A Murine Ommaya Xenograft Model to Study Direct-Targeted Therapy of Leptomeningeal Disease
Summary
Here, we describe a murine xenograft model that functionally resembles an Ommaya reservoir in patients. We developed the Murine Ommaya to study novel therapeutics for the universally fatal leptomeningeal disease.
Abstract
Leptomeningeal disease (LMD) is an uncommon type of central nervous system (CNS) metastasis to the cerebral spinal fluid (CSF). The most common cancers that cause LMD are breast and lung cancers and melanoma. Patients diagnosed with LMD have a very poor prognosis and generally survive for only a few weeks or months. One possible reason for the lack of efficacy of systemic therapy against LMD is the failure to achieve therapeutically effective concentrations of drug in the CSF because of an intact and relatively impermeable blood-brain barrier (BBB) or blood-CSF barrier across the choroid plexus. Therefore, directly administering drugs intrathecally or intraventricularly may overcome these barriers. This group has developed a model that allows for the effective delivery of therapeutics (i.e., drugs, antibodies, and cellular therapies) chronically and the repeated sampling of CSF to determine drug concentrations and target modulation in the CSF (when the tumor microenvironment is targeted in mice). The model is the murine equivalent of a magnetic resonance imaging-compatible Ommaya reservoir, which is used clinically. This model, which is affixed to the skull, has been designated as the "Murine Ommaya." As a therapeutic proof of concept, human epidermal growth factor receptor 2 antibodies (clone 7.16.4) were delivered into the CSF via the Murine Ommaya to treat mice with LMD from human epidermal growth factor receptor 2-positive breast cancer. The Murine Ommaya increases the efficiency of drug delivery using a miniature access port and prevents the wastage of excess drug; it does not interfere with CSF sampling for molecular and immunological studies. The Murine Ommaya is useful for testing novel therapeutics in experimental models of LMD.
Introduction
Leptomeningeal disease (LMD) is an aggressive late-stage metastasis of the CNS, in which tumor cells access the CSF and infiltrate the surface of the brain and spinal cord1. The most common cancers that cause LMD include those of the breast and lung as well as melanoma2. LMD results in a number of neurologic symptoms and signs such as headaches, cranial nerve palsies, stiff neck, and radiculopathies. The prognosis for patients with LMD is generally very poor (average survival is measured in weeks) and is universally fatal3,4,5,6,7. Treatment with surgery, radiation, and systemic chemotherapy is palliative. Systemic therapy for LMD may fail because of inadequate drug penetration into the CSF across an intact BBB or blood-CSF barrier across the choroid plexus1.
Therefore, administering cancer therapeutics (e.g., drugs and antibody-based treatments including checkpoint inhibitors and cellular therapies) directly into the CSF may overcome this limitation8. Accessing and sampling CSF from patients is possible through an Ommaya reservoir that is implanted beneath the scalp. This device allows for the administration of cancer agents (e.g., methotrexate and trastuzumab) as well as the sampling of CSF for diagnostic studies (e.g., the cytological diagnosis of LMD to monitor for responses to treatment) without performing a spinal tap. A murine Ommaya reservoir was designed to mimic those used clinically. The reservoir requires the assembly of an accessing port and spacer parts and a modification of the mouse cannulation technique, which allows the device to remain permanently intact throughout the duration of the drug study. This device has been designated as the "Murine Ommaya".
In contrast to the osmotic infusion pump technique, which requires the preparation of excess liquid volumes to prefill the empty space in the tubing and continuous infusion over frequent injections9, the Murine Ommaya minimizes the wastage of drug solutions. It permits effective administration of multiple single doses of treatments at any given time in small quantities (3-7 µL) into the CSF using a Hamilton syringe, a miniature access port, and an automatic injector. In real time, the efficacy of test drugs against LMD can be determined by imaging. Using this approach, a variety of chemotherapies, antibodies, and cell immunotherapies (as single or combined agents) can be tested against LMD to translate in vivo findings into rational treatment strategies for patients. To further improve the imaging capacity for a patient-derived xenograft (PDX) model of LMD, a collaboration was undertaken with a manufacturer to develop a magnetic resonance imaging (MRI)-compatible version of the Murine Ommaya, which requires no assembly and is ready to use. MRI capability is beneficial, especially for PDX models in which the quantity of circulating tumor cells (CTCs) from CSF is sometimes the limiting factor, and often when prelabeling CTCs is infeasible.
This paper describes a detailed protocol that begins with the injection of CTCs to render mice with LMD . The Murine Ommaya is then surgically implanted, and multiple drug treatment steps via the Murine Ommaya are performed. As a proof of concept for demonstration, an in vivo side-by-side comparison was performed, in which the murine human epidermal growth factor receptor 2 (Her2) antibody called clone 7.16.4 (the human equivalent of trastuzumab) was delivered10. The antibody targets Her2+ breast cancer cells either via the Murine Ommaya (direct-targeted or intrathecal therapy) or by intraperitoneal injection (systemic therapy). The results showed that mice with LMD that received direct intrathecal immunotherapy lived significantly longer than those treated with the same therapy systemically. The CNS metastases in mice treated via the Murine Ommaya were almost completely regressed by the third dose of the third week of treatment, resulting in improved overall survival.
Protocol
The protocol was approved by the University of South Florida Institutional Animal Care and Use Committee (IS00005974).
1. Injection of CTCs into CSF to generate a mouse LMD model
- Preparation of CTCs
- Calculate the number of CTCs needed for injection, and prepare a single-cell suspension at 1.0 × 104 cells/µL in sterile phosphate-buffered saline (PBS). Place the cell suspension on ice or at 4 °C throughout the procedure.
NOTE: When using cell lines that require trypsinization, be sure to wash cells twice with sterile PBS to remove trypsin. Perform cell count using a hemocytometer or an automated cell counter. If a large cohort (>50 mice) is used, recount the cells and validate cell viability between injections to ensure a consistent number of cells are administered per mouse.
- Calculate the number of CTCs needed for injection, and prepare a single-cell suspension at 1.0 × 104 cells/µL in sterile phosphate-buffered saline (PBS). Place the cell suspension on ice or at 4 °C throughout the procedure.
- Presurgical procedure
- Inject the mouse subcutaneously with 1 mg/kg buprenorphine sustained-release (Bup-SR).
NOTE: Do not inject the area of proposed incision; choose an injection site well away from the scapula. - Anesthetize the mouse with 2-3% isoflurane until it shows no signs of the righting reflex. In addition, check for tail and/or paw pinch reflex to confirm the state of anesthesia.
- Prepare the mouse for surgery in a location remote from the operating area. Clip the surgical site (i.e., the dorsal surface of the skull) with enough border area to keep fur from contaminating the incision site; then, saturate the site with germicidal skin antiseptic, working from the center of the site to the periphery, and then allow to dry. Apply either sterile drape or a sterile adhesive-backed plastic drape material to protect the surgical site from contamination.
NOTE: Instruments are to be autoclaved in advance and tips re-sterilized between animals using a glass bead sterilizer. - Shave the fur of the entire ventral surface of the head, and prepare the skin using sterile technique.
- Situate the nose with a modified L-shaped nose cone of the stereotactic apparatus, ensuring the nares remain clear and open. Using tape across the ventral surfaces of both pinnae, gently pull the skin forward to secure it to the nose cone, and bend the neck at an approximately 90° angle once secured. Administer 1.5% isoflurane to maintain anesthesia.
NOTE: Proper positioning will result in the incision area being presented in a manner that allows for the easy identification of the caudal ridge of the occipital bone. - Position the body to ensure the spine is kept level with the cisterna magna, and while applying slight traction to the tail, place tape at the tail base to secure.
- Inject the mouse subcutaneously with 1 mg/kg buprenorphine sustained-release (Bup-SR).
- Surgical cisterna magna injection
- With the neck in full extension, and beginning just between the pinnae, run the surgical scissor tips downward with slight pressure across the occipital bone.
NOTE: While in this midline position, a small depression is noticeable when the scissor tips dip into the concave area over the cisterna magna. - Make a small 3-5 mm midline incision just above the palpated concavity. Draw 5 µL of cell suspension at 1.0 × 104 cells/µL (total 5.0 × 104 cells) into a 30 G Hamilton syringe.
- Use blunt-tipped forceps with 1-2 mm tips to gently press down on the cisterna magna. Introduce tips in a closed position and open them while applying downward pressure on the dura.
- Repeat blunt dissection described in the previous step until the dural membrane is easily identified, and the associated blood vessels are visible in the exposed area.
NOTE: The resulting injection window ensures blood vessels are undamaged during needle insertion. - While holding the forceps open to retract surrounding musculature, introduce a 30 G non-coring needle under the dura to visualize the bevel. Ensure the needle is only introduced just beyond the bevel itself. Slowly deploy a syringe plunger, and deliver cells just below the dura.
- Properly place the bevel to observe the injection beneath the dura. Carefully administer the technique, and use the 30 G non-coring needle to prevent damage to the membrane and ensure minimal leakage. If leakage is noted, apply gentle pressure with a cotton-tipped applicator.
- Close the skin by applying a wound clip or micro-drop of skin adhesive. Once the injection is successfully performed, allow the mice to recover from anesthesia on a warm blanket, observing them continuously until they can maintain sternal position and demonstrate purposeful movement.
- Monitor the mice daily after the surgery for the first week. If a mouse appears to be in pain or distress, treat it with 10 mg/kg subcutaneous injection of carprofen once every 12 to 24 h for up to 5 days based on veterinary consultation and directive. Allow mice to recover and monitor them for at least 48 to 72 h before proceeding to the next step.
NOTE: Bup-SR, given preemptively, will last up to 72 h, so additional analgesics are not normally necessary. However, animals will be provided additional analgesic as necessary (if lethargic, not eating, ruffled). If a surgical site develops signs of postoperative infection (i.e., redness, swelling, tenderness, allodynia, hyperalgesia, hyperpathia, or suppuration), or if the mouse is guarding the affected area, euthanize the mouse. If cancer cells are successfully injected into the CSF, LMD and tumor progression will develop in the CNS within 1 or 2 weeks (depending on the types of CTCs or cell line used) (Figure 1).
- With the neck in full extension, and beginning just between the pinnae, run the surgical scissor tips downward with slight pressure across the occipital bone.
2. Murine Ommaya assembly and implantation
- Preparation of station
- Disinfect the station surface. Place a blue covering over the surface, and keep ready all sterilized tools and supplies indicated in Figure 2.
- Presurgical procedure
- Apply analgesia (Bup-SR), and maintain sterile technique as described in section 1.2.
- Surgical implantation of Murine Ommaya
- Assemble the Murine Ommaya injection device using a 25 G (0.51 mm outer diameter) miniature injection port and a 1 mm spacer disc. Use a cyanoacrylate sterile adhesive to ensure the penetration of approximately 2.5 mm of the metal cannula into the right cerebral hemisphere (Figure 3A-C).
NOTE: An MRI-compatible Mouse Ommaya prototype was developed in this laboratory, which already has both parts (miniature injection port and spacer) 3D-printed together as a single unit (Figure 3D). The single unit version was tested by MRI and can save time by eliminating the assembly step. - Anesthetize the mouse with 2-3% isoflurane until there are no signs of the righting reflex. Further, check for tail and/or paw pinch reflex to confirm the state of anesthesia.
- Shave the entire ventral surface of the head of fur, and prepare the skin according to the sterile technique, as previously described in step 1.2.3. Place the mouse in the stereotactic apparatus with a nose cone to continue isoflurane administration during the procedure; decrease the isoflurane to 1.5%. Gently tighten the ear bars to secure the head, and apply eye lubricant to to cover the eyes of the mouse.
- Make a small skin incision (3 mm), followed by blunt dissection of the underlying subcutaneous tissues to expose the skull. Dry the skull using hydrogen peroxide-soaked cotton-tipped applicator sticks.
- Drill a burr hole in the skull 0.5 mm posterior and 1.1 mm lateral of the bregma-the anatomical point on the skull where the coronal suture is intersected perpendicularly by the sagittal suture (Figure 3A)-as well as a 0.9 mm burr hole to expose the dura mater. Move the microdrill aside, and gently score the bone immediately surrounding the burr hole prior to inserting an injection port (depth of approximately 2.5 mm), affixed to the skull using a cyanoacrylate sterile adhesive. State suture around the injection spot using 4-0 no-absorbance nylon sutures in an interrupted stitch pattern or purse string suture11.
- House post-surgery mice in individual cages for surgery recovery.
NOTE: It is possible that the Murine Ommaya may come off when multiple surgery-recovered mice are housed in the same cage, possibly due to constant tampering interactions. It is recommended that Murine Ommaya-implanted mice are housed individually (or no more than 2 mice per cage) over the course of the drug efficacy trial.
- Assemble the Murine Ommaya injection device using a 25 G (0.51 mm outer diameter) miniature injection port and a 1 mm spacer disc. Use a cyanoacrylate sterile adhesive to ensure the penetration of approximately 2.5 mm of the metal cannula into the right cerebral hemisphere (Figure 3A-C).
3. Murine Ommaya treatment
- Dosing mice using the Murine Ommaya
- Anesthetize the mouse with 2-3% isoflurane, until there are no signs of righting reflex. Further, check for tail and/or paw pinch reflex to confirm maintenance of anesthesia.
- Access the Murine Ommaya using a port injection adapter and a Hamilton syringe. Using forceps, hold the top of the miniature injection port and gently insert the port injector adaptor fully into the port's septum.
NOTE: The port injector pierces the septum of the port in a manner that reduces dead space to a minimum and allows for controlled injection via a motorized syringe pump (Figure 4A). Targeted/novel treatments are injected at a flow rate of 1 µL/min while under anesthesia. The treatment is to be given at specified intervals (daily/weekly) for a set duration (weeks/months), according to the researcher's discretion.
A volume between 3 and 7 µL is optimal, as a volume < 3 µL is unreliable, and a volume > 7 µL may cause too much pressure. The size of the Hamilton syringe can range from 10 to 100 µL, depending on the number of mice in each treatment arm. Preloading the appropriate volume into the Hamilton syringe will prevent repeated replacement of the syringe, thereby minimizing errors. It is best to dedicate 1 Hamilton syringe per treatment arm. - Once the injection is made, detach the Murine Ommaya from the port injection adaptor using forceps, and return the mouse to the cage to recover from anesthesia, as described above in step 1.3.7.
- Euthanasia
- Euthanize the mice by inhalation of carbon dioxide (CO2) from a compressed tank source. Expose the mice to increasing concentrations of CO2 (i.e., a displacement rate from 10% to 30% of the chamber volume/min is to be used) to avoid or minimize discomfort or distress. Monitor the mice until assurance of cessation of cardiovascular and respiratory movements.
Representative Results
In mice, the total volume of CSF is approximately 35-40 µL and is produced at a rate of about 350 nL/min; it turns over 12-13 times a day12. For the purpose of visualizing the route of the injection, 2% Evans Blue was injected via the Murine Ommaya model, following which 15 min and 30 min were allowed to elapse before harvesting the brains for analysis. The dye successfully infiltrated the ventricles and the brain in 15 min. Within 30 min, the dye became visible on the spinal cord (Figure 4).
As a proof of concept, BALB/c mice were injected with a luciferase-labeled Her2+ TUBO breast cancer cell line intracisternally, and the Murine Ommayas were implanted. Approximately 1 week after the injection of cancer cells, the mice began to develop LMD. These mice were treated once a week for up to 4 weeks with Her2-antibody immunotherapy, either through systemic therapy via intraperitoneal injection or intrathecally via the Murine Ommaya (Figure 5A).
Although untreated mice died by day 19, all mice that received intrathecal therapy through the Murine Ommaya survived (P = 0.004). By week 4, a complete regression of tumors was observed. In comparison to mice treated with systemic therapy, which had moderate success in treating LMD, mice that received intrathecal therapy had a much longer overall survival (Figure 5B).
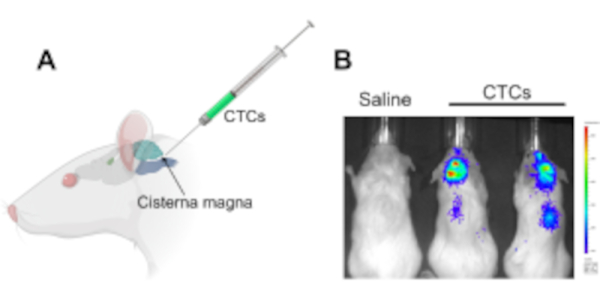
Figure 1: Injection of circulating tumor cells into the cisterna magna in a murine xenograft model to study leptomeningeal disease and central nervous system metastases. (A) An illustration showing the location of the cisterna magna and the CSF accessing site, in which CTCs are injected using a Hamilton syringe. (B) A representative IVIS image of mice that had developed leptomeningeal disease and central nervous system metastases (brain and along the spinal cord) after 2 weeks of injection with circulating tumor cells. Cells were labeled with a luciferase reporter gene. Control animals injected with saline did not develop tumors (n = 3), and the experiment was performed in triplicate. Abbreviations: CTCs = circulating tumor cells; IVIS = in vivo imaging system. Please click here to view a larger version of this figure.
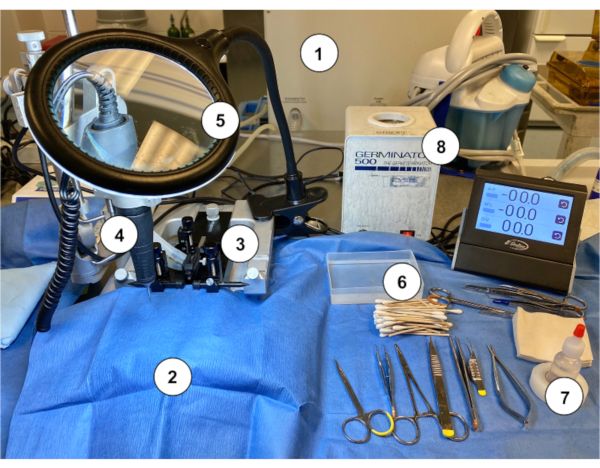
Figure 2: An example of a workstation setup for performing the Murine Ommaya implantation in mice. (1) Gas anesthesia machine/vaporizer. (2) Sterile blue paper drape covering a stereotaxic stand. (3) Stereotaxic device (stand/stage, ear bars, nose cone). (4) Microdrill. (5) Magnifying glass with light. (6) Sterile cotton tapped applicator sticks with sterile saline rinse container. (7) Hydrogen peroxide. (8) Bead sterilizer. Please click here to view a larger version of this figure.
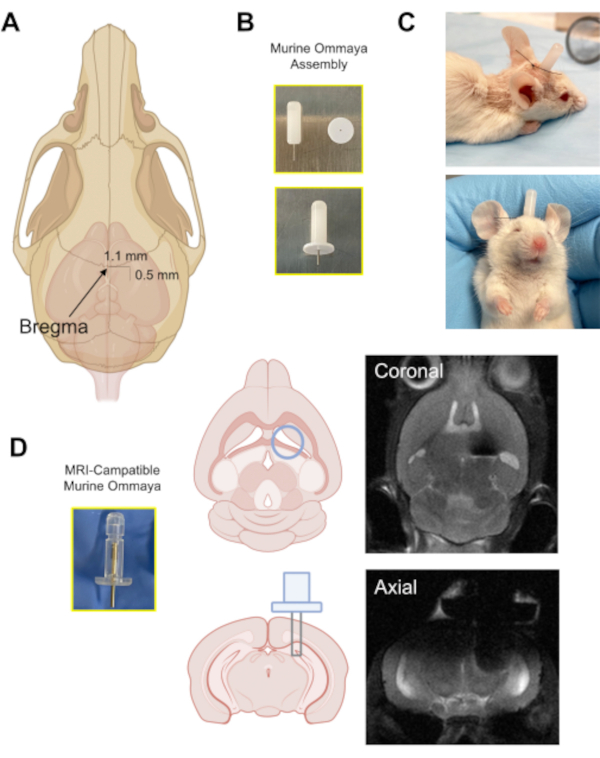
Figure 3: The implantation of the Murine Ommaya device. (A) An illustration with an arrow pointing at the location of the bregma on the skull, and the approximate distance at which a burr hole is drilled in the skull (0.5 mm posterior/1.1 mm lateral) from the bregma using a microdrill. (B) A Murine Ommaya is assembled by combining a metal cannula and a 1 mm spacer as the base for glue attachment to the skull. (C) Representative images of mice that had Murine Ommayas implanted; these mice are monitored to ensure they are bright, alert, and reactive before receiving any injections. (D) An example of the prototype magnetic resonance imaging-compatible Murine Ommaya and representative brain magnetic resonance imaging images of Murine Ommaya implants. Please click here to view a larger version of this figure.
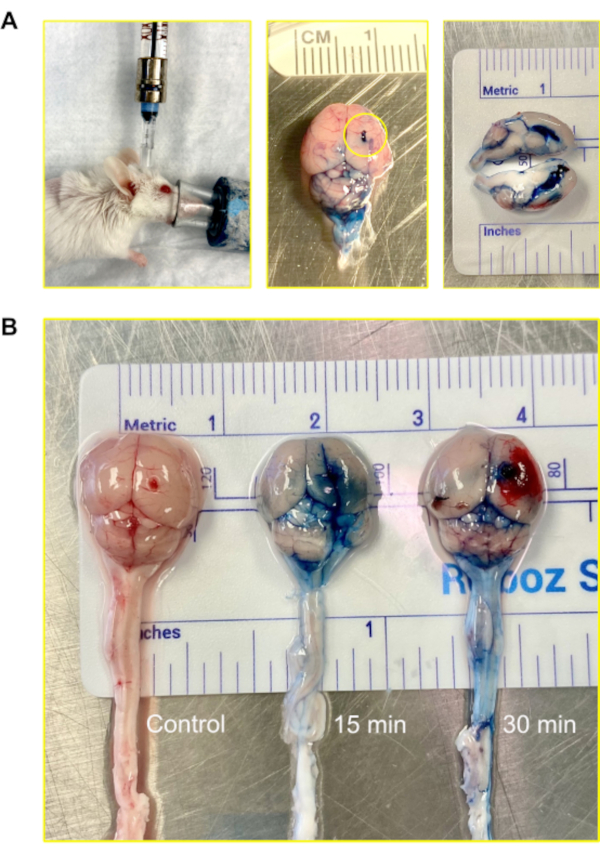
Figure 4: Intraventricular (central nervous system) injection using the Murine Ommaya. (A) An image of an injection, accessing the ventricle and central nervous system via the Murine Ommaya. Mice remain under anesthesia during the injection. In the example, the Murine Ommaya is connected to the miniature port that is attached to a prefilled Hamilton syringe. Injections are performed using an automatic injection set at an infusion rate of 1 µL/min and a volume of 5-7 µL. An image of a mouse brain injected with Evans Blue is shown. The circle shows where the Murine Ommaya was attached. No leakage of the dye was observed on the exterior of the brain. A cross section of the brain shows the lateral ventricles were filled with the dye; the dye did not penetrate brain parenchyma. (B) Images of mouse brains after 15 and 30 min following the injection of Evans Blue dye. The dye infiltrated the brain (15 min) and began to circulate on the spinal cord (30 min). Out of 5 mice, 4 received dye for visualization, and 1 served as control. The experiment was repeated in triplicate. Please click here to view a larger version of this figure.
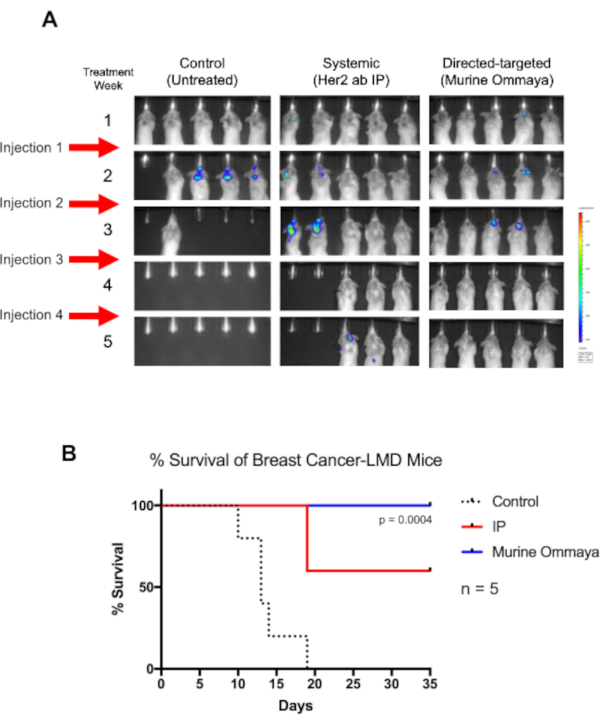
Figure 5: Direct-targeted immunotherapy using the Murine Ommaya increases the overall survival of breast cancer-associated leptomeningeal disease mice. (A) BALB/c mice were injected with luciferase reporter-labeled human epidermal growth factor receptor 2-positive TUBO cells, a murine breast cancer cell line. Three days after cisterna magna injections, Murine Ommayas were implanted. Mice began to develop leptomeningeal disease (LMD) 1 week after injection. LMD mice were treated with either a human epidermal growth factor receptor antibody systemically via intraperitoneal injection or via intrathecal (Murine Ommaya) as a direct-targeted approach. Injections were given once a week for up to 4 weeks. Compared to untreated mice, mice receiving immunotherapy survived much longer. Murine Ommaya mice had complete disease regression by the fourth week, and these mice were eventually cured of disease. (B) These mice also had significantly better median survival (Mantel-Cox test; P = 0.004; n = 5 mice per treatment arm) and better overall survival than systematically treated LMD mice. Abbreviations: LMD = leptomeningeal disease; IP = intraperitoneal. Please click here to view a larger version of this figure.
Discussion
Here, the Murine Ommaya has been described as a reliable model, which allows for repeated administration of anti-cancer agents into the CSF space in preclinical models of LMD and other CNS-related diseases. CSF was sampled from mice while the device was still attached without interruption. This direct-targeted therapy xenograft model is an important step in developing and testing rational treatment strategies for LMD. The time from cisterna magna injections to the initial sign of LMD development varies depending on the cancer cell type. LMD and CNS metastases begin to take form around 1 or 2 weeks after CTC inoculation. If a highly proliferative cancer cell line is used, it is possible that metastasis can occur in <7 days. In this case, vascularization due to tumor growth can sometimes makes implantation of the Murine Ommaya challenging. One solution to this challenge is to reduce the number of cancer cells for injection into the CSF space, allowing for more time before tumor development. Furthermore, this protocol has been optimized to implant the Murine Ommaya no later than 72 h after the cisterna magna injection to ensure enough time for mice to recover from surgery before the first treatment. Researchers should calculate the growth rate of the CTCs in xenograft models before planning the treatment regimen.
Although there are other direct intraventricular delivery methods, such as using an osmotic pump system or an intracerebroventricular (ICV) bolus injection, as described previously9, there are several advantages to using the Murine Ommaya model. For example, an ICV bolus injection is done in a single delivery, whereas the Murine Ommaya allows for multiple doses of treatment at any time, whether given as a single agent or as combined therapies. The osmotic pump is designed to sustain for up to 14, 28, or 42 days before the pump needs replacement, and sometimes more frequently if a smaller mouse with a smaller pump is used. Changing the osmotic pump requires a surgical procedure, which adds stress to tumor-bearing mice. A Murine Ommaya replacement is not necessary for long-duration experiments as long as the device remains intact. It also minimizes potential variability that results from changing the pump9. Implanted Murine Ommayas in the experimental mice remained intact for longer than 42 days, and this duration allowed for long-lived treatment regimens.
Previous findings suggest that a pulsatile intermittent dosing into the CSF has better efficacy against LMD than a prolonged drug delivery process by infusion13. It would be impossible to perform repeated single-dose injections using the osmotic pump system. There is no easy way to flush out the remaining trapped liquid after each injection. The osmotic pump is also limited to delivering mixtures of compatible or single drugs and typically requires higher volumes of drug preparation for continuous infusion. In contrast, the Murine Ommaya is designed for accurate micro-injections as little as 3 to 7 µL, without having to account for dead space, and there is no limitation on the type of drugs researchers can use, including immune cell therapy. The Murine Ommaya also minimizes reagent waste if a particular sample is precious and maximizes the use of that resource. For any treatment regimen that requires multiple doses of anti-cancer therapies, the Murine Ommaya is easy to use, and there is minimal risk of infection or surgical misadventure, with the alternative approaches of repeatedly accessing the CSF surgically or via repeated delivery with a needle. The Murine Ommaya provides researchers with the flexibility to adjust drug concentrations and dosing frequencies and to assess target modulation and duration of the study according to the research of interest.
A limitation of the Murine Ommaya is that researchers may find it difficult to implant the device in smaller mice. Hence, it is better to use mice that are at least 8 to 10 weeks of age. It is possible for the Murine Ommaya to come off during the treatment trial if the device is not secured to the skull during the implantation steps and the glue wears off, or if the mice have abrasively tampered with it. The latter scenario occurs more frequently when multiple mice were housed in the same cage. Hence, it is recommended to house no more than two Murine Ommaya-implanted mice per cage for the duration of the treatment schedule. This protocol was modified to apply cyanoacrylate sterile adhesive on the spacer, which was found to be the most effective glue for adhering the spacer to the skull surface and preventing the Murine Ommaya from coming off. The results showed that LMD mice benefited from direct intrathecal therapy via the Murine Ommaya, with increased overall survival. Single microliter volumes could be safely administered, bypassing the BBB, thereby reducing the amount of drug preparation. Most importantly, the mice that were cured of CNS metastases from the Her2-antibody immunotherapy study have remained healthy.
A collaboration with a manufacturer aimed to develop an MRI-compatible version of the Murine Ommaya for the PDX model of LMD. Because this prototype version has a spacer incorporated, no assembly is required, allowing for better adherence to the skull. A limitation of this prototype is that although the device is MRI-compatible, it generates a shadow where the device is inserted, which decreases the visibility of the image for quantification analyses. The MRI-compatible version is a good alternative tool when ex vivo CTC sampling is a limiting factor, and prelabeling cells is not feasible. The combination of an LMD xenograft model and the Murine Ommaya technique is beneficial for studying direct-targeted drug efficacy bypassing the BBB. The results from these in vivo studies are clinically relevant to design rational therapeutic strategies for patients with LMD.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We would like to thank Michele L. Danielson, Tricia Favors-Watson, and the rest of the Comparative Medicine team at the University of South Florida for their technical support and maintaining our animals. We thank Instech Laboratories, Inc. for their effort working with us based on our request to develop an MRI-compatible Murine Ommaya. This work is supported by the National Institutes of Health (NIH) R21 CA216756 (to K.S.M. Smalley), Department of Defense (DOD) W81XWH1910675 (to B. Czerniecki and P. Kalinski), and the Moffitt Cancer Center CBMM Innovative Awards (to P. Forsyth and D. Duckett). Editorial assistance was provided by the Moffitt Cancer Center's Office of Scientific Writing by Dr. Paul Fletcher and Daley Drucker. No compensation was given beyond their regular salaries.
Materials
| 1 mm spacer disc | Alzet, Durect Corporation | #0008670 | Spacer disc only |
| 4-0 ethilon nylon suture | Any vendor | n/a | |
| Automatic syringe pumps | Harvard Syringe Pumps (or any vendor) | #70-4505 | Pump 11 Elite |
| Bead sterilizer | Braintree Scientific Inc. (or any vendor) | #GER 5287-120V | Germinator 500 |
| Buprenorphine Sustained-Release (Bup-SR) | Zoopharm | DEA controlled | |
| Cyanoacrylate sterile adhesive | Any vendor | ||
| Gas inhalation anestehsia system | VeteEquip | #901812 | COMPAC5 |
| Hamilton microliter syringes | Hamilton | 10, 25, 50, and 100 μL | 30 G for cisterna magna injection |
| Hydrogen peroxide | Any vendor | n/a | |
| IVIS 200 imaging system | Caliper Life Sciences | n/a | |
| Magnifying glass with light | Any vendor | n/a | |
| Microdrill | Stoelting (or any vendor) | #51555M | |
| MRI imaging | Bruker | BioSpec series | Optional |
| Murine Ommaya (MRI-compatible) prototype | Instech Laboratories, Inc. | #VAB620-25MRI-3.3 | |
| Phosphate-buffered saline (PBS) | Any vendor | n/a | 0.1 mm Sterile-Filtered |
| PinPort injector | Instech Laboratories, Inc. | #PNP3M-50 | |
| PinPort | Instech Laboratories, Inc. | #1-PNP3F28-50 | |
| Rodent Surgical Instruments (Scissors, Forceps) | Roboz Surgical Instrument (or any vendor) | ||
| Stereotaxic device | Stoelting (or any vendor) | #51730M | |
| Sterile blue paper/ drape covering | Any vendor | n/a | n/a |
| Sterile cotton sticks | Any vendor | n/a |
References
- Chamberlain, M. C. Leptomeningeal metastasis. Current Opinion in Neurology. 22 (6), 665-674 (2009).
- Nayar, G., et al. Leptomeningeal disease: current diagnostic and therapeutic strategies. Oncotarget. 8 (42), 73312-73328 (2017).
- Shapiro, W. R., Johanson, C. E., Boogerd, W. Treatment modalities for leptomeningeal metastases. Seminars in Oncology. 36 (4), 46-54 (2009).
- Davies, M. A., et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 117 (8), 1687-1696 (2011).
- Znidaric, T., et al. Breast cancer patients with brain metastases or leptomeningeal disease: 10-year results of a national cohort with validation of prognostic indexes. Breast Journal. 25 (6), 1117-1125 (2019).
- Glitza, I. C., et al. Leptomeningeal disease in melanoma patients: An update to treatment, challenges, and future directions. Pigment Cell & Melanoma Research. 33 (4), 527-541 (2020).
- Raizer, J. J., et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro-oncology. 10 (2), 199-207 (2008).
- Taillibert, S., et al. Leptomeningeal metastases from solid malignancy: a review. Journal of Neurooncology. 75 (1), 85-99 (2005).
- DeVos, S. L., Miller, T. M. Direct intraventricular delivery of drugs to the rodent central nervous system. Journal of Visualized Experiments. (75), e50326 (2013).
- Kodumudi, K. N., et al. Sequential anti-PD1 therapy following dendritic cell vaccination improves survival in a HER2 mammary carcinoma model and identifies a critical role for CD4 T cells in mediating the response. Frontiers in Immunology. 10, 1939 (2019).
- Dunn, L., et al. Murine model of wound healing. Journal of Visualized Experiments. (75), e50265 (2013).
- Simon, M. J., Iliff, J. J. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochimica et Biophysica Acta. 1862 (3), 442-451 (2016).
- Shackleford, G. M., et al. Continuous and bolus intraventricular topotecan prolong survival in a mouse model of leptomeningeal medulloblastoma. PLoS One. 14 (1), 0206394 (2019).

