Application of Lucilia sericata Larvae in Debridement of Pressure Wounds in Outpatient Settings
Summary
Maggots of Lucilia sericata were used in the debridement of deep pressure sores in outpatient settings for the treatment of pressure sores involving the complete skin thickness. The technique presented in the article does not pose risks for the patient.
Abstract
Biological therapy using Lucilia sericata larvae has numerous advocates worldwide, yet it is still fairly unknown and not commonly applied in daily practice because of the limited awareness and insufficient experience of medical and nursing personnel. There are case reports suggesting that maggot therapy can be applied and supported by lay caregivers, provided they are supervised and informed by physicians/nurses. The foregoing observation suggests that the method should be considered for implementation by a wider group of caregivers if accepted and meticulously supervised by trained and experienced medical staff. The concerns related to the therapeutic use of maggots in certain regions seem understandable, but are not supported by scientific facts. It should be noted that many therapeutic agents (including brood) used in medicine are of natural origin, and are associated with low production costs and high possibilities of implementation in the course of therapy. By analyzing the literature and using our own clinical and research experience, we have come to conclusions related to using larvae therapy, as a quick and safe method providing cleaning and revitalization in the process of treating wounds of various etiologies, especially pressure ulcers. In the current study, medical-grade Lucilia sericata maggots were applied to remove necrotic tissue from deep pressure sores. The treatment is mostly accepted by both caregivers and patients. In most cases, it is conducted by trained and experienced medical personnel in home and outpatient settings. Over the course of the conducted analyzes involving the collected specimens, there were no statistically significant relationships (p> 0.05) confirmed between the wound surface successfully cleared by brood and variables, such as time from wound formation, location, surface size, and the depth of damage to the tissue structure. The lack of statistical dependence may result from the small size of the studied group. Based on the current findings, we have formulated the following conclusions: Maggot Debridement Therapy (MDT) is a fast and effective method enabling the preparation of the wound bed. The use of MDT in outpatient settings is safe and acceptable for patients and their caregivers.
Introduction
Aging of society and a longer life expectancy determine a need for professional care for the patient. Chronic diseases may lead to a greater risk of skin damage, mainly associated with defective blood circulation and lymphostasis, often resulting in local infections caused by strains of pathogenic microorganisms1. The traditional process of treating pressure ulcers takes months and comes at a high cost for the patient and the healthcare system2,3. Wound debridement is the first and one of the most important stages of local wound care. A concept of wound bed preparation was presented by Sibbald and Falang5,6, and its effectiveness was confirmed with evidence reported in numerous scientific studies1,2,3. Necrotic tissue can be removed by surgical, autolytic, or biological methods. In hospital conditions, some cases of wounds are not qualified for surgical intervention, for various reasons usually related to the patient's condition (or the lack of consent to such a procedure). Furthermore, it is usually not the wound itself that precludes surgical intervention, but the patient's comorbidities. Not all patients are fit for surgical debridement (e.g., because of underlying health conditions). Alternative forms of debridement are required in such cases1,3,7. Autolytic debridement (with gels or active dressings) is expensive and time-consuming; it also carries a risk of infection due to dissolution (autolysis) of necrotic tissue that may be traced or already infected8. This type of treatment, as a rule, takes several weeks, dramatically increasing the costs related to medical personnel work, as well as medical wound care products.
By implementing the biological method of Maggot Debridement Therapy (MDT), it is possible to reduce the duration of wound cleansing to just a few days. Using MDT is associated with a large potential for the effective cleansing of tissues and for reducing bacterial counts and biofilms (seraticin, chymotrypsin, lucifensin), as well as stimulating the repair processes through the activation of defensins (trypsin- and chymotrypsin-like serine, metalloproteinases, aspartyl proteases) produced by maggots9,10. Over the course of just a few days, maggot therapy rapidly removes dead tissue and controls infection. Large wounds or wounds with lots of necrotic tissue may require repeat treatments, which extends the treatment period to one or two weeks. It is possible to remove dead tissue and bacteria from the wound, thanks to which the duration of this cleansing stage is reduced, allowing for further stimulation and accelerating the growth of granulation tissue in the wound11,12,13. This report presents the detailed description of a methodology and a review of our earlier research findings related to the application of flies in pressure sore treatment.
The protocol for the preparation and debridement of pressure injury UPI/3-4° NPIAP was developed based on a specially designed patient care model, using a model of innovative methods11 recommended by the PTLR (Polish Wound Management Association) in 202014, Qualification for the study was carried out according to the following criteria: over 18 years of age, average high level of accepting the method according to the questionnaire assessment, pressure ulcer stage 3/4 according to NPIAP, pain level not exceeding 4 points according to VAS/NRS, no history of allergies to chitin. The implementation of the protocol is based on the qualification of the patient in the morning for cleaning with MDT. The method acceptance questionnaire, general examination (including pain assessment) and local examination of the wound are the first steps of the activities. This is followed by preparation of the wound for mechanical preparation using tools (removal of scab, skin necrosis), then application of the larvae and inspection within 72-96 hours, evacuation, utilization and further activities related to the evaluation of the wound and the procedure depending on the area of tissue cleaning. Treatment then occurs using active dressings or negative pressure wound therapy (NPWT). Assessment of the wound and the condition of the subjects was conducted by a designated team member with extensive clinical experience in the treatment of chronic wounds.
Protocol
The study was conducted in compliance with the Declaration of Helsinki. The study design was reviewed and approved by the Bioethics Commission at the University of Rzeszów on 30/6/2017.
NOTE: Pain management was also provided in an overall evaluation prior to starting MDT. Patients with signs of hyperalgesia and allodynia did not undergo therapy before achieving pain normalization to the level of 2-3 NRS. Regular painkillers were suggested during the therapy. Under the protocol, the preferred choice is treatment with agents from step 2 of the pain ladder – a combination of tramadol and paracetamol (in case of intolerance, other preparations from this group were recommended). Patients with symptoms of allodynia/hyperalgesia had consultations. In the pain management clinic, coanalgesics – pregabalin or gabapentin – were additionally used for a minimum of one week before using MDT.
1. Assessment of the patient's status and qualification
- Diagnose chronic wound etiology (pressure injury, ulceration of the lower leg) based on evaluation of the patient's condition and physical examination, in accordance with clinical pressure sore classification of the NPIAP, as well as RYB (Red-Yellow-Black wound classification) and WAR (Wound At Risk score). Based on visual inspection and palpation, diagnose UPI that meet the criteria of a full-thickness Stage 3-4 wound (Figure 1).
- Apply the following inclusion criteria: over 18 years of age, voluntary consent, pressure sore with full-thickness skin loss and a surface over 30 cm² with necrotic tissue (yellow or black according to the RYB classification), moderate to high level of acceptance in a questionnaire-based assessment (MDT acceptance questionnaire).
- Apply the following exclusion criteria: age below 18 years, lack of consent for participation in the study, pressure sore without full-thickness skin loss, with no symptoms of necrosis, allergic reaction to chitin, low level of acceptance in a questionnaire-based assessment.
2. Mechanical/surgical procedure
NOTE: In some cases, wound debridement does not require surgical intervention due to the earlier debridement intervention. The presented case of using MDT is special due to the simultaneous presentation of demarked tissues preceding the implementation of MDT. In this case, the wound was prepared for the preparation of mechanically dead tissue (a written consent for the type of therapy).
- To achieve demarcation of the dead tissue, apply dressings with addition of PVP-I (povidone-iodine), cover with foam dressing (e.g., Hydrotac, Allevyn Clasic). Apply a protective remedy to wound edges and prevent pressure sore development (alternating pressure mattress, preferably with tubular structure, change of body position in sequence 2-8 hours).
- Remove demarcated dead tissue from the wound bloodlessly, using sterile surgical tools (tweezers, scalpels, or scissors).
- Apply wound dressing with the addition of an antiseptic gel and with Hydrofiber. Continue prevention of pressure sore development (discontinue antiseptic 12-24 hours before larvae application and dress the wound with a moist dressing with NaCl 0.9% or hydrogel on a hydrofiber dressing).
- Assess acceptance for MDT prior to application using questionnaire. Explain the protocol of the therapy procedure and obtain a written consent for the type of therapy.
3. Applying MDT
- Secure wound edges against secretions released during cleansing. Use various methods of skin protection (e.g., hydrocolloid, stoma paste). 25% zinc ointment was preferred in our protocol.
- Subsequently, apply loose larvae to the wound (5-10 larvae per 1 cm2 of the surface). Secure the wound with non-woven fabric dressing, wet dressings followed by dry dressings. Keep the larvae on the wound for 3-4 days (72-96 hours) (Figure 2).
- After applying the larvae, secure the wound with a non-woven fabric dressing (e.g., Vliwasorb or Matovlies) soaked in 0.9% NaCl. Then dry and secure with a 15 cm wide non-woven fabric patch to protect the entire dressing against the potential larvae migration from the wound.
- Inspect the wound after 22-24 hours. Change the top dressing and inspect the colony. Then rinse/moisturize with 0.9% NaCl.
- Monitor larvae viability and assess the quantity and quality of exudate and skin. Secure the skin with zinc ointment and the wound with non-woven fabric dressing using a patch.
- Inspect the wound after 46-48 hours. Change the top dressing, inspect the colony and the wound-cleansing process. Rinse/moisturize the skin with 0.9% NaCl and secure with zinc ointment. Secure the wound with non-woven fabric dressing with a patch (Figure 3).
- Inspect the wound after 70-72 hours. Change the top dressing, inspect the colony and the wound-cleansing process, rinse/moisturize the skin and secure with zinc ointment. Secure the wound with non-woven fabric dressing.
- Decide whether to remove the larvae or to leave them for the next 24 hours based on the amount of live tissue in the wound, the size of the wound, and the activity of the larvae within the wound (Figure 4).
- Inspect the wound after 94-96 hours.
- Remove the dressing with the larvae (prepared for disposal) and assess the wound debridement (after palpation and visual assessment of the wound). Remove larvae from the wound by rinsing with 0.9% NaCl. Note that mature maggots move out of the wound and to the dressing (non-woven fabric).
- Inactivate the larvae with isopropyl alcohol. Secure the wound with Hydrofiber or foam dressing.
- Consider further treatment by re-application of MDT, application of NPWT or application of active dressings (Figure 5). Calculate the purification index as follows:
Debridement index = 100 – x 100,
where:
x1 – percentage of dead tissue and exudate before treatment,
x2 – percentage of dead tissue and exudate after treatment.
The acquired results classified in separate percent ranges, as follows:
0 – no dead tissues removed (no therapeutic effect),
10-30% – poor wound debridement (unsatisfactory therapeutic effect),
40-80% – moderate wound debridement (good therapeutic effect),
90-100 % – complete wound debridement (very good therapeutic effect).
4. Statistics
- Perform statistical analyses in Statistica 13.1.
- Apply non-parametric tests due to a failure to meet the assumptions of parametric tests (i.e., agreement of distributions with normal distribution) as verified with the Shapiro-Wilk W-test.
- Assess associations between level of wound debridement and selected quantitative variables using Spearman's rank correlation test.
- Assess differences in the degree of wound debridement in patients with or without selected symptoms, and with or without wounds in a specific location, with a two-tailed test for significance of differences between two means.
- Assume statistical significance at p < 0.05.
Representative Results
Selection of the study group
The trial, which was prospective and based on a series of cases, used short-term, standardized observations and estimates. From a group of 67 patients treated for chronic wounds, 30 patients were selected for MDT (loose larvae) application. In the latter group, 20 cases of pressure ulcers were confirmed; however, two cases were omitted because the patients did not accept the cleansing protocol. Ultimately, 18 patients receiving 72 to 96 hours of treatment with this method were included in the statistical analyzes. The remaining patients who did not qualify for the main group were treated according to a standard protocol with the use of active dressings.
Study group characteristics
The patients' mean age was 76.72 years ± 12.56 years. The study group comprised 12 females (66.7%) and 6 males (33.3%). There were 5 (27.8%) residents of urban areas and 13 (72.2%) residents of rural areas. The average patients' performance according to the Barthel scale amounted to 12.78 ± 14.58. The mean duration from the wound onset was 2.69 ± 1.65 months. On average, the wound was 54.28 ± 31.25 cm2 in size. The most common wounds were heel and sacral bone pressure ulcers (38.9% each). Most frequently, according to the RYB scale, the wounds were yellow (72.2%) and they were more often classified as Stage 3 (61.1%) than Stage 4 (38.9%), based on the NPIAP score. Pain was reported by the majority of the patients. Measurements taken at four time points, on the day of the therapy and on Day I, Day II, and Day III, showed that on average, pain in the wound area in the study group did not exceed a score of 2 according to the VAS. No concerning symptoms were observed in 27.8% of the patients. The most common problems included large amounts of exudate (50.0%), foul smell (33.3%), and fever (22.2%). Wound edges were most frequently demarked (covered with granulation) – 55.6% or irregular (with signs of undermined and devitalized tissues) (38.9%). The wound debridement level was rated moderate (62.78 ± 15.26%; Table 1).
No statistically significant relationships were found between the level of wound debridement and variables, such as time from wound onset and pain on the consecutive days of the therapy (Table 2).
Similarly, there were no differences in the size of the wound effectively debrided in relation to its location or the presence of alarming symptoms (Table 3).
Despite the lack of statistically significant differences among the results obtained in the three groups, the median values for the locations in the sacral region and the trochanteric region were notably higher compared to those for the heel area, which may suggest greater effectiveness of the treatment in the former body regions. The effectiveness of the treatment in the trochanteric region was most uniform, whereas the largest difference between the minimum and the maximum values was observed for the sacral region (Figure 6).
In the scatter plot reflecting the wound area effectively debrided relative to the depth of tissue destruction, the regression line indicates a negative direction, suggesting poorer effects of wound debridement in the case of deep damage of the skin and the subcutaneous tissue. No statistically significant differences were observed (p > 0.05) (Figure 7).
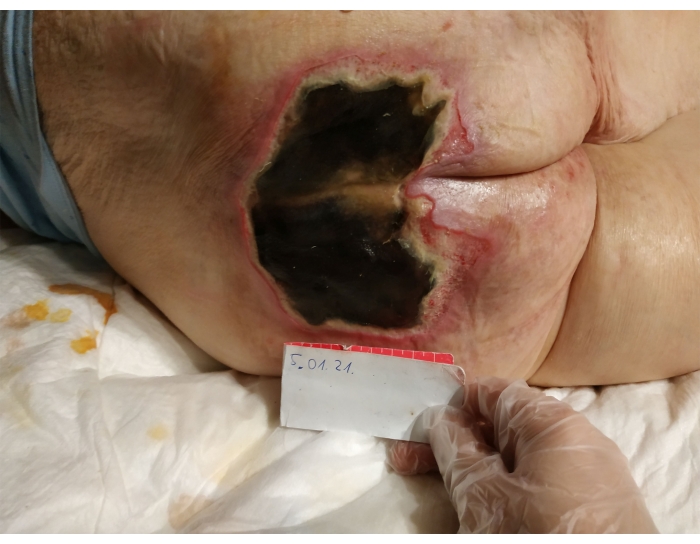
Figure 1. Unstageable Pressure Injury (UPI) before surgical debridement and MDT therapy. Please click here to view a larger version of this figure.

Figure 2. 100 free-range Lucilia sericata larvae from Biolab culture. Please click here to view a larger version of this figure.
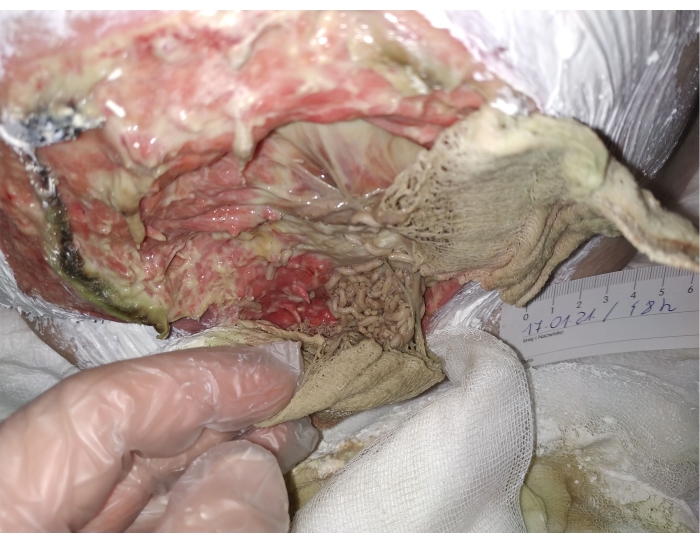
Figure 3. 48 hours after larvae application, abundant exudate, mobility and larvae size suggest a healthy and active colony. Please click here to view a larger version of this figure.
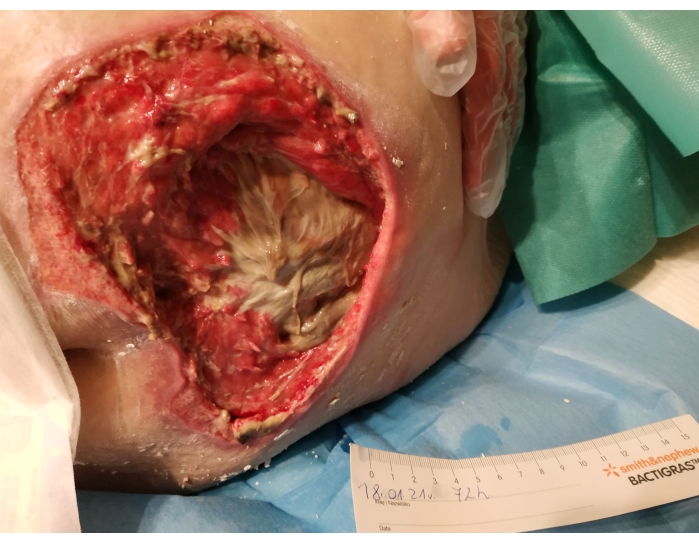
Figure 4. Condition after removing the larvae from the wound (over 72 hours), 70% of the wound was cleaned. Please click here to view a larger version of this figure.
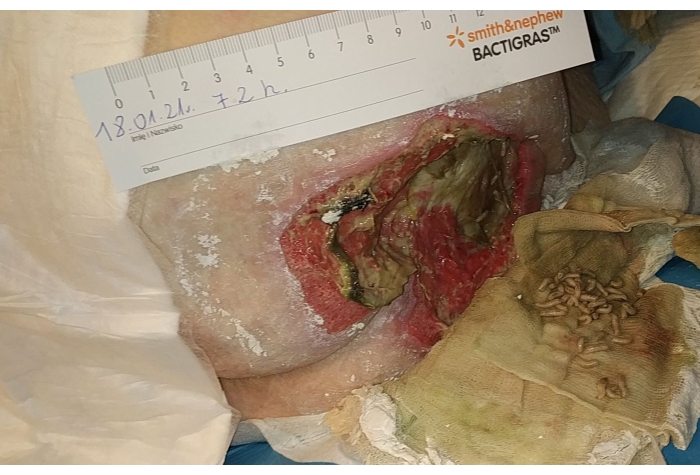
Figure 5. Lucilia larvae removed from the wound on the third day. Please click here to view a larger version of this figure.
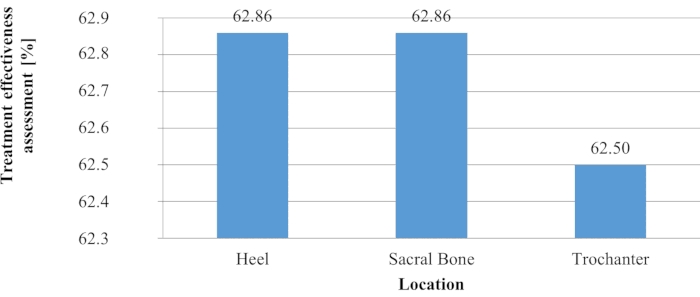
Figure 6. Effectiveness of treatment versus wound location. Please click here to view a larger version of this figure.
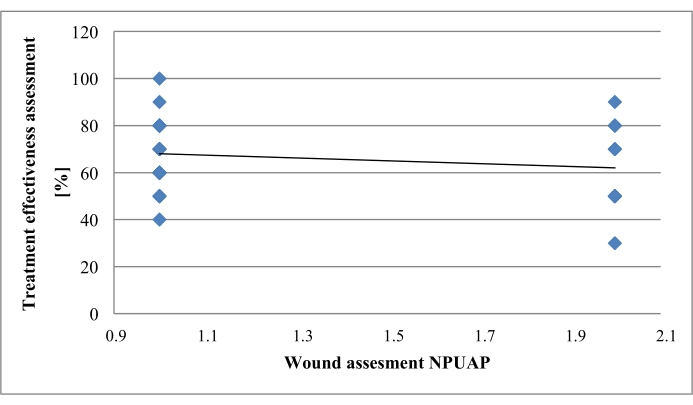
Figure 7. Debrided wound surface versus depth of tissue destruction. Please click here to view a larger version of this figure.
Table 1. Study group characteristics in the context of wound location and size. Please click here to download this Table.
Table 2. Assessment of the relationship between debridement level and selected quantitative variables. Please click here to download this Table.
Table 3. Assessment of the relationship between debridement level and selected qualitative variables. Please click here to download this Table.
Discussion
Wound care treatment in patients with chronic conditions, provided in ambulatory settings and applying novel, as well as natural methods, is frequently discussed in the related literature11,15,16. The increasing availability of advanced technologies and medical products is a determinant for an increasingly more effective application of wide-ranging options in daily clinical practice for specialists in various areas. In modern medicine, there are no doubts whether to treat wounds in outpatient and home settings; instead, discussions focus on methods that may effectively be used to reduce tissue destruction quickly and safely for the patient, in order to enable improvement in his/her functional and health status. The concerns related to the therapeutic use of maggots seem understandable and are mainly related to the concerns arising from the visual aspects and the fear of potential pain. However, it should be remembered that many remedies used in medicine are of natural origin with scientifically proven benefits and medical properties.
Biological therapy methods using Lucilia sericata larvae have many advocates worldwide, yet it is still fairly unknown and not commonly applied in daily practice because of the limited awareness and insufficient experience of medical and nursing personnel11,13,17,18. Mirabzadeh et al. point out that the application of the larvae can be carried out by the family or caregivers, yet it must be carried out under strict medical supervision19. The current study was designed to investigate the effectiveness of pressure sore debridement in patients receiving treatment in home setting (long-term and palliative care). The larvae application model recommended by PTLR experts14 was used, with loose larvae that could freely penetrate and clean the penetrated necrotic tissue, with an average conversion rate of 5-10 larvae per cm². The use of larvae in the biobag was abandoned due to the depth and penetration of the subcutaneous necrosis of the qualified subjects (3/4 NPIAP) and the potentially weaker effect compared to loose larvae in terms of the market price – greater losses compared to the gains for the patient)11,14,17.
The results obtained in a group of 18 patients show that in the course of a 3-day therapy, necrotic tissue was removed, at a rate of 67% on average, in full-thickness wounds (NPIAP Stage 3) and in wounds penetrating the bone (NPIAP Stage 4). The analyses did not confirm statistically significant relations (p > 0.05) between the area of the wound debrided by maggots and variables such as the period from wound onset, location, surface size, and the depth of the tissue structure damage. It has been observed that deep wounds and wounds with complex surface structures have a larger surface area due to their topography. This means that a dose of 5-10 worms per cm2 of visible wound size may not be sufficient for a quick one-time clean-up. This may explain the negative correlation between wound depth and debridement efficiency. The lack of such statistical relationships may result from the small size of the study group.
In a study by Polat et al., involving a group of 36 patients with deep pressure sores, maggots were placed in the wound for 72 hours and then washed away. The procedure was repeated twice a week, and effective wound cleansing was achieved in the majority of the cases (78.9%) with four to six treatment sessions and in seven patients (21.1%) after eight to twelve sessions. In our study, the debridement was faster, and re-debridement was performed in 33% of the subjects. In addition, the cleaning time was not more than 10 days and was related only to the agent being used. The larvae can be ordered once a week.
According to the authors, MDT is a fast option that can effectively be applied to chronic pressure injuries unresponsive to conventional treatments and other therapeutic methods20.
In our study, based on the adopted protocol, the evaluation was performed every 24 hours in order to evaluate larvae viability, the wound cleansing process as well as the replacement of the exuded non-woven fabric. Re-securing the wound was to ensure safety and reduce the risk of skin damage by discharge and larvae migration, which is rare. Larvae migration from the wound occurs mainly due to factors such as: reaching maturity (usually after 3-4 days) and the absence of necrotic tissue in the wound, opening of the purulent reservoir or with exuding fluid present during this treatment method12,14. Despite the fact that there are different methods of skin protection and wound protection against larvae migration used worldwide, there is no single confirmed optimal method. In our study, the basic protection associated with using non-woven fabrics and zinc ointment was implemented, the effectiveness of which has been confirmed on a sample of several hundred people over the last few years. During the observation and treatment of wounds, no adverse effects were noted in the examined patients related to larvae leaving the wound and damaging the skin, although a certain group of respondents (25%) had such concerns.
Sherman investigated the effectiveness of conventional treatments (frequent change of wound dressing, local application of antiseptics or antibiotics, hydrogel or hydrocolloid dressing, surgical wound debridement) in comparison to maggot therapy in patients with a diabetic foot. The author reported that after 5 weeks, wounds subjected to conventional treatments were still covered with necrotic tissue on the surface constituting 33% of the area, whereas all maggot-treated wounds were completely cleansed after 4 weeks (p = 0.001)21.
A study conducted by Steenvoorde et al. in a group of 101 patients demonstrated poorer effectiveness of MDT in individuals with advanced ischemia22. These observations may be linked to hyperalgesia, commonly occurring in patients with atherosclerosis in the lower extremities, and to increased sensation of pain induced by a foreign body present within the wound. In the current study, treatment with pregabalin or gabapentin was introduced not later than 1 week before applying larvae in patients with symptoms of hyperalgesia. On closer examination of the current findings, we also made some interesting observations regarding the so-called "concerning symptoms" during the therapy.
It appears that a larger amount of exudate (or the specific odor referred to as "foul smell") produced by the wound during the therapy corresponds to a more effective cleansing of the wound. Notably, autolytic properties of the larvae are related to the production of proteins and extracorporeal digestion, which explains why large amounts of fluids are discharged by the wound. The foregoing observation requires further investigation in a larger group of patients. The current findings show a low degree of pain experienced by the patients; however, researchers point to mental aspects and sensory perceptions related to the wound, which may increase the experience of pain, specifically in patients with ischemia and symptoms of hyperalgesia. Furthermore, researchers also point to visual and mental aspects, which may be observed among women23. Two studies have suggested that the approval level could be higher if healthcare professionals did not reject the method and did not discourage patients from using it24,25.
The acceptance for the application of maggots in medicine and in health sciences is gradually increasing, which is particularly visible during the current pandemic. The reasons for the acceptance of this method by patients are associated with the long duration of treatments based on other methods, the chronic nature of the wound, as well as poor experiences related to other cleansing methods, ultimately negatively affecting the quality of the patients' life.
Summarizing the described procedures of application and treatment with MDT, we stress that the use of medical maggots in wound debridement in home care settings is safe, inexpensive and effective. Nevertheless, it should be conducted by trained and experienced medical personnel (nurse or doctor). After assessing the patient's condition and the patient's tolerance of the MDT, the wound should be mechanically prepared using selected technique (recommended use of basic surgical instruments)5,6,8,12,14. The application of maggots to dry black necrosis is ineffective and not recommended11,12,14. During ongoing therapy, skin protection and patient surveillance is a key component. We recommend simple methods of skin protection, yet others such as stoma paste and hydrocolloids can be used alternatively9,11,15. We do not recommend the standard use of maggots in a biobag for deep and penetrating pressure ulcer wounds due to low efficacy12. The limitations of the method are very narrow and mainly relate to documented allergic reactions to chitin, neoplastic tissue destruction in the head and neck area (debridement in the hospital setting under supervision due to the risk of hemorrhage), increased pain sensation with ineffective treatment, low level of tolerance in questionnaire assessment12,14,20,21. The use of MDT with subsequent implementation of NPWT reduces the wound healing time and improves the patients' quality of life. Since the relevant wound debridement method is not commonly used, and there are no well-defined criteria for its application and duration, the current study presents results of a small-size group, which may be reflected in the lack of statistical significance of the findings reported in the Results section. Having considered the foregoing, further research focusing on the method described herein will enable more detailed analyses of the presented variables.
In summary, wound debridement using Lucilia sericata larvae is a fast and effective method enabling the preparation of the wound bed. The use of MDT in home and outpatient settings is safe and acceptable for patients and their caregivers.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The study was conducted as a project of the Natural and Medical Centre for Innovative Research at the University of Rzeszow and co-financed by the Regional Operational Program for the Podkarpackie Province for the years 2007-2013, contract number UDA-RPPK.01.03.00-18-004/12-00.
Materials
| Allevyn Non Adhesive | Smith&Nephew | 66927637 | Foam dressing- to apply wound dressing https://www.smith-nephew.com/professional/products/advanced-wound-management/allevyn/allevyn-non-adhesive1/ |
| Aquacel extra | Convatec | 420671 | Hydrofiber -to apply wound dressing https://www.convatec.com/products/pc-wound-skin-tear/aquacel-extra-hydrofiber-dressing# |
| Braunol | B.Brown | 15171 | Povidone-iodine PVP-I- To achieve demarcation of the dead tissue, apply dressings with addition of PVP–I https://www.bbraun-asiapacific.com/en/products/b2/braunol.html |
| Brava* | Coloplast | 120500 | stoma pasta -to protect the entire dressing against potential larvae migration from the wound https://products.coloplast.us/coloplast/ostomy-care/brava/brava-paste/brava-paste/ |
| Doreta | Krk d.d. | painkiller- combination of tramadol and paracetamol | |
| Durafiber | Smith&Nephew | 6680030 | Hydrofiber – to apply wound dressing https://www.smith-nephew.com/professional/products/advanced-wound-management/durafiber/ |
| Hydrocoll* | Hartmann | 9007482 | Hydrocolloid – to protect the entire dressing against potential larvae migration from the wound https://www.hartmann.info/en-gb/our-products/wound-management/advanced-wound-care/hydrocolloids/bevelled-edges/hydrocoll%C2%AE#products |
| Hydrotac | Hartmann | 6858320 | Foam dressing- to apply wound dressing https://www.hartmann.info/en-gb/our-products/wound-management/advanced-wound-care/foam-dressings/hydrotac%C2%AE#products |
| Medical larvae Lucilia Sericata | Biollab | Larvae produced by Biollab®, Poland, loose in an ampoule 50-100 pcs | |
| Omnifix | Hartmann | 9006031 | Non -woven fabric plaster – to protect the entire dressing https://www.hartmann.info/en-gb/our-products/wound-management/adhesive-fixation/adhesive-tape/omnifix%C2%AE-elastic#products |
| Scalpels | Integros | B1583 | to remove bloodlessly demarcated dead tissue |
| Scissors | Hartmann | 9910813 | to remove bloodlessly demarcated dead tissue |
| Sutrisept | ACTO GnbH | 34297 | Antiseptic Gel – to apply wound dressing |
| Tweezers | Hartmann | 9910604 | to remove bloodlessly demarcated dead tissue |
| Vliwasoft | Lohmann& Rauscher International | 12064 | Non- woven fabric dressing – to secure the wound https://www.lohmann-rauscher.com/en/products/wound-care/dressings-swabs-and-packing-rope/vliwasoft/ |
| Zinc ointment 25% | Avena | 2405 | to protect the entire dressing against potential larvae migration from the wound |
| 0.9% NaCl | Fresenius Kabi | Natrii Chloridum – to moisten the dressing | |
| *Can be used, but not in this case |
References
- Kottner, J., et al. Prevention and Treatment of Pressure Ulcers/ Injuries Clinical practice Guideline. The International Guideline 2019; European Pressure Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. , (2019).
- Coleman, S., et al. Patient risk factors for pressure ulcer development: Systematic review. International Journal of Nursing Studies. 50 (7), 974-1003 (2013).
- Sørensen, J. L., Jørgensen, B., Gottrup, F. Surgical treatment of pressure ulcers. American Journal of Surgery. 188, 42-51 (2004).
- Artico, M., et al. Prevalence, incidence and associated factors of pressure ulcers in home palliative care patients: A retrospective chart review. Palliative Medicine. 32 (1), 299-307 (2018).
- Falanga, V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regeneration. 8 (5), 347-352 (2000).
- Sibbald, R. G., et al. Preparing the wound bed – debridement, bacterial balance, and moisture balance. Ostomy Wound Management. 46 (11), 14-35 (2000).
- Atkin, L., et al. Implementing TIMERS: the race against hard-to-heal wounds. Journal of Wound Care. 28, 1-49 (2019).
- McCallon, S. K., et al. Optimizing Wound Bed Preparation With Collagenase Enzymatic Debridement. The Journal of the American College of Clinical Wound Specialists. 6 (1-2), 14-23 (2015).
- Brown, A., et al. Blow fly Lucilia sericata nucle-ase digests DNA associated with wound slough/eschar and with Pseudomonas ae-ruginosa biofilm. Medical and Veterinary Entomology. 26 (4), 432-439 (2012).
- Horobin, A. J., Shakesheff, K. M., Pritchard, D. I. Maggots and wound healing: An investigation of the effects of secretions from Lucilia sericata larvae upon the migration of human dermal fibroblasts over a fibronectin-coated surface. Wound Repair and Regeneration. 13 (4), 422-433 (2005).
- Bazaliński, D., Kózka, M., Karnas, M., Więch, P. Effectiveness of Chronic Wound Debridement with the Use of Larvae of Lucilia Sericata. Journal of Clinical Medicine. 8, 1845 (2019).
- Sun, X., et al. A systematic review of maggot debridement therapy for chronically infected wounds and ulcers. International Journal of Infectious Diseases. 25, 32-37 (2014).
- Sherman, R. A. Mechanisms of maggot-induced wound healing: what do we know, and where do we go from here. Evidence Based Complement Alternative Medicine. , (2014).
- Szewczyk, M. T., et al. Treatment of pressure ulcers – recommendations of the Polish Wound Management Association. Part II. Leczenie Ran. 17 (4), 151-184 (2020).
- Gottrup, F., Jørgensen, B. Maggot debridement: an alternative method for debridement. Eplasty. 11 (33), 290-302 (2011).
- Nasoori, A., Hoomand, R. Maggot Debridement Therapy for an electrical burn injury with instructions for the use of Lucilia Sericata larvae. Journal Wound Care. 26 (12), 734-741 (2017).
- Yan, L., et al. Pharmaco-logical Properties of the Medical Maggot. Evidence-Based Complementary and Alternative Medicine. , (2018).
- Bazaliński, D., Karnas, M., Wołkowicz, M., Kózka, M., Więch, P. The use of Lucilia sericata larvae in the treatment of chronic wounds-A study of three cases. Leczenie Ran. 15, 105-111 (2018).
- Mirabzadeh, A., Ladani, M. J., Imani, B., Rosen, S. A., Sherman, R. A. Maggot therapy for wound care in Iran: a case series of the first 28 patients. Journal of Wound Care. 26 (3), 137-143 (2017).
- Polat, E., Kutlubay, Z., Sirekbasan, S., Gökalp, H., Akarırmak, &. #. 2. 2. 0. ;. Treatment of pressure ulcers with larvae of Lucilia sericata. Turkish Journal of Physical Medicine and Rehabilitation. 63 (4), 307-312 (2017).
- Sherman, R. A. Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care. 26 (2), 446-451 (2003).
- Steenvoorde, P., Jacobi, C. E., Van Doorn, L., Oskam, J. Maggot debridement therapy of infected ulcers: patient and wound factors influencing outcomes: a study on 101 patients with 117 wounds. Annals of the Royal College of Surgons of England. 89 (6), 596-602 (2007).
- Spilsbury, K., et al. Exploring patient perceptions of larval therapy as a potential treatment for venous leg ulceration. Health Expectations. 11 (2), 148-159 (2008).
- Sherman, R. A. Maggot versus conservative debridement therapy for the treatment of pressure ulcers. Wound Repair and Regeneration. 10 (4), 208-214 (2002).
- Turkmen, A., Graham, K., McGrouther, D. Therapeutic applications of the larvae for wound debridement. Journal of Plastic Reconstructive and Aesthetic Surgery. 63 (1), 184-188 (2010).

