Preparation of High-Temperature Sample Grids for Cryo-EM
Summary
This paper provides a detailed protocol for preparing sample grids at temperatures as high as 70 °C, prior to plunge freezing for cryo-EM experiments.
Abstract
The sample grids for cryo-electron microscopy (cryo-EM) experiments are usually prepared at a temperature optimal for the storage of biological samples, mostly at 4 °C and occasionally at room temperature. Recently, we discovered that the protein structure solved at low temperature may not be functionally relevant, particularly for proteins from thermophilic archaea. A procedure was developed to prepare protein samples at higher temperatures (up to 70 °C) for cryo-EM analysis. We showed that the structures from samples prepared at higher temperatures are functionally relevant and temperature dependent. Here we describe a detailed protocol for preparing sample grids at high temperature, using 55 °C as an example. The experiment made use of a vitrification apparatus modified using an additional centrifuge tube, and samples were incubated at 55 °C. The detailed procedures were fine-tuned to minimize vapor condensation and obtain a thin layer of ice on the grid. Examples of successful and unsuccessful experiments are provided.
Introduction
The cryo-EM technology for solving the structures of protein complexes has continued to improve, particularly in the direction of obtaining high-resolution structures1,2. In the meantime, the landscape of its application has also been expanded by varying the sample conditions such as pH or ligands prior to the vitrification process3, which involves the preparation of sample grids followed by plunge freezing4,5. Another important condition is the temperature. Although cryo-EM experiments, like X-ray crystallography, are performed at low temperatures, the structure solved by cryo-EM reflects the structure at the solution state prior to vitrification. Until recently, the majority of single particle analysis (SPA) cryo-EM studies use samples that are kept on ice (i.e., at 4 °C) prior to vitrification6, though a number of studies use samples at around room temperature7,8,9,10 or as high as 42 °C11. In a recent report, we performed temperature-dependent studies of the enzyme ketol-acid reductoisomerase (KARI) from the thermophilic archaeon Sulfolobus solfataricus (Sso) at six different temperatures from 4 °C to 70°C12. Our studies suggest that it is important to prepare sample grids at functionally relevant temperatures and that cryo-EM is the only structural method that is practically feasible for solving the structure of the same protein complex at multiple temperatures.
The major difficulty for vitrification at high temperatures is to minimize vapor condensation and achieve thin ice. Here we report the detailed protocol used for preparing sample grids at high temperatures in our previous study of the Sso-KARI12. We assume that the readers or viewers are already experienced in the overall sample preparation and data processing procedures for cryo-EM experiments and emphasize the aspects relevant to high temperature.
Protocol
NOTE: This protocol aims to use a modified commercial vitrification apparatus to prepare the cryo-electron microscopy (cryo-EM) samples at specific temperatures, especially higher than 37 °C. The overall experimental setup is shown in Figure 1. The protocol uses 55 °C as an example. For the specific conditions at other temperatures, please refer to Supplementary Table 2 in reference12.
1. Preparation of the vitrification apparatus
- Make a 1 cm hole in a 50 mL centrifuge tube on its closed end.
- Place the tube in the vitrification apparatus chamber at the ultrasonic water outlet, as shown in Figure 2.
NOTE: The purpose is to minimize water condensation by guiding water vapor to the heat exchanger through the tube before reaching the entire chamber. - Set up the vitrification apparatus temperature to the specified temperature (e.g., 55 °C, as shown in Figure 3), and allow the vitrification apparatus chamber to reach to 55 °C and 100% relative humidity. Let it stand for at least half an hour to stabilize the conditions before starting the experiment.
2. Warming up the sample and the tools
- Place the water bath on a hotplate and set the hotplate to the desired temperature (here 55 °C). Check with a thermometer to ensure that the water reaches 55 °C.
- Incubate the sample in the water bath and preheat the pipette tip on the edge of the hotplate for 2 min or longer before the blotting experiment.
NOTE: The highest temperature setting for the vitrification apparatus chamber is 60 °C. To prepare higher temperature grids for cyro-EM experiment (e.g., 70 °C), the sample is incubated in the water bath at 80 °C, and the average between the sample temperature and the vitrification apparatus temperature is estimated to be the actual temperature of the sample on the grid (70 °C in this case). See the Discussion section for further details and limitations on this estimation.
3. Preparation for the blotting experiment
- Glow discharge a holey carbon-supported grid at 25 mA for 30 s, or alternative to the values depending on the device used.
- Incubate the tweezers with the grid in the vitrification apparatus for 2 min or longer.
- Fill the ethane container with ethane according to standard procedures. Do not let the ethane overflow.
NOTE: This step takes about 10 min and should be followed immediately with the blotting experiment to avoid freezing. - Place the vitrification filter paper into the vitrification apparatus chamber no sooner than 5 min before the blotting experiment.
NOTE: Placing the filter paper in the chamber too soon will cause it to become too wet.
4. Blotting experiment
NOTE: When holding the grid, ensure that the grid is stable and there is a minimal contact area with the tweezers (Figure 4). This is done to maintain the best cooling efficiency of ethane and to avoid non-vitreous ice.
- Use a pipette tip to apply 7-9 μL of the sample to the grid. Then wait for 1-2 s, blot for 1-1.5 s, and quickly plunge the sample to liquid ethane.
- Transfer the grid from liquid ethane to the cryo box, which is stored in liquid nitrogen.
NOTE: This step must be performed very carefully because the tweezers is still hot, so the entire cooling tank is full of vapor at this time.
5. Quality check for the grids
- Clip the grids and upload them to a cryo-EM instrument.
- Use the display screen of the cryo-EM instrument and the low dose function of the software to screen the ice condition on the grid and the distribution of the sample on the grid.
NOTE: Often, the grids are very dry, or the ice layer is too thick. The success rate of the grids prepared at high temperatures is substantially lower than that at room temperature or 4 °C. Representative results of good grids and non-satisfactory grids are shown in the next section. - If the quality of the resulting grid is not good, repeat the process of grid preparation with varied conditions (such as waiting time, blotting time, etc.). If the quality of the resulting grid is good, repeat the same steps to make grids at a different temperature.
6. Data collection
- Transfer the good quality grids to a high resolution cryo-EM instrument.
- Perform data collection and data analyses according to established procedures.
NOTE: As shown in our previous publication, the resolution of the structure is not affected by the high temperature12.
Representative Results
The low magnification overview is shown in Figure 5A,B. Panel A is an example of a successful grid. There is an ice gradient from top left (thicker) to bottom right (thinner or empty). Such a grid makes it easier to find an appropriate thickness of the ice layer in the middle area suitable for data collection, such as the blue and green boxes. The grid B is too dry. The squares in the grid have bright contrast, which means that the ice layer is too thin or there is no ice layer at all. Only the two squares indicated by the red arrows are suitable for data collection.
Furthermore, examples of the low-dose images from different grids are shown in Figure 5C,D. The image in panel C shows that most of the ice is in the crystalline form, not suitable for data collection. On the other hand, image in panel D shows that the ice layer is mostly in an amorphous state, suitable for data collection.
Please note that this is a short paper focusing on grid preparation at high temperatures. The grid only contains the sample for data collection. A good grid has a good chance, though not a definite chance, to generate good data for solving a high-resolution structure. The real cryo-EM data and final structures for the examples described in this paper are already described in the published paper12. In short, we have obtained grids good enough for data collection, solved the structures of two Sso-KARI complexes at six different temperatures each, and compared the structures from different temperatures for each complex, as well as the structures between the two complexes from the same temperature. The results indicate that the structure of each complex is temperature-dependent and that the temperature-dependent changes are different between the two complexes. Importantly, the successive structural changes correlate well with the successive temperature changes, which is a strong indication for the success of the temperature-dependent sample grid preparation.

Figure 1: The overall experimental setup for high-temperature cryo-EM sample preparation. The items shown include vitrification apparatus, incubator, timer, pipette tip placement, cooling tank, and tweezers. Please click here to view a larger version of this figure.
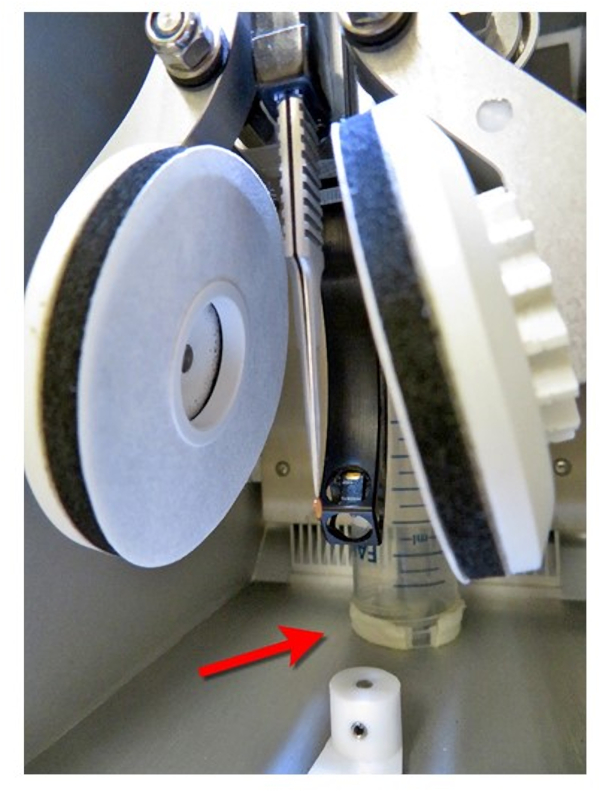
Figure 2: Modification of the vitrification apparatus chamber. A 50 mL tube is installed at the ultrasonic spray outlet as indicated by the red arrow12. Please click here to view a larger version of this figure.

Figure 3: Appearance of the vitrification apparatus during the experiment. The screen shows the temperature at 55 °C and the humidity at 100%. Please click here to view a larger version of this figure.
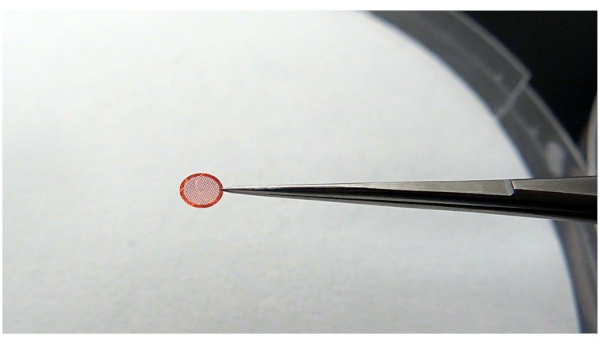
Figure 4: Using tweezers to grab the grid. It is recommended that the tweezers grip the grid with as little contact as possible, but it must be able to hold the grid stably during the process of the operation. Please click here to view a larger version of this figure.
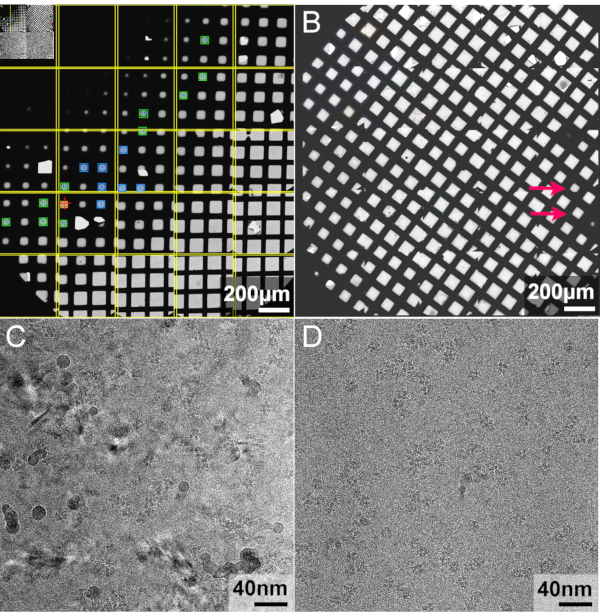
Figure 5: Representative results: Grid checking by Cryo-EM. (A,B) show the overall state of the grid. (C,D) show examples of the low-dose images from different grids. Please click here to view a larger version of this figure.
Discussion
In step 1 of the protocol, make sure that the centrifuge tube has been installed well and does not fall when the experiment is in progress. Due to the accumulation of a large number of water droplets in the chamber, which could change the adsorption capacity of the filter paper, it is recommended that the overall time of the experiment should not exceed 30 min after the vitrification apparatus chamber reached the equilibration temperature. If the operation time exceeds 30 min, the operator needs to replace the filter paper and wait for the cabin to balance the temperature and humidity again. At step 8 of the protocol, the suggested sample volume of 7-9 µL is larger than usual since otherwise, the sample evaporates quickly at high temperature, leading to empty squares on the grid. On the other hand, it is highly recommended that the sample applied does not exceed 9 µL. Otherwise, it is very likely that the sample will drip down during the process of moving the tweezer before blotting. Overall, a key to the success of this technique is stable and fast gripping of grids and correct and stable execution of each time-limited action. Furthermore, it is recommended that each round of experiments deals with only one specific high temperature. Before proceeding to perform the experiment at another temperature, all systems must be fully recovered and reset.
Due to the high temperature and high humidity of the chamber, the window is often covered with fog leading to difficulties in launching the experiment. Use of a little soapsuds is recommended to clear the window. If grids are not good, possible reasons are that the steps described above are not followed precisely and/or that the steps take too long. Try to repeat the preparation of sample grids with precision and swiftness. If the grids are still not good after repeats, then try to adjust the conditions. The more frequent problem observed in this experiment is no ice on the grid at the high temperature. If so, try to reduce the blotting time further. On the other hand, if the ice is too thick, try to increase the blotting time.
A limitation of the high-temperature cryo-EM is that the maximum heating temperature on the vitrification apparatus is 60 °C. To reach to the higher temperature, the sample was heated above 60 °C (e.g., 80 °C), and the average between the sample temperature and the vitrification temperature was estimated to be the real temperature of the sample on the grid (70 °C, in this case). There could be some inaccuracy based on this estimation. A possible future solution is to build a thermocouple to measure the grid temperature accurately right before plunge-freezing. Another potential limitation is the stability of the protein at high temperatures. A separate experiment using circular dichroism should be performed to ensure that the protein is stable at the temperature for the planned cryo-EM experiment.
Another limitation is that only two types of commercially available vitrification apparatus can be heated to above 37 °C and up to 60 °C as mentioned above (e.g., Thermo Fischer Vitrobot and Leica EM-GP). The vitrification apparatuses from other vendors are either limited to room temperature or adjustable only between 4 °C and 37 °C. However, it is possible for research groups to build their own plunging devices with extended temperature ranges in the future.
Our protocol is modified from existing protocols4,5,6, with the purpose of preparing the grids at temperatures higher than room temperature. Without making the modifications described here, the chance of success for making good high-temperature sample grids suitable for cryo-EM image collection is very small.
Two papers in 2019 have demonstrated that protein structures are temperature dependent, in correlation with the temperature dependence of protein functions, in the range of 4 °C to 42 °C for the TRP channel TRPV311 and 4 °C to 70 °C for Sso-KARI12. These reports are likely to encourage a change in cryo-EM research in that more future studies will be performed at functionally relevant temperatures, usually at 37 °C. The stability of the purified protein at this temperature could be a concern. However, it is required to incubate the protein sample at this temperature for only 2 min according to our protocol. Alternatively, physiological conditions can be achieved by imaging proteins in cells using tomography and sub-tomo averaging. Furthermore, cryo-EM can be used to study the mechanism and intermediates of protein unfolding at high temperatures, likely in the range of 40 °C to 80 °C. These studies will all benefit from the protocol described here.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors thank Dr. Hervé Remigy of Thermo Fisher Scientific for useful advice. The cryo-EM experiments were performed at the Academia Sinica Cryo-EM Facility (ASCEM). ASCEM is supported by Academia Sinica (Grant No. AS-CFII-108-110) and Taiwan Protein Project (Grant No. AS-KPQ-109-TPP2). The authors also thank Ms. Hui-Ju Huang for the assistance with the sample preparation.
Materials
| Falcon tube | Falcon | 352070 | size: 50 mL |
| Filter paper | Ted Pella | 47000-100 | Ø55/20mm, Grade 595 |
| HI1210 | Leica | water bath | |
| K100X | Electron Microscopy Sciences | glow discharge | |
| Quantifoil, 1.2/1.3 200Mesh Cu grid | Ted Pella | 658-200-CU-100 | |
| Titan Krios G3 | Thermo Fisher Scientific | 1063996 | low dose imaging |
| Vitrobot Mark IV | Thermo Fisher Scientific | 1086439 | |
| Vitrobot Tweezer | Ted Pella | 47000-500 |
References
- Yip, K. M., Fischer, N., Paknia, E., Chari, A., Stark, H. Atomic-resolution protein structure determination by cryo-EM. Nature. 587, 157-161 (2020).
- Nakane, T., et al. Single-particle cryo-EM at atomic resolution. Nature. 587, 152-156 (2020).
- Chen, C. Y., et al. Use of Cryo-EM to uncover structural bases of pH effect and cofactor bi-specificity of ketol-acid reductoisomerase. Journal of the American Chemical Society. 141, 6136-6140 (2019).
- Cabra, V., Samsó, M. Do’s and don’ts of cryo-electron microscopy: A primer on sample preparation and high quality data collection for macromolecular 3D reconstruction. Journal of Visualized Experiments. (95), e52311 (2015).
- Klebl, D. P., et al. Need for speed: Examining protein behavior during CryoEM grid preparation at different timescales. Structure. 28 (11), 1238-1248 (2020).
- Passmore, L. A., Russo, C. Specimen preparation for high resolution cryo-EM. J. Methods in Enzymology. 579, 51-86 (2016).
- Laughlin, T. G., Bayne, A. N., Trempe, J. -. F., Savage, D. F., Davies, K. M. Structure of the complex I-like molecule NDH of oxygenic photosynthesis. Nature. 566, 411-414 (2019).
- Gao, Y., et al. Structures and operating principles of the replisome. Science. 363, (2019).
- Zhao, Y., Chen, S., Swensen, A. C., Qian, W. -. J., Gouaux, E. Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM. Science. 364, 355-362 (2019).
- Chen, B., et al. Structural dynamics of ribosome subunit association studied by mixing-spraying time-resolved cryogenic electron microscopy. Structure. 23, 1097-1105 (2015).
- Singh, A. K., et al. Structural basis of temperature sensation by the TRP channel TRPV3. Nature Structure and Molecular Biology. 26, 994-998 (2019).
- Chen, C. Y., Chang, Y. C., Lin, B. L., Huang, C. H., Tsai, M. D. Temperature-resolved cryo-EM uncovers structural bases of temperature-dependent enzyme functions. Journal of the American Chemical Society. 141, 19983-19987 (2019).

