Analysis of Transforming Growth Factor ß Family Cleavage Products Secreted Into the Blastocoele of Xenopus laevis Embryos
Summary
When Transforming Growth Factor ß family precursor proteins are ectopically expressed in Xenopus laevis embryos, they dimerize, get cleaved and are secreted into the blastocoele, which begins at the late blastula to early gastrula stage. We describe a method for aspirating cleavage products from the blastocoele cavity for immunoblot analysis.
Abstract
The two arms of the Transforming Growth Factor ß (Tgfß) superfamily, represented by Tgfß/Nodal or Bone morphogenetic protein (Bmp) ligands, respectively, play essential roles in embryonic development and adult homeostasis. Members of the Tgfß family are made as inactive precursors that dimerize and fold within the endoplasmic reticulum. The precursor is subsequently cleaved into ligand and prodomain fragments. Although only the dimeric ligand can engage Tgfß receptors and activate downstream signaling, there is growing recognition that the prodomain moiety contributes to ligand activity. This article describes a protocol that can be used to identify cleavage products generated during activation of Tgfß precursor proteins. RNA encoding Tgfß precursors are first microinjected into X. laevis embryos. The following day, cleavage products are collected from the blastocoele of gastrula stage embryos and analyzed on Western blots. This protocol can be completed relatively quickly, does not require expensive reagents and provides a source of concentrated Tgfß cleavage products under physiologic conditions.
Introduction
Members of the Transforming Growth Factor ß (Tgfß) superfamily are synthesized as inactive, dimerized precursor proteins. The precursors are then cleaved by members of the proprotein convertase (PC) family, either within the secretory pathway or outside of cells. This creates an active, disulfide-bonded ligand dimer and two prodomain fragments1. Although it has been known for over 30 years that the prodomain of Tgfß family precursors is required to generate an active ligand2, the understanding of how prodomains contribute to ligand function is incomplete.
Although the understanding of the process of proteolytic activation of Tgfß family members remains incomplete, there is increasing interest in understanding which PC consensus motif(s) are cleaved in vivo, whether the cleavage occurs in a specific subcellular or extracellular compartment, and whether the prodomain remains covalently or noncovalently associated with the cleaved ligand3. Several studies have shown that the prodomain not only guides ligand folding before cleavage4,5, but can also influence growth factor stability and range of action6,7,8,9, drive the formation of homodimers or heterodimers10, anchor the ligand in the extracellular matrix to maintain ligand latency11, and in some cases, function as a ligand in its own right to activate heterologous signaling12. Heterozygous point mutations within the prodomain of many members of the Tgfß family are associated with eye, bone, kidney, skeletal or other defects in humans3. These findings highlight the critical role of the prodomain in generating and maintaining an active ligand and stress the importance of identifying and deciphering the role of cleavage products developed during proteolytic maturation of Tgfß family precursors.
Here we describe a detailed protocol for aspirating cleavage products generated during maturation of Tgfß family precursors from the blastocoele of X. laevis embryos and then analyzing them on immunoblots. This protocol can be used to determine whether one or more PC consensus motif(s) in a precursor protein are cleaved in vivo10,13, identify the endogenous PC(s) that cleave each motif13,14, compare in vivo formation of Tgfß family homodimers versus heterodimers10 or analyze whether human disease-associated point mutations in Tgfß precursors impact their ability to form functional dimeric ligands.
Protocol
All procedures described are approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Utah. Frogs are housed in an IACUC approved facility. Male frogs are euthanized by immersion in tricaine following by clipping the ventricle of the heart. Female frogs are housed in the laboratory for a maximum of 24 h following hormonal induction of spawning to allow for egg collection and then returned to the care facility.
1. Collection of X. laevis Testes
- Anesthetize a sexually mature male in 0.2% Tricaine (Table 1) for 30-40 min until the animal is unresponsive to claw pinch.
- Use blunt forceps to pull the skin away from the body wall. Make a horizontal incision in the skin across the lower abdomen using surgical scissors. Make a parallel incision in the underlying body wall, and then a third, perpendicular incision through the body wall up through the rib cage.
- Fold back the resulting flaps, locate the ventricle of the heart and clip the base to exsanguinate and ensure death.
- Pull the yellow fat bodies out of the abdomen with blunt forceps and locate the testes on each side, underlying the base of the fat bodies. Remove each testis by clipping it away from the fat bodies and any attached viscera using surgical scissors.
- Transfer testes to a Petri dish containing 1x Modified Barth's Solution (MBS) (Table 1). Rinse and remove any adherent blood vessels or attached viscera.
- Transfer one testis to a 35 mm Petri dish containing testis buffer (Table 1) for immediate use and store the other in a 10 mL conical tube filled with testis buffer for later use. Testis should be stored at 4 °C when not used and will remain viable for at least two weeks.
NOTE: Photographs of testes isolation can be found in Reference 15.
- Transfer one testis to a 35 mm Petri dish containing testis buffer (Table 1) for immediate use and store the other in a 10 mL conical tube filled with testis buffer for later use. Testis should be stored at 4 °C when not used and will remain viable for at least two weeks.
2. Collection and Fertilization of X. laevis Eggs
NOTE: Frogs should be handled with vinyl gloves or hands washed before and after handling the frogs. Latex gloves, lotions and detergent residues on human hands will damage the frogs' fragile skin.
- The evening before spawning, inject 0.3 mL (1200 IU) of human chorionic gonadotropin (Table 1) into the dorsal lymph sac of two or three mature X. laevis females using a 1 mL syringe fitted with a 26 G needle. Use a new needle for each injection.
- After injection, place the females in a sealed container (not air-tight) in an 18 °C biological incubator. Spawning generally begins 14-16 h later.
NOTE: Plastic drawers or plastic containers with air holes cut into the lid work well for holding females overnight. Be sure the lid is on firmly to prevent frogs from escaping and desiccating in the incubator. - Hold the female over a 100 mm Petri dish containing 1x MBS and apply gentle pressure to the frog's abdomen to expel eggs into the dish.
- Cut a small piece of the testis (~1/5) using a razor blade and transfer it into a 1.5 mL tube containing ~500 µL of 1x MBS. Crush with a pellet pestle or tissue homogenizer. Store the resulting sperm suspension on ice or at 4 °C for subsequent fertilization on the same day.
- Tilt the 100 mm Petri dish to pour off as much of the 1x MBS as possible, and remove the remainder using a plastic transfer pipette. Apply approximately 100 µL of sperm suspension, add about 1 mL of 0.1x MBS and swirl to mix.
- After 5 min, flood the dish with dechlorinated tap water or 0.1x MBS and let stand for 30 min.
NOTE: Water can be dechlorinated using a filter or municipal tap water can be left standing uncovered for at least 24 h to allow the chlorine to dissipate in regions where conventional chlorine is added to the water. In areas where chloramine is added to disinfect drinking water, the water must instead be treated with a commercially available conditioner that can break down chloramine.
NOTE: Eggs are collected in a high salt solution (1x MBS) to prevent the formation of a jelly coat, which would provide a barrier to fertilization. After fertilization, the low salt solution (dechlorinated water or 0.1x MBS) allows for jelly coat formation and the eggs will adhere to each other and the dish. Eggs that have been fertilized will rotate such that the pigmented animal pole faces upward within 30 min. If this does not happen, and the eggs look healthy under the microscope, sperm viability is suspect and it may be necessary to harvest new testes.
- After 5 min, flood the dish with dechlorinated tap water or 0.1x MBS and let stand for 30 min.
- Pour off the solution covering the eggs and fill the Petri dish with freshly prepared 2% cysteine solution (Table 1). Swirl the solution in the dish for 3-5 min until the jelly coat is dissolved and the eggs are no longer sticking to each other or the dish.
- Tilt the dish and carefully pour off the cysteine solution, or remove with a plastic pipette. Replace with 1x DeBoer's pond water (Table 1). Swirl to rinse and decant.
- Repeat one wash with DeBoer's solution, one with 0.1x MBS, and refill the dish with 0.1x MBS. After removing the jelly coat, the eggs become more fragile and must not be exposed to the air-liquid interface.
- Move the fertilized eggs to a 16 °C biological incubator or leave them on the bench at room temperature. If left at room temperature, the first cleavage will occur 1-1.5 h after fertilization, and subsequent cleavages will occur approximately every 30 min. By contrast, if embryos are incubated at 15-16 °C, cleavages will occur roughly every hour and it will be possible to inject a larger number of embryos from a given spawn.
3. Microinjection of X. laevis Embryos
- Heat and pull glass capillaries to a fine point using a micropipette puller with the following settings: Two-step pull; Heat 1: 67.4 °C; Heat 2: 62 °C.
NOTE: These settings are specific for the micropipette puller and glass capillaries used here (Table of Materials). - Under a dissecting microscope, clip off the tip of a pulled needle close to the end using Dumont #5 forceps. The needle should be sharp but not so thin that the tip bends when it contacts the embryo. Figure 1 shows an example of an appropriately pulled needle that has not been clipped for microinjection (A) and one that has been clipped (B).
- Insert the needle into a needle holder connected to a microinjector. Attach the needle holder to a micromanipulator. Place 2-4 µL of capped synthetic RNA encoding the Tgfß precursor protein to be analyzed on a small piece of parafilm under a dissecting microscope.
- Using the micromanipulator, lower the needle into the drop and use the fill function on the microinjector to aspirate RNA into the needle. Be sure to keep the needle below the surface of the drop, and watch progress under the microscope to avoid aspirating air into the needle.
NOTE: If antibodies that recognize the prodomain and/or ligand domain of the Tgfß precursor to be analyzed are not available, sequence encoding an epitope tag (e.g., V5, myc, Flag) can be inserted in-frame into the cDNA that will be used as a template for RNA transcription. The in vivo bioactivity of the tagged protein should be compared with that of untagged protein to ensure that the epitope does not interfere with proper folding, cleavage or activity.
- Using the micromanipulator, lower the needle into the drop and use the fill function on the microinjector to aspirate RNA into the needle. Be sure to keep the needle below the surface of the drop, and watch progress under the microscope to avoid aspirating air into the needle.
- Eject a drop of the RNA solution into the air and measure the drop size using a micrometer mounted on the injection stage or in the eyepiece of the microscope. Adjust the drop size to approximately 10 nL utilizing the time and pressure controls on the microinjector.
NOTE: RNA concentrations in the range of 5-20 ng/µL are appropriate to deliver 50-200 pg of RNA into each embryo. This amount of RNA is generally sufficient to detect cleavage products in aspirates from 10-20 embryos. It is best to aim for the lowest dose of RNA that gives a detectable signal on immunoblots. Higher amounts of RNA can cause gastrulation defects, which interfere with the expansion of the blastocoele. - Move embryos to an injection dish or tray containing 5% Ficoll in 0.1x MBS (Table 1) when they reach the 2-4 cell stage. A 35 mm Petri dish fitted with a piece of nylon mesh in the bottom can serve as an inexpensive injection dish that will keep the embryos immobilized. Insert the needle near the animal pole and inject 10 nL of RNA into one cell of each embryo.
NOTE: Embryos can easily be injected at any time between the one and 16 cell stages. Injecting the embryo after it has cleaved ensures that the egg was fertilized and that the embryo is healthy. The exact location of the injection is not essential. A more detailed description of spawning, culture and microinjection of RNAs into X. laevis embryos can be found in Reference 15. - Transfer the injected embryos into a 35 mm Petri dish filled with 5% Ficoll in 0.1x MBS (Table 1). Place the small dishes inside a 150 mm Petri dish and cover loosely with a lid to prevent the culture media from evaporating. Culture the embryos overnight at 15-16 °C.
NOTE: Culturing embryos in Ficoll helps to heal the injection site. If fine needles are used, culturing in 2-3% Ficoll is sufficient, whereas 5% Ficoll is preferred if apparent damage is observed. Keeping the embryos in Ficoll solution for 1-2 h after injection is enough to aid healing, but overnight incubation does not damage the embryo as long as the solution is changed before the onset of gastrulation.
4. Blastocoele Extraction and Analysis of Tgfß Cleavage Products
- The following morning after injection, remove the Ficoll solution and remove any dead or dying embryos. Rinse the embryos once or twice with 0.1x MBS and culture the embryos in 0.1x MBS on the bench at room temperature.
NOTE: Embryos can also be returned to the 16 °C biological incubator to slow down development. - Heat and pull glass capillaries to a fine point using a micropipette puller with the following settings: Two step pull; Heat 1: 67.4 °C; Heat 2: 62 °C.
NOTE: These settings are specific for the puller and glass capillary used here (Table of Materials). - Under a dissecting microscope, clip off the tip of a pulled needle using forceps. To prevent clogging, the opening of a needle used for blastocoele aspiration should be larger than that in a needle used for microinjection. An example of a good pulled and clipped needle to be used for blastocoele aspiration is shown in Figure 1C. Insert the aspiration needle into needle holder connected to a microinjector and attach the needle holder to a micromanipulator.
NOTE: The optimal needle diameter is determined empirically by trial and error. Needles with a large diameter fill too quickly and make it more likely that cellular debris will enter the needle. By contrast, very small diameter needles are likely to become clogged. Once an appropriate needle is generated, it's useful to generate multiple needles clipped at the same position. - Place embryos in an injection tray or dish filled with 0.1x MBS when they reach the early to the mid-gastrula stage (stage 10-11).
- Insert the needle below the surface of the embryo near the animal pole. Press the fill button on the microinjector while looking through the dissecting microscope to keep an eye on the needle and the embryo. Over a few seconds, the level of clear fluid will rise in the needle and the embryo will collapse and become concave.
- If cloudy white matter enters the needle, let go of the fill button and pulse the inject button one or more times to eject any cloudy matter, which consists of cellular debris that is likely to contain proteases.
NOTE: If the blastocoele collapses and cell debris enters the needle as soon as the fill button is pressed, this indicates that the opening in the needle is too large. If the blastocoele fails to collapse but white matter enters the needle immediately, this shows that the needle was inserted too deeply and is touching the blastocoele floor. Eject the white matter, and move to the next embryo. The aspiration rate can be more carefully controlled by pulsing the fill button rather than pressing it continuously. This is particularly important if the opening in the needle is larger than optimal.
- If cloudy white matter enters the needle, let go of the fill button and pulse the inject button one or more times to eject any cloudy matter, which consists of cellular debris that is likely to contain proteases.
- Repeat step 4.5 until the fluid has been aspirated from the blastocoele of 10-20 embryos.
NOTE: Generally, blastocoele fluid aspirated from 10-20 embryos is sufficient to detect cleavage products on immunoblots. However, this can vary depending on antibody quality and must be determined empirically. - Place a piece of paraffin on the injection tray and pipette 1 µL of nuclease-free water onto it. Submerge the needle under the drop of water and press the inject button to dispel the blastocoele fluid into the water.
- Alternatively, eject the fluid directly onto the parafilm, but in this case, pulse the inject button rather than holding it down. This expels the fluid under lower pressure, preventing it from flattening out on the parafilm, making it difficult to draw up in a pipette.
- Transfer the blastocoele fluid into a sterile microcentrifuge tube on ice. Expect to harvest approximately 0.3-0.5 µL of blastocoele fluid per embryo. Add nuclease-free water to adjust the final volume to 30 µL.
- Transfer the blastocoele fluid-depleted embryos to a separate tube on ice. Remove excess 0.1x MBS and add 200 µL of chilled (4 °C) embryo lysate buffer (10 µL per embryo) (Table 1). Pipette the embryos up and down 10-20 times using a micropipette until they are fully homogenized and no clumps remain.
- Centrifuge the homogenized embryos in a refrigerated microcentrifuge at 10,000 x g for 10 min. Remove 160 µL of the supernatant using a P200 pipette and transfer to a new tube on ice, being careful to avoid the white yolk proteins and other cellular debris in the bottom half of the tube.
- Repeat the microcentrifugation once and transfer 128 µL of the clear supernatant to a new tube on ice. At this point, cleared embryo lysates and blastocoele fluid can be stored at -80 °C for as long as desired.
NOTE: With a few exceptions, Tgfß precursor proteins are not secreted into the blastocoele and will only be detected in the depleted embryo lysates. By contrast, Tgfß family cleavage products are primarily seen in the blastocoele fluid. It is helpful to harvest and analyze both the embryo lysates and the blastocoele fluid for most purposes. At a minimum, this provides a positive control to ensure that the precursor was robustly translated and stable. In addition, it allows one to compare relative ratios of cleavage products to precursors among different wild type or mutant proteins, to identify mutations that enable intact precursors to be secreted into blastocoele fluid (e.g., mutations within the PC consensus motif that allow for proper folding and secretion without cleavage, in which case the intact precursor is detected in both the embryo and the blastocoele fluid) or mutations that generate misfolded, non-cleavable proteins (in which case the precursor will be detected in the embryo lysate, but cleavage products will be absent in the blastocoele fluid).
- Repeat the microcentrifugation once and transfer 128 µL of the clear supernatant to a new tube on ice. At this point, cleared embryo lysates and blastocoele fluid can be stored at -80 °C for as long as desired.
- Deglycosylate proteins present in blastocoele fluid with PNGase F at this point by following the manufacturer's instructions.
NOTE There is no need to deglycosylate precursor proteins present in the embryo lysate since these represent endoplasmic reticulum (ER)–resident forms of the precursor that lack the complex N-linked oligosaccharides removed by PNGase F. By contrast, cleavage products present in blastocoele fluid have moved through the trans-Golgi network (TGN) where carbohydrates are further modified and can now be removed by PNGase F. Following deglycosylation, cleavage products migrate as a more tightly condensed band on SDS gels, which can aid in visualizing, quantitating and accurately identifying cleavage products. This is not strictly required, but it can be helpful, for example, if you are trying to distinguish between homodimers and heterodimers based on migration patterns or if a cleavage product contains heterogeneous carbohydrates that cause it to migrate as a broad fuzzy band. - Add 5 µL of reducing 4x sample buffer (Table 1) to 15 µL of blastocoele fluid and 15 µL of clarified embryo lysate. Add 5 µL of non-reducing 4x sample buffer (Table 1) to the remaining 15 µL of blastocoele fluid. Heat to 100 °C for 5 min and place on ice.
NOTE: Analyzing proteins under non-reducing conditions is essential to analyze the formation of cleaved homodimeric or heterodimeric ligands but is not needed if only analyzing cleavage sites or levels of cleaved proteins. With a few exceptions, prodomain fragments are secreted as monomers. Furthermore, precursor proteins present in the embryo lysate are primarily unfolded, ER resident monomers. Thus, there is no need to analyze these products under non-reducing conditions. - Resolve precursor proteins and cleavage products by electrophoresis on 15% SDS/polyacrylamide gels.
- Transfer proteins from the gel onto a PVDF membrane using electrophoretic transfer.
- Incubate membranes with appropriate antibodies that recognize the prodomain and the ligand domain (or epitope tags inserted into those domains), wash and incubate with proper secondary antibodies. Visualize proteins with chemiluminescence or fluorescent imaging.
Representative Results
The experiment's goal described below was to determine whether Bmp4 and Bmp7 form heterodimers (dimers composed of one Bmp4 ligand and one Bmp7 ligand), homodimers (composed of two Bmp4 or two Bmp7 ligands), or a mixture of each when they are co-expressed in X. laevis. Data shown in Figure 2 are extracted from a previously published study10. Figure 2A is a schematic showing cleavage products generated by proteolytic maturation of Bmp4 or Bmp7 homodimeric precursors or Bmp4/7 heterodimeric precursors. Bmp4 is cleaved at two sites within the prodomain to generate two prodomain fragments (dark green and yellow) and one dimeric ligand fragment (light green) that migrates at ~26 kDa on SDS-PAGE gels. Bmp7 is cleaved at a single site to generate one prodomain fragment (maroon) and one dimeric ligand fragment (pink) that migrates at ~35 kDa. Heterodimeric ligands migrate at ~30 kDa. The silver bar in the ligand dimers represents a myc-epitope tag inserted into this domain.
RNA encoding Bmp4myc or Bmp7myc precursors (200 pg), or both RNAs (100 pg of each) were injected into X. laevis embryos at the 2-4 cell stage. The embryos were cultured overnight at 16 °C until they reached the early gastrula stage. At this point, fluid was aspirated from the blastocele of 20 embryos in each group and sterile water was added to bring the volume to a total of 30 µL. Following deglycosylation, each sample was split into two tubes. Reducing sample buffer was added to one tube, and non-reducing sample buffer was added to the other. Samples were heated to 100 °C for 5 min. Proteins were then separated by SDS/PAGE and immunoblots were probed with antibodies that recognize the myc-epitope tag (Figure 2B). Under reducing conditions, a single band corresponding to cleaved Bmp4 monomers and a more slowly migrating band corresponding to cleaved BMP7 monomers were detected in lysates from embryos expressing only Bmp4 or Bmp7, respectively (Figure 2C, D, lower panel, lanes 1, 2). Both bands were detected in embryos co-expressing Bmp4 and Bmp7 (Figure 2C, D, lower panel, lane 3). Relatively equivalent amounts of Bmp4 and Bmp7 monomers were present in each of the three groups (lower panel, reducing). In this experiment, when proteins were separated under non-reducing conditions, a single mature Bmp4/7 heterodimer band of intermediate mobility was detected along with a trace amount of Bmp4 homodimer in embryos co-expressing Bmp4 and Bmp7 (Figure 2C, upper panel). From these results we conclude that if equivalent levels of Bmp4 and Bmp7 precursor proteins are expressed in Xenopus embryos, they preferentially form heterodimers, rather than either homodimer. In the experiment shown in Figure 2D, significantly more Bmp7 protein is present relative to Bmp4 (lower panel, reducing). As a result, in embryos expressing both Bmp7 and Bmp4 precursor proteins, Bmp4 homodimers are not detected and instead the available Bmp4 is present as a Bmp4/7 heterodimer, and the excess Bmp7 forms homodimers. While this experiment is not optimal, as the goal was to co-express the equivalent amount of Bmp4 and Bmp7 precursor, the results are still consistent with the conclusion that Bmp4 and Bmp7 preferentially form heterodimers over either homodimer when co-expressed.
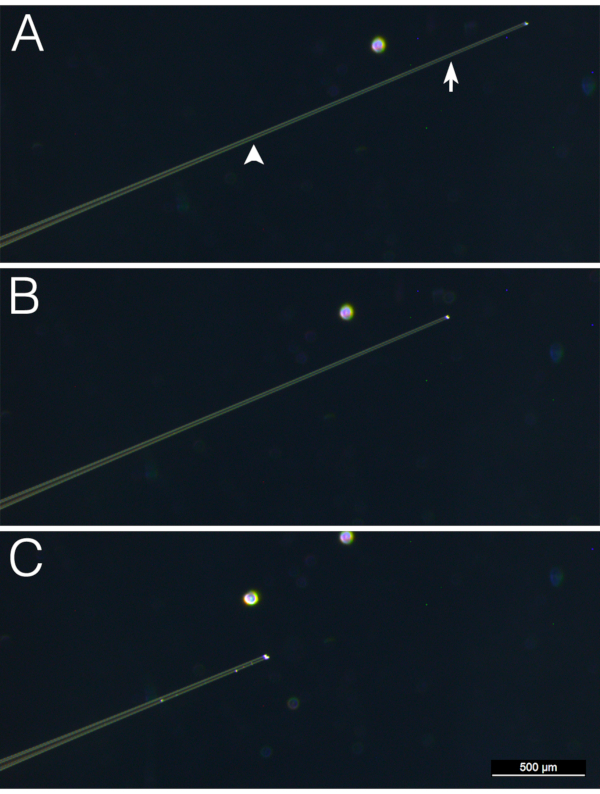
Figure 1. Injection and aspiration needles. Photographs of representative needles that have been pulled but not clipped (A) pulled and clipped for use as an injection needle (B) or pulled and clipped for use as an aspiration needle (C) are shown. The arrow and arrowhead in (A) indicate the point at which the glass was clipped to generate a needle for injection and aspiration, respectively. Please click here to view a larger version of this figure.
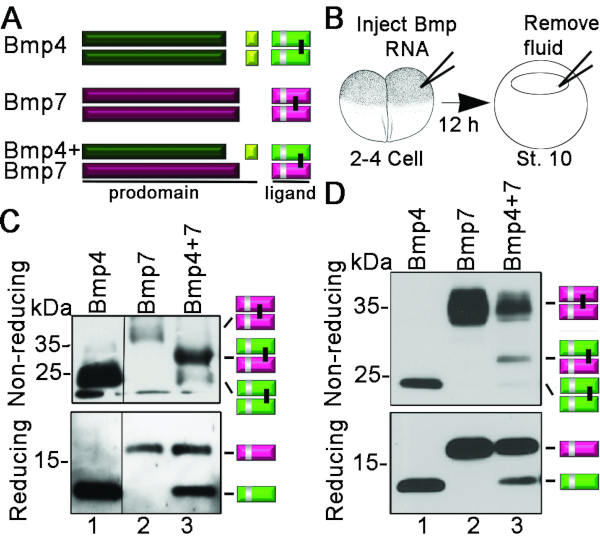
Figure 2. Analysis of cleaved Bmp ligands extracted from X. laevis blastocele fluid. (A) Cleavage products generated from Bmp4 and Bmp7 homodimeric or heterodimeric precursor proteins and position of myc epitope tag (silver bar) is shown schematically (B) Schematic diagram showing the timing of injection and blastocoele fluid extraction. (C-D) Xenopus embryos were injected with 200 pg of Bmp7 or Bmp4 RNA, or with Bmp4 and Bmp7 RNA mixed together (100 pg each). At the gastrula stage, fluid was extracted from the blastocoele of the same number of embryos in each experimental group. Proteins present in the blastocoele fluid were deglycosylated and separated by SDS-PAGE. Antibodies recognizing the myc-epitope tag were used to probe immunoblots. The relative position of immunoreactive ligand monomers and dimers is shown schematically to the right of each gel. Black line in (C) indicates removal of an intervening lane on the blot. Data shown are extracted from a previously published study10. Please click here to view a larger version of this figure.
| Solution | Composition |
| 0.2% Tricaine | 20 g of Tricaine dissolved in deionized water, adjust pH to 7.4 by addition of sodium bicarbonate |
| 10x MBS | 880 mM NaCl, 10 mM KCl, 25 mM NaHCO3, 100 mM HEPES (pH 7.5), 10 mM MgSO4, 0.14 mM Ca(NO3)2, 0.41 mM CaCl2; Adjust to pH 7.5 with NaOH. The 10x MBS stock can be stored at 4 °C and diluted as needed. |
| 2% cysteine solution | 2 g of cysteine per 100 mL of deionized water, adjust pH to 7.8-8.0 with sodium hydroxide. Make fresh each day. |
| 20x DeBoer's pond water | 100 mM NaCl, 1.3 mM KCl, 0.44 mM CaCl2; Adjust to pH 7.4 with NaHCO3. 20x stock of DeBoer’s pond water can be stored at 4 °C and diluted as needed. |
| 4x non-reducing sample buffer | 200 mM Tris pH 6.8, 8% SDS, 0.4% bromophenol blue, 40% glycerol. |
| 4x reducing sample buffer | 200 mM Tris pH 6.8, 8% SDS, 0.4% bromophenol blue, 40% glycerol, 14.4 M ß-mercaptoethanol |
| 5% Ficoll Solution | 25 g of Ficoll in 500 mL 0.1x MBS, adjust pH to 7.5 with sodium hydroxide. Store at 4 °C. |
| Embryo lysate buffer | 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 2.5 % NP-40. Store at 4 °C. |
| Human chorionic gonadotropin | Using a 3 mL syringe attached to a 19 G needle, inject 2.5 mL of sterile water through the rubber stopper of a vial of human chorionic gonadotropin (10,000 IU ). Store at 4 °C . |
| Testis buffer | 10% fetal bovine serum, 1% pen/strep (100U/mL penicillin, 100 mg/mL streptomycin in 1x MBS |
Table 1.
Discussion
The main advantages to the protocol described here are that it can be completed relatively quickly, does not require expensive reagents and provides a source of concentrated Tgfß cleavage products under physiologic conditions. Another advantage is that it allows one to analyze epitope-tagged proteins and thus circumvent the shortage of commercially available antibodies that recognize most Tgfß family prodomain. Although it is also possible to analyze the cleavage of epitope-tagged Tgfß precursor proteins in transfected cultured mammalian cells, there are several advantages to using X. laevis. Early X. laevis embryos express all four members of the PC family that are candidates for endogenous Tgfß convertases (Furin, Pcsk5, Pcsk6 and Pcsk7)13,14,16. Some mammalian cell lines do not express one or more PC, requiring the ectopic expression of both the precursor protein and its convertase to examine cleavage17. A more important consideration is that very high levels of precursor proteins generated by transient transfect of transformed cells can lead to artifacts, such as the secretion of misfolded cleavage products, which can occur if precursor levels exceed the capacity for quality control in the ER18. This can mask the effect of deleterious mutations on precursor dimerization and cleavage since the misfolded cleavage products appear in the media and misfolding would not be detected unless the activity is assayed. By contrast, experiments utilizing X. laevis embryos readily detect defects in folding, leading either to loss of precursor due to the misfolded protein response in the ER or aberrant migration of cleavage products under non-reducing conditions10,18.
During the blastocoele aspiration procedure, it is essential to try to extract an approximately equivalent amount of blastocoele fluid from each embryo, particularly if, for example, the goal was to compare cleavage products generated from wild type and mutant precursors. This is an inexact science but aspirating for an approximately equal amount of time from each embryo helps to normalize the volume. In addition, aspirating fluid from a greater number of embryos in each group helps to normalize any differences in volume between individual embryos.
In vitro cleavage reactions are an alternative procedure that can be used to determine whether a particular PC motif present in a Tgfß precursor protein is cleaved and to identify and/or rule out potential PCs as endogenous convertases for a given substrate. Existing protocols describe a simple assay in which radiolabeled precursor proteins are generated in vitro, using rabbit reticulocytes, for example, or in vivo, using X. laevis oocytes. Substrates are then incubated in vitro with candidate recombinant PCs and cleavage products analyzed by electrophoresis and autoradiography19. While this protocol provides a fast and easy way to determine whether a protein is a PC substrate and to identify which potential cleavage sites are utilized in vivo, the precursor proteins are unlikely to be adequately folded or dimerized and thus cleavage information obtained from these experiments may not reflect the in vivo situation.
One shortcoming of the protocol we describe here is that it is impossible to visualize properly folded, dimerized precursor protein in X. laevis embryos. The same is true in other ectopic expression systems, such as transiently transfected mammalian cells, since uncleaved monomeric precursor protein accumulates within the ER at much higher levels than post-ER dimerized and folded precursors. The dimerized precursor is rapidly cleaved and secreted once it leaves the ER. If it is essential to analyze dimerization and folding of precursor proteins, a useful alternative procedure is to examine the trafficking and cleavage of radiolabeled precursors in X. laevis oocytes20. The high sensitivity of autoradiography, coupled with the fact that proteins move through the secretory system more slowly in oocytes than they do in cultured mammalian cells or embryos allows one to detect dimerized precursor proteins within oocytes and to test whether the proteins are properly folded and have left the ER by examining their glycosylation status18,21.
In summary, this protocol provides a rapid and straightforward method to analyze cleavage products generated by the maturation of Tgfß family precursor proteins.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Mary Sanchez for excellent animal care. The authors' research is supported by the National Institute of Child Health and Human Development of the National Institutes of Health (NIH/NICHD) grants R01HD067473-08 and R21 HD102668-01 and by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK/NIH) grant R01DK128068-01.
Materials
| ß-mercaptoethanol | Fisher | AC125470100 | |
| Bromophenol Blue | Fisher | B392-5 | |
| Calcium Chloride | Fisher | C79-500 | |
| Calcium Nitrate | Fisher | C109-500 | |
| Disposable Pellet Pestle/Tissue Grinder | Fisher | 12-141-364 | |
| Dumont #5 forceps | Fine Science tools | 11251-10 | |
| Fetal Bovine Serum | Atlanta Biologicals | S11150H | |
| Ficoll 400 | Sigma Aldrich | F9378-500G | |
| Glass capillary, 1 X 90 mm | Narshige | G-1 | |
| Glycerol | Fisher | G33-4 | |
| HEPES | Fisher | BP310-500 | |
| Human chorionic gonadotropin | Sigma Aldrich | CG10-10VL | |
| Injection Syringe, 1 mL | Fisher | 8881501368 | |
| L-Cysteine | Sigma Aldrich | C7352 | |
| Magnesium Sulfate | Fisher | M63-500 | |
| Needle, 26 G | Fisher | 305111 | |
| Penicillin/Streptomycin | Gibco | 15140148 | |
| Picoliter Microinjector | Warner Instruments | PLI-100A | |
| Pipette Puller | Narashige | PC-100 | |
| Potassium Chloride | Fisher | P217-500 | |
| PVDF Membrane | Sigma Aldrich | IPVH00010 | |
| Sodium Bicarbonate | Fisher | S233-500 | |
| Sodium Chloride | Fisher | S271-10 | |
| Sodium Dodecyl Sulfate | Fisher | BP166-500 | |
| Sodium Hydroxide | Fisher | S318-500 | |
| Tricaine-S | Pentair | TRS5 | |
| Tris | Fisher | BP152-5 |
References
- Harrison, C. A., Al-Musawi, S. L., Walton, K. L. Prodomains regulate the synthesis, extracellular localisation and activity of TGF-beta superfamily ligands. Growth Factors. 29 (5), 174-186 (2011).
- Gray, A. M., Mason, A. J. Requirement for activin A and transforming growth factor–beta 1 pro-regions in homodimer assembly. Science. 247 (4948), 1328-1330 (1990).
- Constam, D. B. Regulation of TGFbeta and related signals by precursor processing. Seminars in Cell and Developmental Biology. 32, 85-97 (2014).
- Wang, X., Fischer, G., Hyvonen, M. Structure and activation of pro-activin A. Nature Communications. 7, 12052 (2016).
- Zhao, B., Xu, S., Dong, X., Lu, C., Springer, T. A. Prodomain-growth factor swapping in the structure of pro-TGF-beta1. Journal of Biological Chemistry. 293 (5), 1579-1589 (2018).
- Cui, Y., et al. The activity and signaling range of mature BMP-4 is regulated by sequential cleavage at two sites within the prodomain of the precursor. Genes & Development. 15 (21), 2797-2802 (2001).
- Goldman, D. C., et al. Mutation of an upstream cleavage site in the BMP4 prodomain leads to tissue-specific loss of activity. Development. 133 (10), 1933-1942 (2006).
- Le Good, J. A., et al. Nodal stability determines signaling range. Current Biology. 15 (1), 31-36 (2005).
- Tilak, A., et al. Simultaneous rather than ordered cleavage of two sites within the BMP4 prodomain leads to loss of ligand in mice. Development. 141 (15), 3062-3071 (2014).
- Neugebauer, J. M., et al. The prodomain of BMP4 is necessary and sufficient to generate stable BMP4/7 heterodimers with enhanced bioactivity in vivo. Proceedings of the National Academy of Science U S A. 112 (18), 2307-2316 (2015).
- Robertson, I. B., Rifkin, D. B. Regulation of the Bioavailability of TGF-beta and TGF-beta-Related Proteins. Cold Spring Harbor Perspectives in Biology. 8 (6), 355-372 (2016).
- Zhou, F., et al. GDF6-CD99 Signaling Regulates Src and Ewing Sarcoma Growth. Cell Reports. 33, 108332 (2020).
- Nelsen, S. M., Christian, J. L. Site-specific cleavage of BMP4 by furin, PC6, and PC7. Journal of Biological Chemistry. 284 (40), 27157-27166 (2009).
- Birsoy, B., et al. XPACE4 is a localized pro-protein convertase required for mesoderm induction and the cleavage of specific TGFbeta proteins in Xenopus development. Development. 132 (3), 591-602 (2005).
- Mimoto, M. S., Christian, J. L. Manipulation of gene function in Xenopus laevis. Methods Mol Biol. 770, 55-75 (2011).
- Nelsen, S., Berg, L., Wong, C., Christian, J. L. Proprotein convertase genes in Xenopus development. Developmental Dynamics. 233 (3), 1038-1044 (2005).
- Constam, D. B., Robertson, E. J. Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. Journal of Cell Biology. 144 (1), 139-149 (1999).
- Sopory, S., Nelsen, S. M., Degnin, C., Wong, C., Christian, J. L. Regulation of bone morphogenetic protein-4 activity by sequence elements within the prodomain. Journal of Biological Chemistry. 281 (45), 34021-34031 (2006).
- Sopory, S., Christian, J. L., Whitman, M. A., Whitman, S. . Analysis of Growth Factor Signaling in Embryos. , 38-55 (2006).
- Kim, H. S., McKnite, A., Christian, J. L. Proteolytic Activation of Bmps: Analysis of Cleavage in Xenopus Oocytes and Embryos. Methods in Molecular Biology. 1891, 115-133 (2019).
- Degnin, C., Jean, F., Thomas, G., Christian, J. L. Cleavages within the prodomain direct intracellular trafficking and degradation of mature bone morphogenetic protein-4. Molecular Biology of the Cell. 15 (11), 5012-5020 (2004).

