Cardiac Response to β-Adrenergic Stimulation Determined by Pressure-Volume Loop Analysis
Summary
Here we describe a cardiac pressure-volume loop analysis under increasing doses of intravenously infused isoproterenol to determine the intrinsic cardiac function and the β-adrenergic reserve in mice. We use a modified open-chest approach for the pressure-volume loop measurements, in which we include ventilation with positive end-expiratory pressure.
Abstract
Determination of the cardiac function is a robust endpoint analysis in animal models of cardiovascular diseases in order to characterize effects of specific treatments on the heart. Due to the feasibility of genetic manipulations the mouse has become the most common mammalian animal model to study cardiac function and to search for new potential therapeutic targets. Here we describe a protocol to determine cardiac function in vivo using pressure-volume loop measurements and analysis during basal conditions and under β-adrenergic stimulation by intravenous infusion of increasing concentrations of isoproterenol. We provide a refined protocol including ventilation support taking into account the positive end-expiratory pressure to ameliorate negative effects during open-chest measurements, and potent analgesia (Buprenorphine) to avoid uncontrollable myocardial stress evoked by pain during the procedure. All together the detailed description of the procedure and discussion about possible pitfalls enables highly standardized and reproducible pressure-volume loop analysis, reducing the exclusion of animals from the experimental cohort by preventing possible methodological bias.
Introduction
Cardiovascular diseases typically affect cardiac function. This issue points out the importance in assessing in vivo detailed cardiac function in animal disease models. Animal experimentation is surrounded by a frame of the three Rs (3Rs) guiding principles (Reduce/Refine/Replace). In case of understanding complex pathologies involving systemic responses (i.e., cardiovascular diseases) at the current developmental level, the main option is to refine the available methods. Refining will also lead to a reduction of the required animal numbers due to less variability, which improves the power of the analysis and conclusions. In addition, combination of cardiac contractility measurements with animal models of heart disease including those induced by neurohumoral stimulation or by pressure overload like aortic banding, which mimics for example altered catecholamine/β-adrenergic levels1,2,3,4, provides a powerful method for pre-clinical studies. Taking into account that the catheter-based method remains the most widely used approach for in depth assessment of cardiac contractility5, we aimed to present here a refined measurement of in vivo cardiac function in mice by pressure-volume loop (PVL) measurements during β-adrenergic stimulation based on previous experience including the evaluation of specific parameters of this approach6,7.
To determine cardiac hemodynamic parameters approaches that include imaging or catheter-based techniques are available. Both options are accompanied by advantages and disadvantages that carefully need to be considered for the respective scientific question. Imaging approaches include echocardiography and magnetic resonance imaging (MRI); both have been successfully used in mice. Echocardiographic measurements involve high initial costs from a high-speed probe required for the high heart rate of the mice; it is a relatively straightforward non-invasive approach, but it is variable among operators who ideally should be experienced recognizing and visualizing cardiac structures. In addition, no pressure measurements can be performed directly and calculations are obtained from combination of size magnitudes and flow measurements. On the other hand, it has the advantage that several measurements can be performed on the same animal and cardiac function can be monitored for example during disease progression. Regarding the volume measurement, the MRI is the gold standard procedure, but similar to echocardiography, no direct pressure measurements are possible and only preload dependent parameters can be obtained8. Limiting factors are also the availability, analysis effort and operating costs. Here catheter-based methods to measure cardiac function are a good alternative that additionally allow for the direct monitoring of intracardiac pressure and the determination of load-independent contractility parameters like preload recruitable stroke work (PRSW)9. However, ventricular volumes measured by a pressure-conductance catheter (through conductivity determination) are smaller than those from the MRI but group differences are maintained in the same range10. In order to determine reliable volume values the corresponding calibration is required, which is a critical step during the PVL measurements. It combines ex vivo measurements of blood conductivity in volume-calibrated cuvettes (conversion of conductance to volume) with the in vivo analysis for the parallel conductance of the myocardium during the bolus injection of the hypertonic saline11,12. Beyond that, the positioning of the catheter inside the ventricle and the correct orientation of the electrodes along the longitudinal axis of the ventricle are critical for the detection capability of the surrounding electrical field produced by them. Still with the reduced size of the mouse heart it is possible to avoid artifacts produced by changes in the intraventricular orientation of the catheter, even in dilated ventricles5,10, but artifacts can evolve under β-adrenergic stimulation6,13. Additional to the conductance methods the development of admittance based method appeared to avoid the calibration steps, but here the volume values are rather overestimated14,15.
Since the mouse is one of the most important pre-clinical models in cardiovascular research and the β–adrenergic reserve of the heart is of central interest in cardiac physiology and pathology, we here present a refined protocol to determine in vivo cardiac function in mice by PVL measurements during β-adrenergic stimulation.
Protocol
All animal experiments were approved and performed according to the regulations of the Regional Council of Karlsruhe and the University of Heidelberg (AZ 35-9185.82/A-2/15, AZ 35-9185.82/A-18/15, AZ 35-9185.81/G131/15, AZ 35-9185.81/G121/17) conform to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. Data shown in this protocol are derived from wild type C57Bl6/N male mice (17 ± 1.4 weeks of age). Mice were maintained under specified pathogen-free conditions at the animal facility (IBF) of the Heidelberg Medical Faculty. Mice were housed in a 12-hour light-dark cycle, with a relative humidity between 56-60%, a 15-times air change per hour and room temperature of 22°C +/- 2°C. They were kept in conventional cages type II or type II long provided with animal bedding and tissue papers as enrichment. Standard autoclaved food and autoclaved water were available to consume ad libitum.
1. Preparation of instruments and drug solutions
- Central venous catheter: Cut the micro tube (0.6 mm outer diameter) into ~20 cm long catheter tubes. Use forceps to pull one end of the tube onto the tip of a 23-gauge cannula. Cut the other end of the tubing diagonally to create a sharp tip that can pierce the femoral vein.
- Endotracheal tube: For an intubation tube cut a 20-gauge venipuncture-cannula 3 cm in length to remove the syringe attachment.
- If the intubation tube does not fit the ventilator connection perfectly, wrap parafilm over the end of the tube where the ventilation device is connected. The connection must be stable and sealed by the thickening (Figure 1A). Shorten the metal guide pin of the 20-gauge venipuncture-cannula to 2.7 cm and use it as an intubation aid. Refined approaches for intubation including light fibers to facilitate visualization of the trachea are also well described, for example by Das and collaborators16.
- Anesthetic mixture used for intubation: Mix 200 µL of heparin (1000 IU/mL) with 50 µL of 0.9% NaCl and 750 µL of 2 mg/mL etomidate from an oil-in-water emulsion based product. Use 7 µL/g body weight (BW) for each mouse (0.1 mg/kg BW Buprenorphine 10 mg/kg BW etomidate).
- Muscle relaxant: Dissolve 100 mg of Pancuronium-bromide in 100 mL of 0.9% NaCl. Use 1.0 µL/g body weight (1 mg/kg BW) for each mouse.
- Isoproterenol solutions: Dissolve 100 mg of isoproterenol in 100 mL of 0.9% NaCl (1 µg/µL). Prepare the following dilutions (Table 1) and transfer each in a 1 mL syringe.
- To obtain dilution 1, dilute the stock 1:1.8. To obtain dilution 2, dilute the stock 1:6. To obtain dilution 3, dilute dilution 1 into 1:10. Finally, obtain dilution 4 by a 1:10 dilution of dilution 2.
- 15% Hypertonic NaCl (w/v): Dissolve 1.5 g of 0.9% NaCl in 10 mL of double distilled H2O. Filter the solution with a 0.45 µm pore syringe filter.
- Preparation of 12.5% albumin solution (w/v): Dissolve 1.25 g of bovine serum albumin in 10 mL of 0.9% NaCl. Incubate the solution at 37 °C for 30 min. Cool down to room temperature and filter the solution with a 0.45 µm pore syringe filter.
- Preparation of the setup: First switch on the heating plate and set it to 39-40 °C. Place a syringe filled with saline on the heating pad and transfer the pressure-volume loop (PVL) catheter into the syringe. Pre-incubate the catheter for at least 30 min before use for stabilization. The setup we use consist of a 1.4-F pressure-conductance catheter, a control unit and the corresponding software, and it is graphically described on Figure 1B and provider references are listed in the Table of Materials.
2. Anesthesia
- Inject buprenorphine (0.1 mg/kg BW intraperitoneally) 30 min before intubation.
- Place the mouse into an acrylic glass-chamber pre-saturated with 2.5% isoflurane and pre-warmed with a heating pad placed on the base of the chamber.
- As soon as the mouse sleeps (lack of reflex), inject the anesthetic mixture (7 mL/kg BW) containing 10 mg/kg etomidate and heparin (1,200 IU/kg BW) intraperitoneally.
3. Ventilation
- Transfer the animal to the intubation platform (Figure 1C) 3-4 minutes after the anesthetic injection. The mouse hangs from the teeth with the dorsal view facing the operator.
- Gently lift the tongue with forceps. To identify the glottis, lift the mouse's lower jaw slightly with second forceps.
- Carefully insert the endotracheal tube (Figure 1A) into the trachea and remove the guide rod.
- Transfer the animal onto the heating plate, place it on the back and connect the intubation tube to the small animal respirator.
- Adjust respiratory rate to 53.5 x (Body weight in grams)-0.26 [min-1], as described by others12, and tidal volumes to peak inspiratory pressures of 11 ± 1 cmH2O. Establish a PEEP of 2 cmH2O.
- Fix carefully the extremities of the mouse on the heating plate with adhesive strips and apply eye ointment on both eyes to prevent dryness.
- Insert a rectal temperature probe and maintain core body temperature at 37 ± 0.2 °C.
- Install a 1-lead ECG and monitor the heart rate on-line as an indicator for anesthesia depth and stability.
- Upon absence of interdigital reflexes, inject 1 mg/kg BW of the muscle relaxant pancuronium-bromide intraperitoneally. This prevents respiratory artifacts during PVL measurements.
4. Surgery
- General recommendations
- During surgery, ventilate with ~1.5-2% isoflurane vaporized with O2. The isoflurane concentration can also depend on variables like mouse strain, gender, age and weight of the animals, but it needs to be individually and experimentally determined and the values here are reference for the C57BL6/N mouse strain. Importantly, the ventilator is connected to an extraction system to prevent the operator from inhaling isoflurane.
- Use a magnification between 1.5-4x from the stereo microscope for surgical procedures.
NOTE: Refer to institutional/local guidance on preparation of the animal for non-survival surgeries.
- Femoral cannulation
- Rinse the hindlimb with 70% ethanol, incise the left inguinal region and expose the left femoral vein.
- Blast the epigastric artery and vein with a cautery.
- Ligate the femoral vein with a suture placed distal to the catheter access.
- Pass a suture underneath the femoral vein and prepare a knot cranial of puncture site. Puncture the femoral vein with the prepared micro tube (see step 1.1) attached to a 1 mL syringe.
- Tie down the knot to fix the tube inside the vessel.
- Counteract fluid loss by the infusion of 0.9% NaCl supplemented with 12.5% albumin at an infusion rate of 15 µL/min with an automatic syringe pump. Additionally, keep exposed tissue humid using pre-warmed 0.9% NaCl.
- Thoracotomy
- Rinse the thorax with 70% ethanol.
- Incise the skin just beneath the xyphoid process and bluntly separate the pectoral muscles from the chest wall with forceps or a cautery.
- Lift the xyphoid process with forceps, and then cut through the chest wall moving laterally on both sides with a cautery until the diaphragm is fully visible from beneath.
- Incise the diaphragm from beneath and expose the cardiac apex. Then carefully remove the pericardium with forceps.
- Perform a limited costotomy on the left side as previously described6.
- Pass a suture beneath the inferior caval vein to perform preload reduction during later stages.
- Gently puncture the cardiac apex with a 25-gauge cannula (maximal 4 mm). Remove the cannula and insert the PV catheter until all electrodes are within the ventricle.
- Adjust the position of the catheter by gentle movements and turns until rectangular shaped loops are obtained (Figure 2A).
- Keep always all exposed tissue humid using pre-warmed 0.9% NaCl.
5. Measurements
- General recommendations
- During measurements, ventilate with ~1.5-2% isoflurane vaporized with 100% O2.
- Perform 2 baseline measurements as well as 2 vena cava occlusions on each step of the dose response protocol.
NOTE: It is important that after the first and second vena cava occlusion, both pressure and volume values return to steady-state values as before the first occlusion. This observation is necessary in order to recognize a shift in catheter position due to serial reductions in intraventricular volume. If a shift in catheter position would be the case, especially volume values would be shifted.
- Perform an on-line analysis of parameters (heart rate, stroke volume, dP/dtmax) and wait until steady-state cardiac function is obtained. For the expected parameter range with the here used setting in C57Bl6/N mice please refer to published results6.
- Stop the respirator at end-expiratory position and record baseline parameters. After 3 to 5 seconds reduce cardiac preload by lifting the suture beneath the inferior caval vein with forceps in order to obtain preload independent parameters (Figure 2B). Turn the ventilator on. Wait at least 30 seconds for the second occlusion until hemodynamic parameters are stabilized.
- After obtaining the measurements under basal conditions proceed to the dose-response of isoproterenol by switching to the prepared syringes. Here the infusion rate stays unchanged in order to avoid modifications of the cardiac preload. Take care not to infuse air bubbles when changing the syringe.
- Wait at least 2 minutes until new steady-state cardiac function is obtained than again stop the respirator at end-expiratory position and record baseline parameters. After 3 to 5 seconds reduce cardiac preload by lifting the suture beneath the inferior caval vein in order to obtain preload independent parameters.
- Wait at least 30 seconds for the second occlusion. Afterwards switching to the prepared syringe with the next isoproterenol concentration and repeat the recordings of baseline and preload independent parameters.
NOTE: Artifacts like the end-systolic pressure-spike (ESPS, Figure 2C) can occur during the increase in the dosage of isoproterenol, which results from catheter entrapment. Artefacts that occur before the start of basal parameters can be easily corrected via re-positioning of the catheter.
6. Calibration
NOTE: Calibration procedures may vary depending on the PVL system used.
- Parallel-conductance calibration
- Connect a syringe containing a 15% NaCl solution to the femoral cannula after the last measurement from the isoproterenol dose-response. Carefully infuse 5 µL of the hypertonic solution remaining in the tube until PVL slightly shift to the right during on-line visualization. Then wait until the loops come back to steady-state.
- Stop the respirator at end-expiration and inject one bolus of 10 µL of 15% NaCl within 2 to 3 seconds. Check if PVL largely broaden and are shifted to the right during on-line visualization.
- Conductance-to-volume calibration
- Wait 5 min, no less, so that the hypertonic saline bolus is completely diluted. Afterwards remove the catheter and draw at least 600 μL blood from the left ventricle of the beating heart using a 1 mL syringe and a 21-gauge cannula. At this time point the animal is euthanized under deep anesthesia and analgesia by massive bleeding, by stopping the ventilation and removal of the heart.
- Transfer the blood into the pre-warmed (in a water bath at 37 °C) calibration cuvette with cylinders of known volume. Place the PV catheter centrally in each cylinder and record the conductance. By calculating a standard curve for each animal, the conductance units can be converted into absolute volume values.
7. Analysis
- After successful PVL measurements under basal conditions and isoproterenol stimulation, visualize, digitalize, calculate and extract parameters characterizing cardiac function (like PRSW, dP/dt, end-diastolic pressure and volume, end-systolic pressure and volume, relaxation constant Tau, among others) using an appropriate PVL analysis software. Further statistical analysis and graphical representations can be performed with standard analysis software.
- Analysis of preload independent parameters
NOTE: For this step it is crucial to standardize the procedure.- Select the first 5-6 PVLs showing decreasing preload throughout all measurements for the analysis of preload independent parameters (Figure 2D). A constant number of PVLs selected for analysis during preload reduction will decrease the variability among measurements of the obtained parameters.
- Calculate the mean value of the two measurements on each step of the protocol.
Representative Results
The pressure volume-loop (PVL) measurement is a powerful tool to analyze cardiac pharmacodynamics of drugs and to investigate the cardiac phenotype of genetically modified mouse models under normal and pathological conditions. The protocol allows the assessment of cardiac β-adrenergic reserve in the adult mouse model. Here we describe an open-chest method under isoflurane anesthesia combined with buprenorphine (analgesic) and pancuronium (muscle relaxant), which focuses on the cardiac response to β-adrenergic stimulation by infusing isoproterenol concentrations through a femoral vein catheter. Some representative data shown in this protocol are derived from wild type C57Bl6/N adult male mice (Figure 3 and Table 2). As indicator of the variability of some important parameters measured by our PVL analysis we performed a power analysis (α error probability of 0.05 and power of 0.8) using the results from the WT group and the free available G*Power software17. In Table 3 the calculated effect sizes and required sample sizes for heart rate, PRSW, stroke volume, the relaxation constant Tau, dP/dtmax and dP/dtmin assuming changes between 10% to 30% for each parameter under 0, 0.825 and 8.25 ng/min isoproterenol are depicted.
Graphical analysis of pressure-volume relations is done by plotting volume (µL) on the Y- and pressure (mmHg) on the X-axis. If the catheter is correctly placed within the ventricle, a full cardiac cycle is represented by a rectangular shaped PVL (Figure 2A and Figure 3A). Shortly, systole begins with a phase of isovolumetric contraction (characterized by dP/dtmax), during which both cardiac valves are closed (right vertical edge). When ventricular pressure exceeds aortic pressure, the aortic valve opens and blood is pumped into the aorta during ejection phase (upper horizontal). Subsequently, when the aortic pressure exceeds ventricular pressure, the aortic valve closes and diastole begins. During the isovolumetric relaxation (characterized by the parameters dP/dtmin and Tau) ventricular pressure falls until the atrial pressure exceeds the ventricular pressure and the mitral valve opens (left vertical edge). Now passive diastolic filling, characterized by end-diastolic pressure-volume relationship (EDPVR), takes place until the next cardiac cycle begins (bottom horizontal) (Figure 2A-B).
PVL analysis provides detailed insights into cardiac function since it is capable of determining cardiac function independent from cardiac preload. Thus, it has been described as the gold-standard for determining cardiac function in experimental setups5. In the described protocol using C57Bl6/N mice, we evaluated the response to isoproterenol produced on general parameters of cardiac function such as heart rate, cardiac output, stroke volume and stroke work. A significant effect of isoproterenol on each parameter is observed in the dose response under different isoproterenol concentrations (Figure 3B). Parameters of cardiac contractility like PRSW and dP/dtmax showed the expected increase in dose-response under isoproterenol infusion (Figure 3A-B). On the other hand, a reduction in diastolic parameters (constant of relaxation Tau and dP/dtmin) with increasing isoproterenol concentrations were recorded (Figure 3C) as being expected from a positive lusitropic effect produced by catecholamines in the healthy heart. Further parameters from those shown in Figure 3 (i.e., end-systolic pressure and volume, end-diastolic pressure and volume, maximal pressure, among others) are obtained from PVL analysis as well and can also be analyzed depending on the scientific question, the genetic or disease model and observations obtained. Additional and detailed values for the most common parameters of cardiac function in PVL on each step during incremental β-adrenergic stimulation, including the time point of calibration for parallel-conductance with hypertonic saline which highly influences cardiac volume parameters, but also cardiac inotropy and relaxation, have been previously reported1,6.
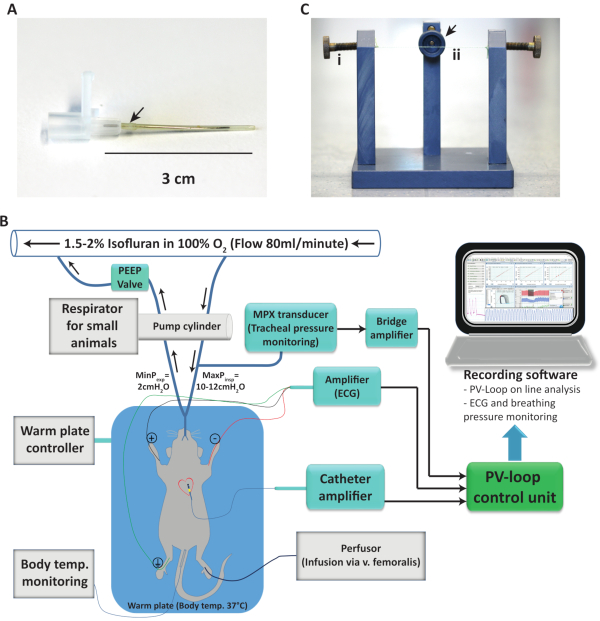
Figure 1. Anesthesia and pressure-volume loop setup. (A) 20-gauge venipuncture-cannula adapted for mouse intubation. (B) Diagram showing the organization and connection of the different components of the pressure-volume measurement setup used, including the flow direction of the anesthetic gas. (C) Intubation platform used to hang the mice for a rapid and safe intubation. Screws (i) at both sides at the end of the hanging thread (ii) are included to tighten the threat depending on the mouse weight. The arrow indicates a connection possibility for isoflurane exposure. Temp.: Temperature; ECG: Electrocardiogram; MinPexp: Minimal expiratory pressure; MaxPexp: Maximal expiratory pressure; PV: Pressure-volume. Please click here to view a larger version of this figure.
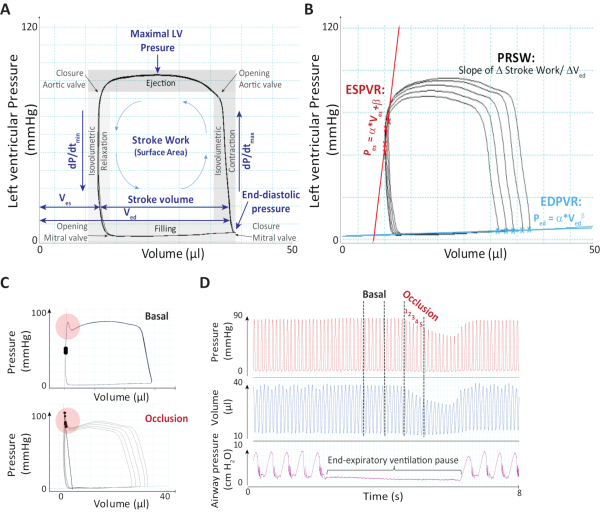
Figure 2. Representative pressure-volume analysis. (A) Exemplary pressure-volume recordings where parameters analyzed during basal measurement are shown and main events during the cardiac cycle are depicted. (B) Parameters ESPVR, EDPVR and PRSW are depicted during preload-reduction. (C) End-systolic pressure-spikes during basal measurements (upper panel) or during the occlusion maneuver (lower panel) both under isoproterenol stimulation are presented. LV: Left ventricular; dP/dtmin: Minimum dP/dt; dP/dtmax: Maximum dP/dt; Ves: End-systolic volume; Ved: End-diastolic volume; ESPVR: End-systolic pressure-volume relationship; PRSW: Preload recruitable stroke work; EDPVR: End-diastolic pressure-volume relationship. Figure was adapted from the supplement of our previous work 20196. Please click here to view a larger version of this figure.
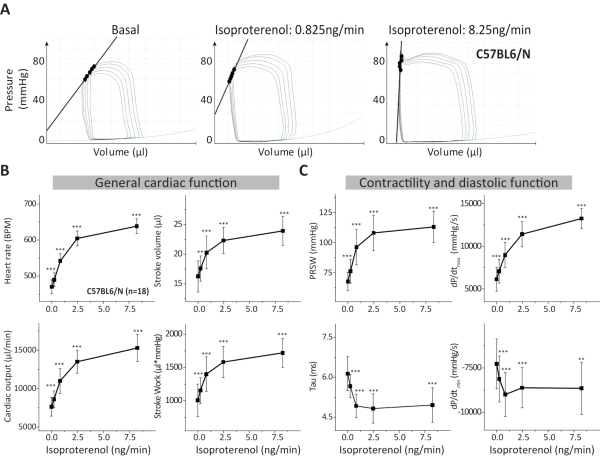
Figure 3. Analysis of PVL-measurements in C57BL6/N mice. (A) Representative PVLs during inferior caval vein occlusion from C57BL6/N control mice and subjected to increasing isoproterenol concentrations. (B) General cardiac function during basal conditions and during isoproterenol is described by the analysis of heart rate, cardiac output, stroke volume and stroke work. (C) Additional parameters were analyzed to assess cardiac contractility and diastolic function like PRSW, the constant of relaxation Tau (Weiss Equation18) and the maximal and minimal dP/dt. Data are presented as mean ± standard deviation. BPM: Beats per minute; PRSW: Preload recruitable stroke work; n: number of mice. **p < 0.01: p-values from the paired Student's t-test against the basal condition (isoproterenol = 0 ng/min). Please click here to view a larger version of this figure.
| Isoproterenol | Concentration (pg/µL) | Infusion rate (µL/min) | Doses (ng/min) |
| Stock | 1000 | ||
| Dilution 1 | 550 | 15 | 8.25 |
| Dilution 2 | 165 | 15 | 2.475 |
| Dilution 3 | 55 | 15 | 0.825 |
| Dilution 4 | 16.5 | 15 | 0.2475 |
Table 1. Dilution of isoproterenol for increasing β-adrenergic stimulation. Please click here to download this table.
| Isoproterenol (ng/min) | |||||
| 0 | 0.2475 | 0.825 | 2.475 | 8.25 | |
| Global Parameters and Volumes | |||||
| Heart Rate (bpm) | 470 ± 19.6 | 490 ± 19.3 | 542 ± 20.6 | 605 ± 20.5 | 638 ± 20.5 |
| Stroke Volume (µl) | 16.2 ± 2.6 | 17.6 ± 2.1 | 20.3 ± 2.8 | 22.3 ± 2.2 | 23.9 ± 2.5 |
| Cardiac Output (µl/min) | 7627 ± 1210 | 8609 ± 1097 | 11000 ± 1616 | 13502 ± 1494 | 15291 ± 1761 |
| End-systolic Volume (µl) | 13 ± 3.1 | 10.5 ± 3.5 | 4.81 ± 2.3 | 1.94 ± 1.9 | 1.5 ± 1.7 |
| End-diastolic Volume (µl) | 27.4 ± 3 | 26.6 ± 3.0 | 24.1 ± 3.1 | 23.8 ± 2.6 | 24.8 ± 2.7 |
| Mean Pressure (mmHg) | 27.4 ± 2.2 | 28.6 ± 2.2 | 29.2 ± 1.9 | 29.7 ± 1.9 | 30.5 ± 1.9 |
| Arterial Elastance (mmHg/µl) | 4.44 ± 0.6 | 4.18 ± 0.7 | 3.46 ± 0.5 | 2.78 ± 0.9 | 2.91 ± 1 |
| Systolic Parameters | |||||
| Preload Recruitable Stroke Work | 67.8 ± 7.62 | 76.3 ± 9.85 | 96.1 ± 14.62 | 108 ± 14.56 | 113 ± 13.02 |
| ESPVR | 4.96 ± 1.29 | 5.15 ± 1.16 | 7.2 ± 2.28 | 17.3 ± 42.04 | 40 ± 107.55 |
| Ejection Fraction (%) | 52.59 ± 9.57 | 60.9 ± 9.94 | 80.23 ± 8.65 | 92.16 ± 7.2 | 94.18 ± 6.15 |
| Stroke Work (mmHg x µl) | 1007 ± 244.26 | 1153 ± 193 | 1399 ± 261 | 1582 ± 234 | 1720 ± 216 |
| Maximum dP/dt (mmHg/s) | 6128.7 ± 1398.39 | 7087 ± 1401 | 8982.4 ± 1481 | 11422 ± 1477 | 13256 ± 1165 |
| Minimum dV/dt (µl/s) | – 523 ± 105.58 | – 613 ± 102 | – 835 ± 151 | – 1103 ± 165 | – 1273 ± 177 |
| End-systolic Pressure (mmHg) | 70.8 ± 6.98 | 72.5 ± 7.42 | 69 ± 6.28 | 61.2 ± 17.36 | 68.2 ± 19.72 |
| Maximum Power (mmHg x µl/s) | 3009 ± 955.31 | 3541 ± 1188 | 4185 ± 1058 | 4272 ± 959 | 4918 ± 1418 |
| Diastolic Parameters | |||||
| EDPVR | 1 ± 0.93 | 1.23 ± 0.88 | 1.5 ± 0.86 | 1.87 ± 0.92 | 1.96 ± 0.99 |
| Tau (ms, Weiss' equation) | 6.14 ± 0.64 | 5.67 ± 0.44 | 4.92 ± 0.44 | 4.83 ± 0.55 | 4.96 ± 0.65 |
| Minimum dP/dt (mmHg/s) | – 7272 ± 1403 | – 8119 ± 1295 | – 8998 ± 1240 | – 8618 ± 1129 | – 8648 ± 1468 |
| End-diastolic Pressure (mmHg) | 5.29 ± 1.01 | 5.74 ± 1.07 | 5.6 ± 1.51 | 5.37 ± 1.13 | 5.76 ± 1.15 |
| Maximum dV/dt (µl/s) | 765 ± 174 | 817 ± 178 | 972 ± 156 | 1158 ± 163 | 1264 ± 153 |
Table 2. Analysis of PVL-measurements in C57BL6/N mice. PVL parameters of cardiac function during basal conditions and during isoproterenol infusion. Data are presented as mean ± standard deviation from 18 male adult mice. PV: Pressure volume; BPM: Beats per minute; ESPVR: Slope of End-systolic PV-Relationship, insufficient calculation at low intra-ventricular volumes (2.475 and 8.25 ng/min Isoproterenol); EDPVR: End-diastolic PV-Relationship, exponential regression (alpha coefficient). Please click here to download this table.
| Delta (%) | Effect size | Sample size per group | ||||
| Isoproterenol ng/min | Isoproterenol ng/min | |||||
| 0 | 0.825 | 8.25 | 0 | 0.825 | 8.25 | |
| Heart rate | ||||||
| 10 | 2.4 | 2.6 | 3.1 | 4 | 4 | 3 |
| 15 | 3.6 | 3.9 | 4.6 | 3 | 3 | 3 |
| 20 | 4.8 | 5.3 | 6.2 | 3 | 3 | 3 |
| 25 | 6.0 | 6.6 | 7.8 | 3 | 3 | 3 |
| 30 | 7.2 | 7.9 | 9.3 | 3 | 3 | 3 |
| Stroke volume | ||||||
| 10 | 0.6 | 0.7 | 1.0 | 42 | 30 | 18 |
| 15 | 0.9 | 1.1 | 1.5 | 20 | 15 | 9 |
| 20 | 1.2 | 1.5 | 2.0 | 12 | 9 | 6 |
| 25 | 1.5 | 1.8 | 2.4 | 8 | 6 | 4 |
| 30 | 1.8 | 2.2 | 2.9 | 6 | 5 | 4 |
| Preload recruitable stroke work | ||||||
| 10 | 0.9 | 0.7 | 0.9 | 21 | 38 | 22 |
| 15 | 1.3 | 1.0 | 1.3 | 10 | 18 | 11 |
| 20 | 1.8 | 1.3 | 1.7 | 7 | 11 | 7 |
| 25 | 2.2 | 1.6 | 2.2 | 5 | 7 | 5 |
| 30 | 2.7 | 2.0 | 2.6 | 4 | 6 | 4 |
| dP/dtmax | ||||||
| 10 | 0.4 | 0.6 | 1.1 | 83 | 44 | 14 |
| 15 | 0.7 | 0.9 | 1.7 | 38 | 20 | 7 |
| 20 | 0.9 | 1.2 | 2.3 | 22 | 12 | 5 |
| 25 | 1.1 | 1.5 | 2.8 | 15 | 8 | 4 |
| 30 | 1.3 | 1.8 | 3.4 | 11 | 6 | 3 |
| Tau | ||||||
| 10 | 1.0 | 1.1 | 0.8 | 19 | 14 | 28 |
| 15 | 1.4 | 1.7 | 1.2 | 9 | 7 | 13 |
| 20 | 1.9 | 2.2 | 1.5 | 6 | 5 | 8 |
| 25 | 2.4 | 2.8 | 1.9 | 4 | 4 | 6 |
| 30 | 2.9 | 3.4 | 2.3 | 4 | 3 | 5 |
| dP/dtmin | ||||||
| 10 | 0.5 | 0.7 | 0.6 | 60 | 31 | 47 |
| 15 | 0.8 | 1.1 | 0.9 | 27 | 15 | 22 |
| 20 | 1.0 | 1.4 | 1.2 | 16 | 9 | 13 |
| 25 | 1.3 | 1.8 | 1.5 | 11 | 6 | 9 |
| 30 | 1.6 | 2.2 | 1.8 | 8 | 5 | 7 |
| End-systolic pressure-volume relationship | ||||||
| 10 | 0.4 | 0.3 | 0.04 | >100 | >100 | >100 |
| 15 | 0.6 | 0.5 | 0.06 | 48 | 73 | >100 |
| 20 | 0.8 | 0.6 | 0.07 | 28 | 41 | >100 |
| 25 | 1.0 | 0.8 | 0.09 | 19 | 27 | >100 |
| 30 | 1.2 | 1.0 | 0.11 | 13 | 19 | >100 |
| End-diastolic volume | ||||||
| 10 | 0.9 | 0.8 | 0.9 | 20 | 27 | 20 |
| 15 | 1.4 | 1.2 | 1.4 | 10 | 13 | 10 |
| 20 | 1.8 | 1.6 | 1.8 | 6 | 8 | 6 |
| 25 | 2.3 | 2.0 | 2.3 | 5 | 6 | 5 |
| 30 | 2.8 | 2.4 | 2.8 | 4 | 5 | 4 |
Table 3. Estimated effect size and required sample size for selected parameters based on values observed in C57BL6/N male mice. Delta depicts a hypothetic difference in the parameter between a control (i.e., wild type) and a treatment group. Effect size and required sample size per group is calculated using control data (mean and standard deviation), alpha error (0.05) and power (0.8) via G*Power 19. Bold values (green backgrounds in the online version of the table) indicate a suggested threshold effect size (1≤) and sample size for each parameter on each dose of isoproterenol. dP/dtmin: Minimum dP/dt; dP/dtmax: Maximum dP/dt. Please click here to download this table.
Discussion
Here, we provide a protocol to analyze the in vivo cardiac function in mice under increasing β-adrenergic stimulation. The procedure can be used to address both, baseline parameters of cardiac function and the adrenergic reserve (e.g., inotropy and chronotropy) in genetically modified mice or upon interventions. The most prominent advantage of pressure-volume loop (PVL) measurements as compared to other means of determining cardiac function is the analysis of intrinsic, load-independent cardiac function. All other methods (e.g., MRI and echocardiography) can only assess load-dependent parameters of cardiac function and especially cardiac contractility cannot be reliably determined. This makes PVL measurements the gold standard for end-point measurements of in depth analysis of cardiac function5. However, the methods named before allow for sequential analysis of cardiac function, bringing them to the forefront for longitudinal observations (e.g., during disease progression). Further, intraventricular volumes, and subsequently stroke volume and other derived parameters, may be underestimated in PVL measurements compared to MRI in mice20.
There are four critical steps during the protocol that are crucial to obtain valid PVL data: 1) Intubation, 2) placement of the femoral vein catheter, 3) placement of the pressure-conductance catheter and 4) the periprocedural regimen. Non-invasive intubation of mice requires some experience and is complicated when using isoflurane as the time frame for intubation is narrow (20 – 40 s). Thus, after intubation the correct tube placement should be carefully checked by examining murine chest movements when altering the ventilators respiratory rate. To widen the window for intubation, we here described the concomitant use of the short acting hypnotic etomidate. Furthermore, light fibers to facilitate visualization of the glottis are available16. Proper placement of the femoral vein catheter is essential for the application of isoproterenol during later stages. During this step, air embolism can severely harm the animals inducing pulmonary embolism. Correct placement of the femoral catheter can initially be checked by a careful aspiration of venous blood. When proper catheter placement is uncertain during later stages, end-diastolic volume can be examined, which should increase in response to the slightest bolus when visualizing PVL on-line. Contrary to most other investigators, we here describe cannulation of the femoral vein, whereas others most often used the jugular vein as the target vessel for central venous access12,21. This approach has the advantage of not manipulating close to the vagal nerve, as done in the close chest approach when the carotid is prepared, and thus we assume that potential stimulation of the parasympathetic system by simply touching/damaging the nerve is avoided. Proper placement of the PV catheter within the ventricle is crucial to obtain meaningful data especially concerning volume parameters. When electrodes are not completely inside the ventricle or the catheter is not properly placed along the longitudinal axis of the ventricle, volume parameters are highly underestimated. Further, contact between the endocardium and the pressure transducer causes end-systolic pressure spikes which should not be tolerated during baseline measurements6. Lastly, the periprocedural regimen including anesthesia depth and fluid management has a significant impact on the reliability of PVL data in mice. Anesthetic under- or overdosing can both severely affect hemodynamic parameters, most often resulting in reduced cardiac function. Fluid loss, which is mostly due to blood loss and evaporation, must be counteracted with the constant infusion of suitable solutions such as 12.5% albumin dissolved in 0.9% NaCl, which we recommend. Being that the approach is very invasive, no less important is the inclusion of a potent analgesic like Buprenorphine to minimize influences on cardiovascular functions evoked by insufficient pain avoidance. We inject the analgesic drug before intubation. It is important to perform the injection ~30 minutes before starting the whole procedure, especially if the operator is experienced, and thus fast, in order to reach a proper analgesic effect avoiding any pain during the investigation phase. Additionally, when working with obese models probably higher doses should be considered due to the high lipophilicity of this substance. Finally, this protocol may also be modified in determining response to other catecholaminergic stimuli such as dobutamine or epinephrine; as for example done by Calligaris and colleagues22 who described the analysis in intraventricular pressure during dobutamine stimulation.
Regarding the recording and analysis of PVL measurements there are several steps that need to be considered. First, it is of overwhelming importance to consistently analyze PVL recordings across an experimental data set. Respiratory artifacts that evolve due to alternating pulmonary pressure resulting in alternating cardiac preload during mechanical ventilation need to be avoided by switching off the ventilator during the recordings. To further eliminate respiratory artifacts, we recommend using the muscle relaxant pancuronium in order to prevent contractions of the diaphragm that are frequently seen during isoflurane anesthesia. In addition, it makes feasible to stop the ventilation at end-expiration and to analyze all of the selected loops, in contrast to other protocols recommending to select 8-10 loops and then identifying 5-6 end-expiratory loops that are subsequently analyzed23. Importantly, periods of apnea should be kept short to avoid hypoventilation resulting in hypercapnia and respiratory acidosis. To improve oxygenation and prevent the formation of atelectasis, we previously examined the use of PEEP-ventilation during PVL measurements in mice6. When selecting loops for the analysis of preload independent data, select the first 5-6 loops showing decreasing end-diastolic volume and avoid including loops where only pressure is declining, but volume is constant. Further, extra beats should not be included into analysis, as they crucially affect PVL parameters. Remarkably, most often arrhythmic beats occur owing to contact between the occlusion suture and the murine heart. Calibration for parallel conductance via infusion of hypertonic saline has a tremendous impact on parameters of cardiac function and should, to our understanding, be performed at the end of an experiment6. Notably, due to its impact on cardiac function, calibration for parallel conductance is performed only once during the protocol. However, parallel conductance changes slightly during the protocol, owing to changes in the ventricles shape upon adrenergic stimulation. Admittance systems for PVL assessments in mice are available that have no need for saline calibrations and can calculate parallel conductance dynamically throughout PVL recordings. However, the accuracy of this method is still under debate5,8,24,25.
We determined from our observations that when using this protocol in adult healthy wild type male mice (i.e., C57Bl6/N), systolic pressure is in the range of 70 mmHg to 90 mmHg at baseline and between 80 and 100 mmHg during maximum stimulation with the β-adrenoreceptor agonist isoproterenol. Likewise, stroke volume was observed to be in the range of 13 µL to 20 µL at baseline and between 20 µL and 35 µL during maximum stimulation. Heart rate was around 450 to 520 beats per minute at baseline and can well exceed 650 beat per minute during maximum stimulation. Concerning preload-independent cardiac contractility, the most robust parameter preload recruitable stroke work (PRSW) was considered to be adequate between 60 mmHg to 80 mmHg at baseline and between 100 mmHg and 140 mmHg during maximum stimulation. If baseline parameters significantly diverge from the ones usually obtained, or when cardiac function inappropriately reacts to β-adrenergic stimulation, complications (e.g., unobserved blood loss, drop/rise in body temperature or anesthetic over/under dose) should be taken into consideration.
Moreover, some artifacts may arise during PVL measurements in mice. The most common artifact is the end-systolic pressure-spike (ESPS, Figure 2C), which results from catheter entrapment and it is easily rectifiable by re-positioning the catheter prior to the basal measurements at 0 ng/min isoproterenol. Measurements should not start before ESPSs are eradicated at baseline conditions in order to obtain meaningful data, as ESPS can affect several parameters of cardiac function6. However, when an ESPS occurs during incremental stimulation with isoproterenol due to altered ventricular morphology in measurements unaffected at baseline, this is not rectifiable, since catheter repositioning would alter parallel conductance during the dose response protocol. One has to examine this closely, since, likewise those at baseline, these ESPSs have been shown to significantly alter parameters of cardiac function not only through a significantly increased maximum pressure13,26, but also through reduced volume detection6.
Representative values for hemodynamic parameters obtained by PVL measurements under baseline conditions and during incremental stimulation with isoproterenol in mice vary widely with different methodological approaches and in different mouse strains27,28. Beyond that, one should be aware that phenotypes of genetically altered mice may also be restricted to distinct genetic backgrounds. Methodologically, there are two paramount approaches of performing pressure-volume analysis in mice. Each method has its (dis)advantages and the method of choice often depends on the experiences of the lab and its investigators. We here focus on the open-chest procedure, in which the catheter is placed via a puncture on the apex. This approach has the advancement of catheter placement under vision which allows for precise catheter positioning, an essential predictor for the recording of meaningful data of cardiac function in mice. This is particularly true for the recording of volume parameters in the range of microliters. In contrast, a critical aspect of this approach is the loss of physiological intra-thoracic pressures, resulting in collapsing lungs and atelectasis formation and a higher loss of body fluid. However, by using positive end-expiratory pressure (PEEP) ventilation, we here describe a strategy that has proven to counteract pulmonary damage during open-chest PVL in mice6. The second experimental approach is to insert the catheter via the carotid artery and then retrogradely through the aortic valve. By using this technique, intra-thoracic pressures can be held rather normal, although mechanical ventilation is still needed, which weakens this advantage. Further, the closed-chest approach limits the investigators possibilities for precise catheter positioning. Moreover, PV catheters used in mice have diameters ranging from 1 to 1.4 French (0.33 mm to 0.47 mm), which implies a significant obstruction of the murine outflow tract when using the closed-chest approach, as aortas of adult mice typically have diameters between 0.8 mm to 1.2 mm29,30. Concerning the use of PVL in heart failure models, the open-chest approach is of particular importance for transverse aortic constriction models, where the constriction is located between the innominate artery and the left carotid artery. Here the catheter cannot be placed via the carotid artery. On the other hand, the closed-chest approach is of interest for researchers investigating murine models of dilated ventricles, such as after the induction of myocardial infarction, where puncture of the apex is not feasible.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We are thankful to Manuela Ritzal, Hans-Peter Gensheimer, Christin Richter and the team from the Interfakultäre Biomedizinische Forschungseinrichtung (IBF) from the Heidelberg University for expert technical assistance.
This work was supported by the DZHK (German Centre for Cardiovascular Research), the BMBF (German Ministry of Education and Research), a Baden-Württemberg federal state Innovation fonds and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Project-ID 239283807 – TRR 152, FOR 2289 and the Collaborative Research Center (SFB) 1118.
Materials
| 1.4F SPR-839 catheter | Millar Instruments, USA | 840-8111 | |
| 1 ml syringes | Beckton Dickinson, USA | REF303172 | |
| Bio Amplifier | ADInstruments, USA | FE231 | |
| Bridge-Amplifier | ADInstruments, USA | FE221 | |
| Bovine Serum Albumin | Roth, Germany | 8076.2 | |
| Buprenorphine hydrochloride | Bayer, Germany | 4007221026402 | |
| Calibration cuvette | Millar, USA | 910-1049 | |
| Differential pressure transducer MPX | Hugo Sachs Elektronik- Harvard Apparatus, Germany | Type 39912 | |
| Dumont Forceps #5/45 | Fine Science tools Inc. | 11251-35 | |
| Dumont Forceps #7B | Fine Science tools Inc. | 11270-20 | |
| Graefe Forceps | Fine Science tools Inc. | 11051-10 | |
| GraphPad Prism | GraphPad Software | Ver. 8.3.0 | |
| EcoLab-PE-Micotube | Smiths, USA | 004/310/168-1 | |
| Etomidate Lipuro | Braun, Germany | 2064006 | |
| Excel | Microsoft | ||
| Heparin | Ratiopharm, Germany | R26881 | |
| Hot plate and control unit | Labotec, Germany | Hot Plate 062 | |
| Isofluran | Baxter, Germany | HDG9623 | |
| Isofluran Vaporizer | Abbot | Vapor 19.3 | |
| Isoprenalinhydrochloride | Sigma-Aldrich, USA | I5627 | |
| Fine Bore Polythene tubing 0.61 mm OD, 0.28 mm ID | Smiths Medical International Ltd, UK | Ref. 800/100/100 | |
| MiniVent ventilator for mice | Hugo Sachs Elektronik- Harvard Apparatus, Germany | Type 845 | |
| MPVS Ultra PVL System | Millar Instruments, USA | ||
| NaCl | AppliChem, Germany | A3597 | |
| NaCl 0.9% isotonic | Braun, Germany | 2350748 | |
| Pancuronium-bromide | Sigma-Aldrich, USA | BCBQ8230V | |
| Perfusor 11 Plus | Harvard Apparatus | Nr. 70-2209 | |
| Powerlab 4/35 control unit | ADInstruments, USA | PL3504 | |
| Rechargeable cautery-Set | Faromed, Germany | 09-605 | |
| Scissors | Fine Science tools Inc. | 140094-11 | |
| Software LabChart 7 Pro | ADInstruments, USA | LabChart 7.3 Pro | |
| Standard mouse food | LASvendi GmbH, Germany | Rod18 | |
| Stereo microscope | Zeiss, Germany | Stemi 508 | |
| Surgical suture 8/0 | Suprama, Germany | Ch.B.03120X | |
| Venipuncture-cannula Venflon Pro Safty 20-gauge | Beckton Dickinson, USA | 393224 | |
| Vessel Cannulation Forceps | Fine Science tools Inc. | 00574-11 | |
| Water bath | Thermo Fisher Scientific, USA | ||
| Syringe filter (Filtropur S 0.45) | Sarstedt, Germany | Ref. 83.1826 |
References
- Bacmeister, L., et al. Inflammation and fibrosis in murine models of heart failure. Basic Research in Cardiology. 114 (3), 19 (2019).
- Hartupee, J., Mann, D. L. Neurohormonal activation in heart failure with reduced ejection fraction. Nature Reviews Cardiology. 14 (1), 30-38 (2017).
- Hasenfuss, G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovascular Research. 39 (1), 60-76 (1998).
- Lefkowitz, R. J., Rockman, H. A., Koch, W. J. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 101 (14), 1634-1637 (2000).
- Cingolani, O. H. K. Pressure-volume relation analysis of mouse ventricular function. The American Journal of Physiology-Heart and Circulatory Physiology. 301, 2198-2206 (2011).
- Bacmeister, L., et al. Assessment of PEEP-Ventilation and the Time Point of Parallel-Conductance Determination for Pressure-Volume Analysis Under beta-Adrenergic Stimulation in Mice. Frontiers in Cardiovascular Medicine. 6, 36 (2019).
- Segin, S., et al. Cardiomyocyte-Specific Deletion of Orai1 Reveals Its Protective Role in Angiotensin-II-Induced Pathological Cardiac Remodeling. Cells. 9 (5), (2020).
- Clark, J. E., Marber, M. S. Advancements in pressure-volume catheter technology – stress remodelling after infarction. Experimental Physiology. 98 (3), 614-621 (2013).
- Glower, D. D., et al. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 71 (5), 994-1009 (1985).
- Winter, E. M., et al. Left ventricular function in the post-infarct failing mouse heart by magnetic resonance imaging and conductance catheter: a comparative analysis. Acta Physiologica. 194 (2), 111-122 (2008).
- Krenz, M. Conductance, admittance, and hypertonic saline: should we take ventricular volume measurements with a grain of salt. Journal of Applied Physiology. 107 (6), 1683-1684 (2009).
- Pacher, P., Nagayama, T., Mukhopadhyay, P., Batkai, S., Kass, D. A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nature Protocols. 3 (9), 1422-1434 (2008).
- Wei, A. E., Maslov, M. Y., Pezone, M. J., Edelman, E. R., Lovich, M. A. Use of pressure-volume conductance catheters in real-time cardiovascular experimentation. Heart, Lung and Circulation. 23 (11), 1059-1069 (2014).
- van Hout, G. P., et al. Admittance-based Pressure-Volume Loops versus gold standard cardiac magnetic resonance imaging in a porcine model of myocardial infarction. Physiological Reports. 2 (4), 00287 (2014).
- Wei, C. L., Shih, M. H. Calibration Capacity of the Conductance-to-Volume Conversion Equations for the Mouse Conductance Catheter Measurement System. IEEE Transactions on Biomedical Engineering. 56 (6), 1627-1634 (2009).
- Das, S., MacDonald, K., Chang, H. Y., Mitzner, W. A simple method of mouse lung intubation. Journal of Visualized Experiments. (73), e50318 (2013).
- Faul, F., Erdfelder, E., Lang, A. G., Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 39 (2), 175-191 (2007).
- Weiss, J. L., Frederiksen, J. W., Weisfeldt, M. L. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. Journal of Clinical Investigation. 58 (3), 751-760 (1976).
- Faul, F., Erdfelder, E., Lang, A. G., Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavioral Research Methods. 39 (2), 175-191 (2007).
- Jacoby, C., et al. Direct comparison of magnetic resonance imaging and conductance microcatheter in the evaluation of left ventricular function in mice. Basic Research in Cardiology. 101 (1), 87-95 (2006).
- Georgakopoulos, D., Kass, D. A. Estimation of parallel conductance by dual-frequency conductance catheter in mice. The American Journal of Physiology-Heart and Circulatory Physiology. 279 (1), 443-450 (2000).
- Calligaris, S. D., Ricca, M., Conget, P. Cardiac stress test induced by dobutamine and monitored by cardiac catheterization in mice. Journal of Visualized Experiments. (72), e50050 (2013).
- Abraham, D., Mao, L. Cardiac Pressure-Volume Loop Analysis Using Conductance Catheters in Mice. Journal of Visualized Experiments. (103), e52942 (2015).
- Pearce, J. A., Porterfield, J. E., Larson, E. R., Valvano, J. W., Feldman, M. D. Accuracy considerations in catheter based estimation of left ventricular volume. Conference proceedings – IEEE engineering in medicine and biology society. 2010, 3556-3558 (2010).
- Nielsen, J. M., et al. Left ventricular volume measurement in mice by conductance catheter: evaluation and optimization of calibration. The American Journal of Physiology-Heart and Circulatory Physiology. 293 (1), 534-540 (2007).
- Townsend, D. Measuring Pressure Volume Loops in the Mouse. Journal of Visualized Experiments. (111), e53810 (2016).
- Barnabei, M. S., Palpant, N. J., Metzger, J. M. Influence of genetic background on ex vivo and in vivo cardiac function in several commonly used inbred mouse strains. Physiological Genomics. 42 (2), 103-113 (2010).
- Oosterlinck, W., Vanderper, A., Flameng, W., Herijgers, P. Glucose tolerance and left ventricular pressure-volume relationships in frequently used mouse strains. Journal of Biomedicine and Biotechnology. 2011, 281312 (2011).
- Guo, X., Kono, Y., Mattrey, R., Kassab, G. S. Morphometry and strain distribution of the C57BL/6 mouse aorta. The American Journal of Physiology-Heart and Circulatory Physiology. 283 (5), 1829-1837 (2002).
- Weiss, R. M., Ohashi, M., Miller, J. D., Young, S. G., Heistad, D. D. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 114 (19), 2065-2069 (2006).

