Summary
Here, we present a protocol to establish a new rat model of active HIV infection using chimeric HIV (EcoHIV), which is critical for enhancing our understanding of HIV-1 viral reservoirs in the brain and offering a system to study HIV-associated neurocognitive disorders and associated comorbidities (i.e., drug abuse).
Abstract
It has been well studied that the EcoHIV infected mouse model is of significant utility in investigating HIV associated neurological complications. Establishment of the EcoHIV infected rat model for studies of drug abuse and neurocognitive disorders, would be beneficial in the study of neuroHIV and HIV-1 associated neurocognitive disorders (HAND). In the present study, we demonstrate the successful creation of a rat model of active HIV infection using chimeric HIV (EcoHIV). First, the lentiviral construct of EcoHIV was packaged in cultured 293 FT cells for 48 hours. Then, the conditional medium was concentrated and titered. Next, we performed bilateral stereotaxic injections of the EcoHIV-EGFP into F344/N rat brain tissue. One week after infection, EGFP fluorescence signals were detected in the infected brain tissue, indicating that EcoHIV successfully induces an active HIV infection in rats. In addition, immunostaining for the microglial cell marker, Iba1, was performed. The results indicated that microglia were the predominant cell type harboring EcoHIV. Furthermore, EcoHIV rats exhibited alterations in temporal processing, a potential underlying neurobehavioral mechanism of HAND as well as synaptic dysfunction eight weeks after infection. Collectively, the present study extends the EcoHIV model of HIV-1 infection to the rat offering a valuable biological system to study HIV-1 viral reservoirs in the brain as well as HAND and associated comorbidities such as drug abuse.
Introduction
Biological systems have enhanced our understanding of HIV-1 associated neurocognitive disorders (HAND) and their underlying neural mechanisms2. Determining which biological system is most appropriate for any given study is often dependent upon the question of interest2. The limitation of the range of host animal models challenges studies of HIV-1 disease development. To investigate HIV-1 viral replication and pathogenesis, Potash et al.3 created a mouse model of active HIV-1 infection, replacing the coding region of HIV surface envelope glycoprotein, gp120, with ecotropic MLV gp80, which led to successful viral replication in mice4. After tail vein injections in chimeric HIV (EcoHIV) mice, many characteristics were observed resembling those of HIV-1 seropositive individuals (e.g., infected lymphocytes and macrophages, targeted for antiviral immune responses, and inflammation3,5,6).
Although mice and rats are both members of the Muridae, fundamental species differences may influence their suitability for specific experimental questions7. Therefore, the extension of the EcoHIV infection model to rats (commonly used in studies of drug abuse and neurocognitive disorders) would be advantageous in the study of neuroHIV. For example, their larger size makes jugular catheter implantation for drug self-administration procedures more practical8. Drug self-administration techniques in rats have been utilized to evaluate motivation in HIV-19. Furthermore, many neurocognitive/behavioral tasks were initially designed for rats10. Here, we report the utilization of stereotaxic injections of EcoHIV in rats to extend the EcoHIV infection model and afford a key opportunity to address novel questions related to neuroHIV and HAND.
Protocol
All animal protocols were reviewed and approved by the Animal Care and Use Committee at the University of South Carolina (federal assurance number: D16-00028). Six adult male F344/N rat was pair housed in a controlled environment under a 12/12 light: dark cycle with ad libitum access to food and water. All animals were cared for using guidelines established by the National Institutes of Health in the Guide for the Care and Use of Laboratory Animals.
1. Virus packaging in 293 FT cells
- Culture the 293 FT cells (5×105/mL) in gelatin coated 75 cm2 flasks with DMEM plus 10% FBS11. Grow cells at 37 °C to be 50% confluent at transfection.
- Dilute 22.5 µL of transfection reagent (e.g., Lipofectamine 3000) in 750 µL of medium (e.g., Opti-MEM) in a 1.5 mL micro-centrifuge tube and vortex for 3 s.
- Dilute 20 µg of EcoHIV plasmid DNA in 750 µL of medium in a 1.5 mL micro-centrifuge tube and mix well.
- Add diluted DNA into the tube of diluted transfection reagent and mix gently. Incubate for 15 min at room temperature.
- Add the mixture to 10 mL of prewarmed DMEM medium in 75 cm2 flask. Incubate cells for 2 days at 37 °C.
- Harvest and combine 24 mL of conditional medium from the two flasks that include the packaged lentivirus.
- Centrifuge all 24 mL of conditional medium at 500 x g for 10 minutes at 4 °C. Transfer the clarified supernatant to a sterile 50 mL tube.
- Combine 8 mL of Lenti-x concentrator with 24 mL of clarified supernatant (1:3 ratio). Mix by gentle inversion. Incubate mixture at 4 °C for two days.
- Centrifuge mixture at 1,500 x g for 45 minutes at 4 °C. Carefully remove the supernatant.
- Gently re-suspend the pellet with 100 µL of 100 mM PBS.
- Titer virus concentration with a p24 ELISA kit.
2. EcoHIV-EGFP virus stereotaxic surgeries
- Anesthetize rats using 3% sevoflurane. Proceed to step 2.2 when the rats are not responsive to noxious stimuli and reflexes are absent.
- Shave the hairs from the brain region and sterilize the skin twice with 70% ethanol and a chlorhexidine-based scrub. Secure the rat in a prone position in the stereotaxic apparatus.
- Make an incision (5-6 cm) through the skin along the scalp midline.
- Mark two drilling positions at 0.8 mm lateral, 1.2 mm rostral to bregma. Drill a hole (diameter 0.4 mm) in each skull position.
- Fill titered EcoHIV lentivirus solution (1.04 × 106 TU/mL) in a 10 µL injection syringe. Secure the syringe to the stereotaxic apparatus.
- Move down the needle close to the surface of drilling hole. Measure and move 2.5 mm in depth.
- Infuse 1 µL of virus solution at a rate of 0.2 µL/min. Keep the needle inside of the injection area for 5 min. Slowly move the needle up until it is outside of the rat skull.
- Suture the skin with a 4-0 silk thread.
- Sterilize the incision with 70% ethanol once. Subcutaneously inject butorphenol (Dorolex, 0.1 mg/kg body weight).
- Transfer the rat to a recovery chamber with a heating pad until it wakes up.
3. Visualization of brain sections
NOTE: Wait one to eight weeks after EcoHIV viral infusion.
- Anesthetize the rat with 5% sevoflurane. Continue to step 3.2 when the rats are not responsive to noxious stimuli and reflexes are absent.
- Fix the rat in a supine position inside a fume hood.
- Incise the skin along the thoracic midline. Cut the diaphragm and open the thoracic cavity.
- Insert a 20 G × 25 mm needle into the left ventricle.
- Immediately open the right atrium with scissors.
- Perfuse 50 mL of prechilled 100 mM PBS at a rate of 5 mL/min.
- Perfuse 100 mL of cold 4% paraformaldehyde at a rate of 5 mL/min.
- Decapitate the rat, open the scalp and remove the brain.
- Postfix overnight with 4% paraformaldehyde.
- Transfer the brain to 40 mL of 30% sucrose in 100 mM PBS in 50 mL tube until the brain floats down to the bottom (about 3 days).
- Snap freeze the brain in methylbutanol for 2 min at – 80 °C.
- Secure the brain tissue on a metal platform inside a -20 °C cryostat.
- Cut 50 µm thick coronal sections using the cryostat.
- Transfer the brain slices onto glass slides with a fine brush.
- Mount sections in 0.3 mL of antifade medium and cover with 22 mm 50 mm coverslips.
- Keep the glass slides in the dark at room temperature till dry.
- Image the targeted neurons with a confocal microscope using Z-stack based on brain region boundaries and morphological characteristics of neurons.
NOTE: The confocal microscope settings used were: magnification of 60 X (A/1.4, oil), and a Z-plane interval of 0.15 µm (pinhole size 30 µm; back-projected pinhole radius 167 nm) using a wavelength of 488 nm.
Representative Results
The conditioned medium was collected from lentivirus of EcoHIV-EGFP infected 293FT cells. Next, it was concentrated and titered, then stereotaxically injected into the brain (cortical region) of F344/N rats. Seven days post-injection, rats were sacrificed and images were taken from coronal brain slices ranging from bregma 5.64 mm to bregma -4.68 mm. In Figure 1A, there are significant EcoHIV-EGFP signals throughout the brain, especially in the cortex and the hippocampal dentate gyrus. Furthermore, dual-labeling with Iba1and EcoHIV-EGFP probes provided strong evidence that microglia were the predominant cell type harboring EcoHIV expression in the brain (Figure 1B). Notably, the distribution pattern of EcoHIV-EGFP is consistent with the relative regional concentrations of microglia in the rat brain (i.e., cortex and dentate gyrus of the hippocampus).
In a subsequent study, we validated the utility of EcoHIV infection in rats to model key aspects of HAND. Using the protocol detailed above, F344/N rats were stereotaxically injected with either EcoHIV-EGFP or saline. First, eight weeks post-infection, temporal processing, a potential elemental dimension of HAND12, was evaluated using visual gap prepulse inhibition (Figure 2). EcoHIV animals exhibited a relative insensitivity to the manipulation of interstimulus interval (ISI), evidenced by a relatively flatter ISI function compared to saline controls. Specifically, significant differences in the slope of the ISI function from the 50 ms ISI to the 200 ms ISI were observed (Semilog Line-X is Log, Y is Linear, R2s ≥ 0.99; F(1,2)=642.9, p≤0.001). Second, ballistic labeling was used to investigate the impact of EcoHIV-EGFP injections on the morphology of dendritic spines in medium spiny neurons (MSN) of the nucleus accumbens (NAc; Figure 3); parameters which can be utilized to draw inferences about synaptic function13. EcoHIV rats displayed profound alterations in dendritic spine morphology, evidenced by an increased relative frequency of shorter dendritic spines (Genotype x Bin Interaction, F(16, 218) = 4.3, p ≤ 0.001) with an increased head diameter (Genotype x Bin Interaction, F(12, 96) = 18.7, p ≤ 0.001) and increased neck diameter (Genotype x Bin Interaction, F(15, 120) = 16.3, p ≤ 0.001) relative to control animals. Detailed methodology for the assessment of temporal processing14 and ballistic labeling13 have been previously reported.
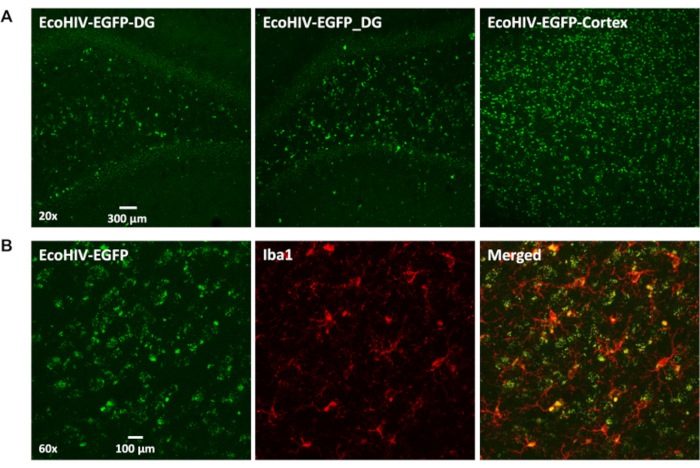
Figure 1. The EcoHIV-EGFP infected cells distributed in rat brain. (A) The representative confocal images (20x) of EcoHIV-EGFP expression in hippocampal dentate gyrus or cortex regions at 7 days after injection. (B) The representative confocal images (60X) of co-localization of Iba1 immunostaining with EcoHIV-EGFP infected cells at 7 days after injection. Please click here to view a larger version of this figure.
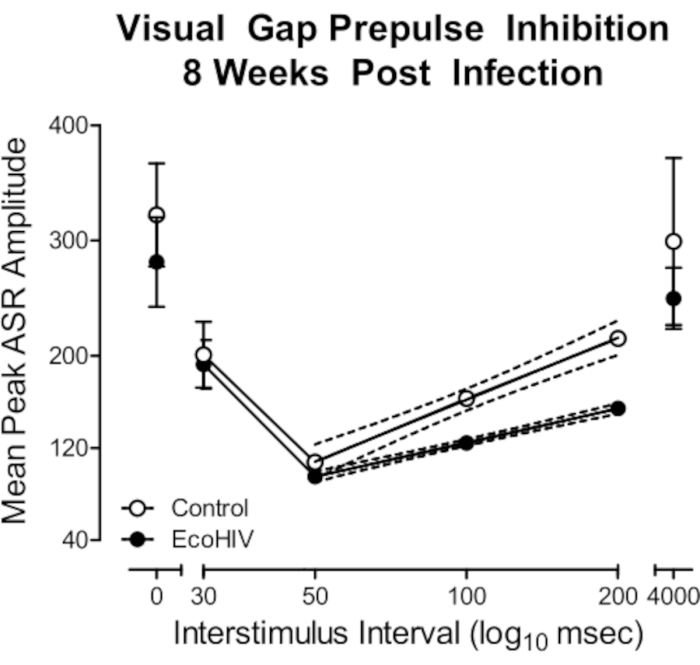
Figure 2. EcoHIV infection induced prominent neurocognitive deficits in temporal processing. Visual gap prepulse inhibition was conducted eight weeks after stereotaxic injections of either EcoHIV or saline. EcoHIV infection induced prominent alterations in temporal processing evidenced by the relative insensitivity to the manipulation of interstimulus interval relative to control rats. Detailed methodology described in McLaurin et al.13. Please click here to view a larger version of this figure.
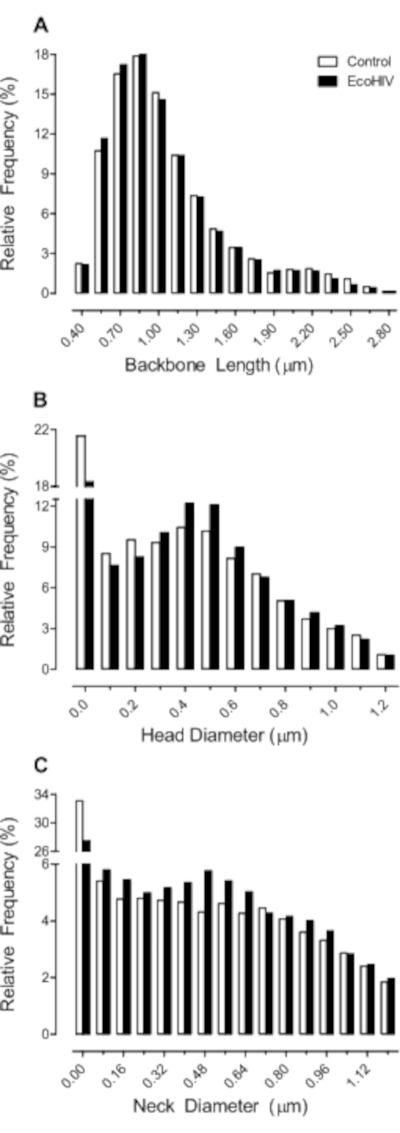
Figure 3. Infectivity with EcoHIV-EGFP altered the morphological parameters of dendritic spines, supporting profound synaptic dysfunction. EcoHIV rats displayed profound alterations in dendritic spine morphology, evidenced by an increased relative frequency of shorter dendritic spines (A) with an increased head diameter (B) and increased neck diameter (C) relative to control animals. Please click here to view a larger version of this figure.
Discussion
In this protocol, we established an EcoHIV-induced HIV infection model in rats. Specifically, we described a bilateral stereotaxic injection of EcoHIV into the cortex which successfully induced active HIV infection in the rat brain 7 days post-injection. Futhermore, we demonstrate that EcoHIV infection in rats could be a good biological system to study key aspects of HAND. Eight weeks post- EcoHIV infection, rats exhibited significant neurocognitive impairments, which included the alterations in temporal processing and synaptic dysfunction in MSNs of the NAc. Given the importance of animal models for the study of neuroHIV and HAND2, the development of a new biological system may be advantageous for addressing novel questions within the field. Potash et al.3 first reported the use of EcoHIV to induce active HIV-1 infection in mice. Specifically, mice were inoculated with a 0.1 mL of solution of EcoHIV virus through tail vein injections3. Six weeks after infection, the HIV-1 viral DNA was detected in spleen cells and lymphocytes. Additionally, one inoculation of EcoHIV injection was sufficient to induce infection in more than 75% of the mice. Utilization of bilateral stereotaxic injections, as in this study, successfully infected 100% of the rats (n = 6 or n = 4, respectively) evidenced by the detection of EcoHIV-EGFP in the brain seven days post-injection.
Previous studies have shown that infection by EcoHIV in the mouse strongly implicated that brain microglia are highly susceptible to EcoHIV virus infection3. In this study, combined with Iba1 (a microglial cell marker) immunostaining, a significant co-localization of EGFP signal with Iba1+ cells was observed, strongly suggesting that microglia were the major cell type for EcoHIV infection in rat brain. Observations of significant EcoHIV infection in microglia are consistent with data in EcoHIV infected mice6, as well as HIV-1 seropositive individuals15 and other biological systems commonly utilized to model HIV16,17. We also performed retro-orbital injection of EcoHIV-EGFP into F344/N rats and the data also indicated high expression of EcoHIV-EGFP in both the cortex and hippocampal dentate gyrus after only seven days (data not shown). In contrast, I.P. injection of EcoHIV in F344/N rats led to undetectable viral expression in the rat brain, inspite of high doses of EcoHIV lentivirus.
Regarding this protocol, researchers should ensure that the conditioned medium, including the EcoHIV lentivirus packaging in 293 FT cells, is concentrated and titered before using for stereotaxic injection. These steps are critical to ensuring consistent and replicable results across experiments. Furthermore, we also found that EcoHIV infection was propagated from stereotaxic injection in brain to spleen tissue at 8 weeks after injection. Meanwhile, the temporal processing deficits were observed in EcoHIV-infected rats as early as 14 days and maintained through 8 weeks post-infection (the current experimental terminal time, Figure 2). Compared to the generalized EcoHIV infection model in mice, the more specific regional stereotaxic injection of EcoHIV produced efficient HIV infection in brain areas and produced a temporal processing deficit in rats, which is key for the study of HIV associated neurocognitive dysfunction.
Limitations providing further opportunities for the current protocol include the identification of other cell types such as neurons and astrocytes, a time-course study of changes in EcoHIV-EGFP expression after infection to indicate viral replication and latency in brain, a longitudinal study to address the potential impacts of HIV associated neurocognitive deficits, and evaluating if the stereotaxic injection of EcoHIV evades the immune system further spreading viral expression throughout the body. Additionally, this should be tested on other rat strains to confirm the generalizability of EcoHIV infection in rats.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was funded by NIH grants HD043680, MH106392, DA013137, and NS100624.
Materials
| 293FT cells | ThermoFisher Scientific | R70007 | |
| Antibiotic-Antimycotic solution | Cellgro | 30004CI | 100X |
| Corning BioCoatGelatin 75cm² Rectangular Canted Neck Cell Culture Flask with Vented Cap | Life Technologies | 354488 | |
| Corning DMEM with L-Glutamine, 4.5 g/L Glucose and Sodium Pyruvate | Life Technologies | 10013CV | |
| Cover glass | VWR | 637-137 | |
| drill | |||
| Dumont #5 Forceps | World Precision Instruments | 14095 | |
| Dumont #7 Forceps | World Precision Instruments | 14097 | |
| Eppendorf Snap-Cap Microcentrifuge Biopur Safe-Lock Tubes | Life Technologies | 22600028 | |
| Ethicon Vicryl Plus Antibacterial, 4-0 Polyglactin 910 Suture, 27in. FS-2 | Med Vet International | VCP422H | |
| Hamilton syringe | Hamilton | 1701 | |
| Invitrogen Lipofectamine 3000 Transfection Reagent | Life Technologies | L3000015 | |
| Iris Forceps | World Precision Instruments | 15914 | |
| Iris Scissors | World Precision Instruments | 500216 | |
| Lentivirus-Associated p24 ELISA Kit | Cell Biolabs, inc. | VPK-107-5 | |
| Lenti-X Concentrator | Takara | PT4421-2 | |
| Opti-MEM I Reduced Serum Medium | Life Technologies | 11058021 | |
| Paraformaldehyde | Sigma-Aldrich | 158127-500G | |
| Paraformaldehyde | Sigma | P6148 | |
| ProLong Gold | Fisher Scientific | P36930 | |
| Sevoflurane | Merritt Veterinary Supply | 347075 | |
| stereotaxic apparatus | Kopf Instruments | Model 900 | |
| SuperFrost Plus Slides | Fisher Scientific | 12-550-154% | |
| Vannas Scissors | World Precision Instruments | 500086 |
References
- Illenberger, J. M., et al. HIV Infection and Neurocognitive Disorders in the Context of Chronic Drug Abuse: Evidence for Divergent Findings Dependent upon Prior Drug History. Journal of Neuroimmune Pharmacology. 15 (4), 715-728 (2020).
- Joseph, S. B., Swanstrom, R. The evolution of HIV-1 entry phenotypes as a guide to changing target cells. Journal of Leukocyte Biology. 103 (3), 421-431 (2018).
- Potash, M. J., et al. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proceedings of the National Academy of Sciences U S A. 102 (10), 3760-3765 (2005).
- Albritton, L. M., Tseng, L., Scadden, D., Cunningham, J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 57, 659-666 (1989).
- Geraghty, P., Hadas, E., Kim, B. H., Dabo, A. J., Volsky, D. J., Foronjy, R. HIV infection model of chronic obstructive pulmonary disease in mice. American Journal of Physiology – Lung Cellular and Molecular Physiology. 312 (4), 500-509 (2017).
- Gu, C. J., et al. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathogens. 14 (6), 1007061 (2018).
- Ellenbroek, B., Youn, J. Rodent models in neuroscience research: is it a rat race. Disease Models, Mechanisms. 9 (10), 1079-1087 (2016).
- Feduccia, A. A., Duvauchelle, C. L. Novel apparatus and method for drug reinforcement. Journal of Visualized Experiments. (42), e1998 (2010).
- Bertrand, S. J., Mactutus, C. F., Harrod, S. B., Moran, L. M., Booze, R. M. HIV-1 proteins dysregulate motivational processes and dopamine circuitry. Scientific Reports. 8 (1), 7869 (2018).
- McGaughy, J., Sarter, M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology. 117 (3), 340-357 (1995).
- Li, H., Aksenova, M., Bertrand, S., Mactutus, C. F., Booze, R. M. Quantification of filamentous actin (F-actin) puncta in rat cortical neurons. Journal of Visualized Experiments. (108), (2016).
- McLaurin, K. A., Li, H., Booze, R. M., Mactutus, C. F. Disruption of Timing: NeuroHIV Progression in the Post-cART Era. Scientific Reports. 9 (1), 827 (2019).
- McLaurin, K. A., Moran, L. M., Li, H., Booze, R. M., Mactutus, C. F. The Power of Interstimulus Interval for the Assessment of Temporal Processing in Rodents. Journal of Visualized Experiments. (146), e58659 (2019).
- Li, H., McLaurin, K. A., Mactutus, C. F., Booze, R. M. Ballistic Labeling of Pyramidal Neurons in Brain Slices and in Primary Cell Culture. Journal of Visualized Experiments. (158), (2020).
- Ko, A., et al. Macrophages but not Astrocytes Harbor HIV DNA in the Brains of HIV-1-Infected Aviremic Individuals on Suppressive Antiretroviral Therapy. Journal of Neuroimmune Pharmacology. 14 (1), 110-119 (2019).
- Sopper, S., et al. The effect of simian immunodeficiency virus infection in vitro and in vivo on the cytokine production of isolated microglia and peripheral macrophages from rhesus monkey. Virology. 220 (2), 320-329 (1996).
- Llewellyn, G. N., Alvarez-Carbonell, D., Chateau, M., Karn, J., Cannon, P. M. HIV-1 infection of microglial cells in a reconstituted humanized mouse model and identification of compounds that selectively reverse HIV latency. Journal of NeuroVirology. 24 (2), 192-203 (2018).

