In this protocol, Cas9 proteins and small guide RNAs (sgRNAs) are introduced into ant embryos using microinjection for the purpose of inducing mutagenesis of a target gene. Here, we will describe the microinjection procedure in detail along with directions regarding the care of colonies and injected embryos. These methods are appropriate for inducing mutagenesis in a variety of different genes in H. saltator ants and may be applied to a broader spectrum of hymenopteran insects.
Protocol
1. Regular maintenance of Harpegnathos saltator colonies
- Maintain wild-type colonies of H. saltator in transparent plastic boxes in an ant rearing room at 22-25 °C and a photoperiod of 12 hours light: 12 hours dark (12L:12D) lighting schedule.
- Use small boxes (9.5 x 9.5 cm2) to rear individual workers or small colonies. Use medium boxes (19 x 13.5 cm2) or large boxes (27 x 19 cm2) to rear larger colonies (Figure 1).
- To create nest boxes, use plaster to make floors for the boxes. As the wet plaster is drying in the medium and the large boxes, press a foam block into the plaster a few centimeters deep and a few centimeters from the back of the box to designate a lower nest region. Once the plaster has dried, cover the designated nest region with a square piece of glass.
NOTE: In small boxes, there is no need to designate a lower nest region. If ants are neglecting to use the designated lower nest region, it may help to cover the glass with a square piece of red cellophane. This gives the impression of a dark underground space resembling nests that H. saltator use in the wild and may encourage them to move their brood to the designated region.
- Feed colonies with live crickets twice per week.
NOTE: Colonies should be fed enough that they consume all crickets prior to their next feeding. Feed any isolated ants and mutant ant colonies with crickets pre-stung by workers of a regular colony 2-3 times every week. - Apply water regularly to the plaster nest box flooring using a wash bottle.
NOTE: The plaster should be moist enough that it does not feel dusty to the touch, but it should be dry enough that all added water is absorbed by the plaster. It is important that the nests are not watered excessively. On average, nest boxes will need a small amount of water added once per week. - Whenever feeding occurs, remove trash and dead individuals. Freeze all waste and dead ants overnight at -30 °C before disposing of these materials as regular garbage.
- Add a pinch of dried sawdust to colonies periodically; this helps larvae as they undergo pupation and helps workers keep the nest box clean.
2. Preparation of quartz glass microinjection needles
- Use a micropipette puller to pull glass microinjection needles.
- Select the glass to be pulled. Ensure that the glass being used has been stored in a dust-free and clean environment.
NOTE: Here, thin-walled filamentous quartz glass with an outer diameter of 1.0 mm, inner diameter of 0.5 mm, and length of 7.5 cm has been used to produce microinjection needles. If injecting a soft-bodied embryo, borosilicate needles may also be applicable, but borosilicate needles are not able to penetrate hard chorion. - Set the parameter settings of the puller. Use a two-step process to pull microinjection needles for H. saltator embryos: parameters for the first step include heat of 575, filament of 3, velocity of 35, delay of 145, and a pull of 75; parameters of the second step include heat of 425, filament of 0, velocity of 15, delay of 128, and a pull of 200. Following the second step, ensure that the resulting needle has a 2 mm taper and a tip of 0.5 µm (Figure 2).
NOTE: This set of parameters, along with parameters for other needle types, can be found in the Operation Manual17. Manuals for pipette pullers often provide recommended parameters for a variety of techniques. Some trial and error may be required to determine which parameters generate the best needles for specific needs. A short taper is ideal for H. saltator injections, as it can penetrate the hard chorion of H. saltator embryos. If injecting a soft embryo, such as one that has been dechorionated (e.g., Drosophila), a longer taper around 10 mm may yield better results. Operation manuals for pipette pullers usually provide specific parameters for pulling needles suitable for different needs. It is important that gloves are worn while handling glass filaments and pulling needles. Oils from bare hands may transfer to the glass if gloves are not worn. - Once parameters are set, use a micropipette puller to pull needles for microinjection. Ensure that needles are kept in a dust-free and clean environment until used.
NOTE: It is recommended to use freshly pulled microinjection needles. If pre-pulled needles are used, they should be stored properly in a box to prevent damage of needle tips and potential contamination. Needles that have undergone long-term storage are not recommended for embryo injections.
3. Preparation of microinjector
- Use a microinjector to inject desired materials into ant embryos.
- Prepare the microinjection mixture of Cas9 proteins and in vitro synthesized small guide RNAs (sgRNAs)10. Keep the mixture on ice until it is time to load a microinjection needle. When not in use, store the microinjection mixture at -80 °C.
NOTE: The concentrations vary in different species. High concentration may cause high mortality, whereas low concentration may reduce efficiency. We use 0.2 µg/µL Cas9 proteins and 0.2 µg/µL sgRNAs for H. saltator embryo injection. Design of our sgRNAs followed a previously established protocol18. Gene sequences were obtained from DNA databases. The genome sequence of H. saltator was also previously reported19,20. - Adjust the injection parameters for H. saltator embryos: an injection pressure of 140 hectopascal (hPa), a constant pressure of 70 hPa, and a time of 0.4 seconds. Adjust constant pressure such that material only flows in one direction. Adjust the injection pressure and time only if no material is flowing from the needle into the embryo.
NOTE: If injecting a different type of embryo, the primary parameter that may change is constant pressure, which is responsible for ensuring that fluid from the embryo does not flow back into the needle. Volume is not controlled for in this protocol. Setting the parameters of the microinjector is sufficient for obtaining consistent injections. - Load a microinjection needle with 2 µL of the mixture using microloader pipette tips. Do this slowly to ensure that no bubbles are formed in the mixture.
NOTE: If bubbles form, it may be difficult to maintain consistent microinjections. - Break just the tip of the needle by breaking along the edge of the tape such that a narrow taper is still maintained. Ensure that the needle is broken just enough that the tip is opened, but not so much that the taper is broken off.
NOTE: Importantly, if the opening of the needle is too wide after breaking, the user will see liquid run out of the needle when mounted to the pressurized microinjector prior to application of injection pressure. Some microinjection protocols advise breaking needles by cutting the tip with scissors. This method is not advised, as scissors may cause the tip of the needle to shatter. Injecting embryos with a shattered needle will be highly damaging. - Mount the needle to the micromanipulator
4. Injection of embryos
- Select embryos for microinjection from the syncytial stage: the time during development in which nuclei divide without cytokinesis.
NOTE: This is the ideal time during development for genome editing by microinjection, as discovered previously in Drosophila21. H. saltator embryos pass the syncytial stage and reach cellularization around 36 h after egg deposition10. Higher efficiency is achieved if younger embryos are used for injections. - Line embryos on a piece of double-sided tape stuck to a glass microscope slide. Ensure that embryos are secured well to the tape to prevent movement during injection. Lay the embryos in a vertical orientation, such that the lateral side of the embryo is at the edge of the tape (Figure 3). Place the slide and lined embryos onto the stage of the microscope at a designated microinjection workstation.
NOTE: It is recommended that the embryos are arranged such that successive injections can be performed by moving the slide on the stage instead of adjusting the needle position with every injection. This allows for successive injections to be performed more efficiently. - Align the needle with the first embryo to be injected using the micromanipulator (Figure 4a).
- Laterally puncture the needle into the first embryo along its dorsal/ventral axis under a microscope.
- Inject the microinjection mixture. Look for slight movement of the embryo, indicating an increase in internal pressure due to the injected liquid. Additionally, watch for the formation of a small droplet containing visible trace of tissue and/or lipid on the outer membrane of the embryo (Figure 4b).
NOTE: Presence of trace of tissue and/or lipid in the droplet indicates that the needle has successfully punctured both chorion and vitelline membrane of the embryo. If traces of these materials are not present, the injection was not performed successfully and should be repeated. After a few seconds, the droplet will be reabsorbed by the embryo and will no longer be visible. - Gently remove the needle from the embryo immediately, and proceed to the next by adjusting the position of the microscope slide. Repeat until all the embryos have been injected.
- Once all embryos on the slide have been successfully injected, transfer the slide to a humid box for 1 hour to give the embryos time to recover from the injection process prior to being removed from the slide.
5. Rearing of injected embryos
- After 1 hour of incubation in a humid box, gently remove the injected embryos from the tape using featherweight forceps, and transfer them to a tube filled with a small amount of 70% ethanol. Invert the tube several times to transfer the embryos to the bottom of the tube. Repeat the ethanol wash once, followed by three washes with autoclaved water.
- Using a small and soft paintbrush, transfer all injected embryos to 1% agar plates with 2% Antibiotic-Antimycotic. Apply the Antibiotic-Antimycotic after poured agar plates have cooled by spreading over the surface of the plate using a cell spreader. Seal the agar plate with parafilm to prevent agar desiccation.
NOTE: Do not attempt to return injected embryos to a colony after injections as workers may destroy most injected embryos. Survival is therefore optimized by allowing embryos to develop on agar plates outside of a normal colony environment. - Incubate the agar plates at 25 °C for approximately 4 weeks. Check regularly for hatching.
- Once the first embryo has hatched into a larva, return all embryos and larvae to a nest box with a few young nurse workers to care for the hatchlings. Feed using crickets pre-stung by a larger wild-type colony, remove waste products, and add water following the same protocol discussed in Section 1.
NOTE: Small boxes (9.5 x 9.5 cm2) are ideal for such colonies. H. saltator reproduces well in captivity. Therefore, mutant embryos can be reared to adulthood. Isolation of mutant adult(s) induces transition to the reproductive gamergate stage. Controlled crosses are used to establish mutant colonies with heterozygous or homozygous individuals (Figure 5).
Representative Results
Using the protocol provided here, genome editing in Harpegnathos saltator embryos was performed successfully. These results were validated via polymerase chain reaction and pGEM cloning of DNA extracted from injected embryos followed by DNA sequencing. Efficiency of somatic mutagenesis using this protocol reached approximately 40%. F1 mutant males were mated to wild type females to produce heterozygous F2 females which, if not mated, produced F3 males. Mutant F3 males were mated to heterozygous females to produce F4 homozygous mutant females. Absence of the target peptide was further confirmed via mass spectrometry of these F4 homozygous female individuals. Wings were clipped from males using microdissection scissors and used for genotyping purposes. As workers do not have wings, they are normally sacrificed and genotyped after experiments. As a result of successful mutagenesis, unusual behaviors were observed, which correlated with loss of the target gene. The loss of orco resulted in abnormal behaviors related to loss of pheromone sensing, inability to detect prey, impaired fecundity, and wandering from the colony. Furthermore, orco mutant ants exhibited a decreased number of odorant receptor neurons and antennal lobe glomeruli, suggesting that neuroanatomy in ants is dependent on odorant receptor functionality10.
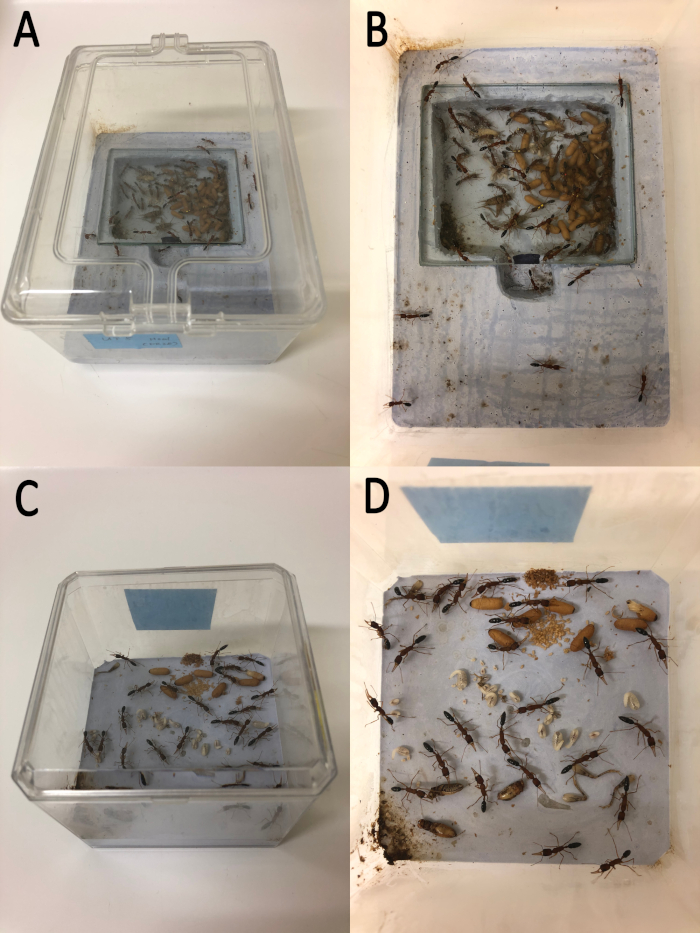
Figure 1: Harpegnathos saltator nests. (A) External features of a 19 x 13.5 cm2 nest box containing an H. saltator colony. (B) Internal features of a 19 x 13.5 cm2 nest box containing an H. saltator colony. Note the presence of a lower nest region under a square piece of glass. (C) External features of a 9.5 x 9.5 cm2 nest box containing a small H. saltator colony. Such a nest box is also suitable for isolated workers and mutant colonies. (D) Internal features of a 9.5 x 9.5 cm2 nest box containing a small H. saltator colony. Please click here to view a larger version of this figure.
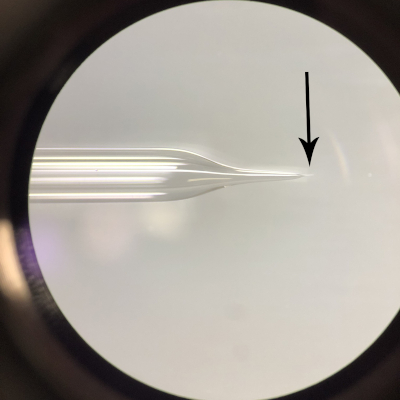
Figure 2: Needle for Harpegnathos saltator embryo microinjection. Note the thin taper of the needle (marked with an arrow). The needle may be opened by breaking the tip slightly. Please click here to view a larger version of this figure.

Figure 3: Alignment of embryos on double-sided tape. Embryos should be aligned so that their length is parallel with the long edge of the tape. This enables successive injections to be performed with ease by moving the slide on the stage. Please click here to view a larger version of this figure.
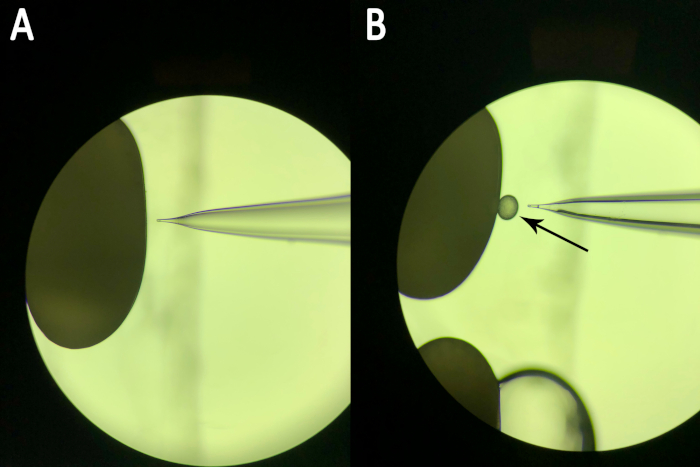
Figure 4: Embryo and needle as seen during microinjection. (A) Proper alignment of the needle with the embryo prior to injection. The needle rests perpendicular to the mid-point of the embryo's side, the typical site of injection. (B) The embryo and needle following a successful injection. A small droplet protrudes from the embryo's side (marked with an arrow). Please click here to view a larger version of this figure.
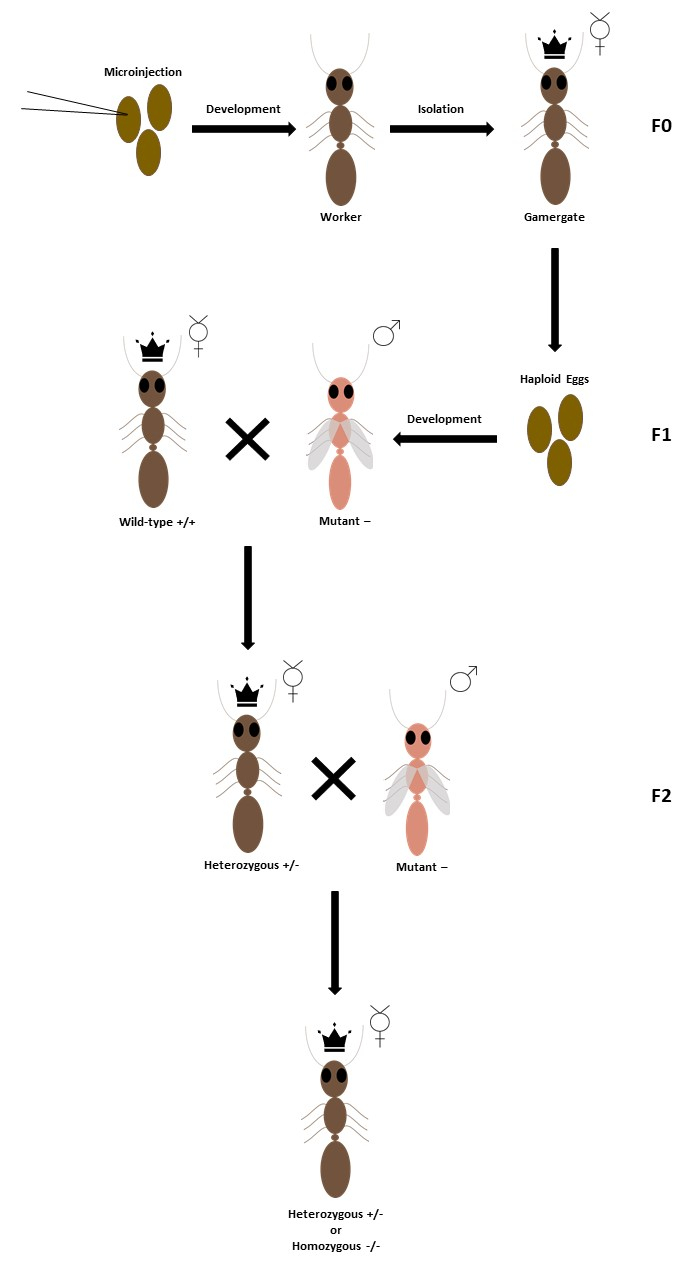
Figure 5: Diagram of basic mutant crosses. CRISPR may induce mutations on the target gene in F0 females, which are subsequently isolated upon adulthood to induce the gamergate transition. If mutations occur in germline cells, unmated gamergates may lay mutant male eggs. The F1 mutant adult males may be mated with wild-type females to generate heterozygous offspring, or they may be mated with heterozygous females to generate homozygous or heterozygous offspring. Please click here to view a larger version of this figure.
Discussion
The evolution of eusociality amongst insects, including ants, bees, wasps, and termites, has resulted in the appearance of novel behavioral and morphological traits, many of which are understood to be influenced by a combination of environmental and genetic factors1,2,3,4. Unfortunately, the attractiveness and usefulness of eusocial insects as research models in the field of genetics has been hindered by the difficulties associated with mutagenesis in this group. This hindrance occurs due to reproductive division of labor, a key trait of eusocial insects in which only a few members of a colony may reproduce. This trait places limitations on the number of mutant offspring and makes the development of genetic lines challenging5. The ponerine ant species, Harpegnathos saltator, provides a solution to this dilemma, as all females are capable of becoming reproductive gamergates when isolated5. Here, we provide methods for mutagenesis in H. saltator using the CRISPR/Cas9 system, delivered via embryo microinjection.
Proper colony maintenance and selection of embryos at the appropriate developmental stage for microinjection are critical. Previous work established that the syncytial stage in insect development is the ideal stage for genome editing21, but the timing of this stage in H. saltator embryonic development was previously unknown. Via nuclear staining of early embryo sections, we were able to determine that the syncytial stage in H. saltator embryos lasts until 36 hours after egg deposition, allowing us to determine when to select new embryos for microinjection10.
Selecting parameters for needle pulling and for microinjection can be challenging. Factors to consider when selecting parameters for needle pulling include (1) the type of glass used, (2) the desired purpose of the needle, (3) the desired tip size, (4) the amount of resistance the needle will experience during injection, (5) the desired taper length, and (6) the type of cell or organism that will be injected. Suggestions and guidelines for pulling various needle types can be found in various operation manuals17. Similarly, there are factors to consider when selecting parameters for microinjection including (1) size of the needle being used and (2) the embryos to be injected22. While this protocol focuses on microinjection in H. saltator embryos, the techniques may vary in other insects with modification to these parameters. In particular, the parameters for needle pulling and injection will differ if the embryo in question is soft or dechorionated. H. saltator embryos have a tough chorion, and injection results are best when a needle with a short taper is used (approximately 2 mm). Embryos that have less firm chorion may be injected with needles with longer tapers (approximately 10 mm).
In H. saltator, injected embryos cannot be returned immediately to a colony, as doing so will risk their destruction by nursing workers. If applying this protocol to other social insect species, this may not be the case. Determining the best post-microinjection rearing methods in other species may require some trial and error. In the case of H. saltator, embryos need to be reared on agar plates until hatching. Once hatched, they can be returned safely to a small colony with a few workers to care for the injected brood10. A similar method of embryo care is utilized in fire ants (Solenopsis invicta) and clonal raider ants (Ooceraea biroi) to increase the survival rate of injected embryos9,15. Upon adult eclosion, mature mutant females may be kept individually or in small colonies to begin their reproductive cycle. Directions for the care of H. saltator mutants are provided in this protocol, but if using a different species, directions for care of injected embryos and mutant adults should be modified to fit the needs of the species.
The protocol provided here is optimized for mutagenesis, in which specifically non-essential genes are targeted. In this case, the orco gene was targeted, and mutations in orco did not affect ant survival to adulthood. Similarly, this protocol may be used to target other non-essential genes, including the ones associated with other sensory receptors. If the target gene is essential, transgenic ants will have to be generated instead, either via CRISPR or transposon. Transposon-mediated transgenesis has been used in honeybees13 and may apply to ants. If the desired outcome is transgenic organisms, the injected materials will have to be different. However, post-injection processes will be similar, and therefore some aspects of this protocol will be beneficial despite the difference in the desired outcome.
Overall, H. saltator is ideal for use as a model organism and for performance of genetic crosses due to its plastic reproductive system in which workers begin reproducing after isolation10. This differentiates this ant species from other ants in which CRISPR/Cas9 technology has been established, for example, O. biroi, a species in which all females reproduce clonally. H. saltator presents unique research opportunities amongst organisms of its kind as genetic lineages can be established and mutations can be maintained across generations in this species. The novelty of this system allows researchers not only to generate mutant ants, but also to develop transgenic lines in the future. This provides opportunities for novel research to study genetic control of advanced eusociality.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors thank Danny Reinberg's and Claude Desplan's labs at New York University and Jürgen Liebig's lab at Arizona State University for their support on ant genetics. Hua Yan acknowledges support from the National Science Foundation I/UCRC, the Center for Arthropod Management Technologies under Grant No. IIP-1821914 and by industry partners. Maya Saar was supported by the United States – Israel Binational Agricultural Research and Development Fund, Vaadia-BARD Postdoctoral Fellowship No. FI-595-19.
Materials
| Antibiotic-Antimycotic (100X) | ThermoFisher | 15240-062 | |
| Cas9 protein with NLS, high concentration | PNA Bio | CP02 | |
| Cellophane Roll 20 inch X 5 feet | Hypogloss Products | B00254CNJA | The product has many color variations. Purchase it in red for use in making ant nests. |
| Eclipse Ci-S upright microscope | Nikon | Ci-S | |
| Featherweight forceps, narrow tip | BioQuip | 4748 | |
| FemtoJet ll microinjector | Eppendorf | 920010504 | This product is no longer sold or supported by Eppendorf. A comparable microinjector may be used instead. |
| Microloader pipette tips | Eppendorf | 930001007 | |
| NCBI database | National Center for Biotechnology Information | Gene ID: 105183395 | |
| P-2000 Micropipette Puller | Sutter Instruments | P-2000/G | |
| Plastic boxes (19 X 13.5 cm2) | Pioneer Plastics | 079C | |
| Plastic boxes (27 X 19 cm2) | Pioneer Plastics | 195C | |
| Plastic boxes (9.5 X 9.5 cm2) | Pioneer Plastics | 028C | |
| Quartz glass without filament | Sutter Instruments | Q100-50-7.5 | |
| Vannas scissors, 8.5 cm | World Precision Instruments | 500086 | |
| Winsor & Newton Cotman Water Colour Series 111 Short Handle Synthetic Brush – Round #000 | Winsor and Newton | 5301030 |
References
- Evans, J. D., Wheeler, D. E. Expression profiles during honeybee caste determination. Genome Biology. 2 (1), 1-6 (2000).
- Keller, L. Adaptation and the genetics of social behaviour. Philosophical Transactions of the Royal Society B: Biological Sciences. 364 (1533), 3209-3216 (2009).
- Cahan, S. H., et al. Extreme genetic differences between queens and workers in hybridizing Pogonomyrmex harvester ants. Proceedings. Biological Sciences. 269 (1503), 1871-1877 (2002).
- Volny, V. P., Gordon, D. M. Genetic basis for queen-worker dimorphism in a social insect. Proceedings of the National Academy of Sciences of the United States of America. 99 (9), 6108-6111 (2002).
- Yan, H., et al. Eusocial insects as emerging models for behavioural epigenetics. Nature Reviews Genetics. 15 (10), 677-688 (2014).
- Liebig, J., Hölldobler, B., Peeters, C. Are ant workers capable of colony foundation. Naturwissenschaften. 85 (3), 133-135 (1998).
- Bonasio, R. Emerging topics in epigenetics: ants, brains, and noncoding RNAs. Annals of the New York Academy of Sciences. 1260 (1), 14-23 (2012).
- Peeters, C., Liebig, J., Hölldobler, B. Sexual reproduction by both queens and workers in the ponerine ant Harpegnathos saltator. Insectes Sociaux. 47 (4), 325-332 (2000).
- Trible, W., et al. orco mutagenesis causes loss of antennal lobe glomeruli and impaired social behavior in ants. Cell. 170 (4), 727-735 (2017).
- Yan, H., et al. An engineered orco mutation produces aberrant social behavior and defective neural development in ants. Cell. 170 (4), 736-747 (2017).
- Kohno, H., Suenami, S., Takeuchi, H., Sasaki, T., Kubo, T. Production of knockout mutants by CRISPR/Cas9 in the European honeybee, Apis mellifera L. Zoological Science. 33 (5), 505-512 (2016).
- Kohno, H., Kubo, T. mKast is dispensable for normal development and sexual maturation of the male European honeybee. Scientific Reports. 8 (1), 1-10 (2018).
- Schulte, C., Theilenberg, E., Müller-Borg, M., Gempe, T., Beye, M. Highly efficient integration and expression of piggyBac-derived cassettes in the honeybee (Apis mellifera). Proceedings of the National Academy of Sciences of the United States of America. 111 (24), 9003-9008 (2014).
- Hu, X. F., Zhang, B., Liao, C. H., Zeng, Z. J. High-efficiency CRISPR/Cas9-mediated gene editing in honeybee (Apis mellifera) embryos. G3: Genes, Genomes, Genetics. 9 (5), 1759-1766 (2019).
- Chiu, Y. K., Hsu, J. C., Chang, T., Huang, Y. C., Wang, J. Mutagenesis mediated by CRISPR/Cas9 in the red imported fire ant, Solenopsis invicta. Insectes Sociaux. 67 (2), 317-326 (2020).
- Zhou, X., et al. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding. PLoS Genetics. 8 (8), 1002930 (2012).
- Sutter, P-2000 Laser Based Micropipette Puller System Operation Manual. 2.2 edn. Sutter Instrument Company. , (2012).
- Perry, M., et al. Expanded color vision in butterflies: molecular logic behind three way stochastic choices. Nature. 535 (7611), 280-284 (2016).
- Bonasio, R., et al. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science. 329 (5995), 1068-1071 (2010).
- Shields, E. J., Sheng, L., Weiner, A. K., Garcia, B. A., Bonasio, R. High-quality genome assemblies reveal long non-coding RNAs expressed in ant brains. Cell Reports. 23 (10), 3078-3090 (2018).
- Henderson, D. S. . Drosophila Cytogenetics Protocols. , (2004).
- Kern, R., Stobrawa, S. . Step-by-Step Guide: Microinjection of Adherent Cells with the Eppendorf Injectman® 4 and Femtojet® 4. , (2019).

