Assessment of Sensory Thresholds in Dogs Using Mechanical and Hot Thermal Quantitative Sensory Testing
Summary
This work describes a standard protocol for mechanical and hot thermal quantitative sensory testing to evaluate the somatosensory system in dogs. Sensory thresholds are measured using an electronic von Frey anesthesiometer, pressure algometer, and hot contact thermode.
Abstract
Quantitative sensory testing (QST) is used to evaluate the function of the somatosensory system in dogs by assessing the response to applied mechanical and thermal stimuli. QST is used to determine normal dogs' sensory thresholds and evaluate alterations in peripheral and central sensory pathways caused by various disease states, including osteoarthritis, spinal cord injury, and cranial cruciate ligament rupture. Mechanical sensory thresholds are measured by electronic von Frey anesthesiometers and pressure algometers. They are determined as the force at which the dog exhibits a response indicating conscious stimulus perception. Hot thermal sensory thresholds are the latency to respond to a fixed or ramped temperature stimulus applied by a contact thermode.
Following a consistent protocol for performing QST and paying attention to details of the testing environment, procedure, and individual study subjects are critical for obtaining accurate QST results for dogs. Protocols for the standardized collection of QST data in dogs have not been described in detail. QST should be performed in a quiet, distraction-free environment that is comfortable for the dog, the QST operator, and the handler. Ensuring that the dog is calm, relaxed, and properly positioned for each measurement helps produce reliable, consistent responses to the stimuli and makes the testing process more manageable. The QST operator and handler should be familiar and comfortable with handling dogs and interpreting dogs' behavioral responses to potentially painful stimuli to determine the endpoint of testing, reduce stress, and maintain safety during the testing process.
Introduction
Quantitative sensory testing (QST) assesses the responses elicited by externally applied stimuli; it is used to evaluate the function of the somatosensory system in humans and animals1. Mechanical stimuli in the form of punctate pressure or deep pressure are applied as a ramped stimulus. The sensory threshold is determined as the force that evokes a psychophysical response1. Hot or cold thermal stimuli can be used as a ramped stimulus or as a fixed intensity stimulus. The sensory threshold is determined as the temperature at which there is a response or the latency to respond to the stimulus. Punctate pressure sensory thresholds are measured using electronic von Frey anesthesiometers or von Frey hair filaments, deep pressure is measured using handheld pressure algometers, and thermal sensory thresholds are determined using a variety of contact thermode systems.
QST provides information about the functioning of both peripheral and central sensory pathways and can be used to evaluate alterations in these sensory pathways (algoplasticity) in various disease processes, particularly those that cause chronic pain1. Meissner's corpuscles detect punctate pressure, and the sensation is transmitted by Aβ afferent fibers at non-noxious levels and Aδ afferent fibers when the stimulus is of a noxious intensity1,2. Deep pressure is detected by Pacinian corpuscles and transmitted by C afferent fibers, noxious heat is detected by Ruffini corpuscles and transmitted by Aδ and C afferent fibers, and noxious cold is detected by Krause corpuscles and transmitted by C afferent fibers1,2. QST can be used to detect both inhibition (decreased sensitivity, hypoesthesia) and facilitation (increased sensitivity, hyperesthesia) of these receptors and pathways. In dogs, QST has been used to evaluate alterations in sensory thresholds secondary to acute spinal cord injury3,4,5, Chiari-like malformation and syringomyelia6, cranial cruciate ligament rupture5,7, and osteoarthritis (OA)8,9,10. Additionally, some studies have used QST to assess pain alleviation provided by certain analgesics6,11,12,13 and surgical procedures14. These studies have provided important insights into the mechanisms of pain sensation in dogs, such as evidence for peripheral and central sensitization after surgery and diseases causing chronic pain states such as cranial cruciate ligament rupture and OA. This information can help improve the detection and treatment of pain in dogs.
Validation studies of mechanical and hot thermal QST in dogs have shown good feasibility, repeatability, and reliability of QST results over time in normal dogs and dogs with chronic pain from OA8,9,15,16. However, several studies have found poor repeatability and reliability of cold thermal and occasionally von Frey QST1,15,17. These studies used different equipment and methodology but provided evidence that mechanical and hot thermal QST is an accurate, semi-quantitative method of measuring sensory thresholds in dogs. However, attention to precise details, including the setting of the measurements, is critical to optimizing QST in dogs, necessitating a standardized protocol for QST. Sanchis-Mora et al. detailed a sensory threshold examination protocol (STEP) for mechanical and hot and cold thermal QST but encountered difficulty with dogs not responding to the cold thermal QST or the highest gram force von Frey filament used in the study17. The following protocol provides a standard method for mechanical and hot thermal QST in dogs; this protocol can assess sensory thresholds in normal dogs or dogs with various disease processes affecting the somatosensory system. The development of standardized protocols may allow for comparing results across studies and meta-analyses of data to improve the utility of QST in veterinary medicine.
Protocol
All procedures were approved by the Institutional Animal Care and Use Committee of North Carolina State University.
1. Room set-up and study subject acclimatization
- Perform QST in a dedicated space where there is ample room for a QST operator, handler, and dog of any size to move about comfortably. Minimize potential auditory and visual distractions and use a white noise machine to block out ambient sound.
- Place a large yoga mat or similar padding on the floor to ensure that the dogs are comfortable in lateral recumbency during testing.
- Allow the dog at least 10 min to freely explore and acclimate to the room and become comfortable with the QST operator and handler. Offer fresh water ad libitum in the room, and give occasional food rewards.
- Randomize the testing site (left or right side) by a coin flip. Clip an approximately 2 x 4 cm section of fur centered around the space between the dorsal surface of the third and fourth metatarsals halfway between the tarsometatarsal joint and the metatarsophalangeal joint. Clip an approximately 1 x 2 cm fur section on the lateral antebrachium just proximal to the antebrachiocarpal joint over the ulna.
2. Electronic von Frey anesthesiometer
- Instrument set-up
- Gently apply a rigid 0.9 mm von Frey tip to the load cell and ensure that the load cell is securely screwed into the handpiece. Connect the cord from the handpiece to the recording device through the M0 channel (Figure 1A,B).
- Turn on the recording device and press the MAX button so that the device will record and display the maximum force achieved when the dog responds to the applied stimulus (Figure 1C).
- Zero the instrument by pressing the CLR button.
- Data collection
- Place the dog in lateral recumbency for measuring thresholds.
NOTE: Dogs are placed in right lateral recumbency for measuring thresholds on the left limbs or placed in left lateral recumbency for measuring thresholds on the right limbs. If the dog will not willingly lay in lateral recumbency when given verbal cues, the QST operator and handler can manually lay the dog in lateral recumbency. - Apply minimal to moderate restraint as needed to maintain the dog in lateral recumbency and relatively still.
NOTE: The handler performs this step. - Apply the stimulus once the dog is calm and relaxed and the limb being tested is in at least 70% extension. Provide gentle manual support to the limb being tested to keep the limb off the floor and provide stable backing to apply force against while not preventing the dog from withdrawing the limb.
NOTE: The QST operator performs this step. - Apply the von Frey tip perpendicular to the skin of the area being tested. If the dog exhibits reflex movements (e.g., twitching of the paw or withdrawal of the limb before force is being applied) from the sensation of the von Frey tip on the skin, allow the dog to relax the limb again before reapplying the von Frey tip. Take a measurement when the skin does not cause reflex movements by applying the von Frey tip.
- Apply steadily increasing force with the von Frey tip (~20 g/sec) until the dog withdraws the limb, vocalizes, turns to look at the stimulus, or exhibits other movements or behavioral responses that indicate the conscious perception of the stimulus. Remove the stimulus when the dog withdraws the limb, or the maximum force is reached.
NOTE: Do not exceed 1,000 g of force. - Record the maximum force applied that is displayed on the recording device.
NOTE: If the safety cut-off of 1,000 g of force is reached, 1,000 g is recorded as the sensory threshold, and it is noted that there was no response before the safety cut-off. - Repeat the measurements for a total of five trials, allowing 1 min between each measurement (inter-trial interval). Zero the instrument between each step by pressing the CLR button.
- Allow the dog to remain in lateral recumbency during the inter-trial intervals if they remain relatively calm and relaxed with no or minimal restraint. Otherwise, allow the dog to sit, stand, or move about the QST room to maintain their comfort. Place the dog back in lateral recumbency before the subsequent measurement.
- Record a feasibility score of 0-5 to indicate the ease with which the data was collected.
NOTE: Feasibility scores are as follows: 0 = no problem, 1 = mild difficulty, 2 = moderate difficulty, 3 = significant difficulty, 4 = extremely difficult, 5 = impossible. The rubric used for assigning feasibility scores is provided in Table 1. - Give the dog a 5 min break before starting measurements with the blunt probed pressure algometer.
- Place the dog in lateral recumbency for measuring thresholds.
3. Blunt probed pressure algometer
- Instrument set-up
- Ensure that the small blunt probe is securely screwed into the device (Figure 2A).
- Turn on the recording device and press the MAX button to continue when prompted on the screen. Press the UNIT button until the unit is displayed as grams (g) at the top of the screen (Figure 2B).
- Zero the instrument by pressing the ZERO button.
- Data collection
- Place the dog in lateral recumbency for measuring thresholds.
NOTE: Dogs are placed in right lateral recumbency for measuring thresholds on the left limbs or placed in left lateral recumbency for measuring thresholds on the right limbs. If the dog will not willingly lay in lateral recumbency when given verbal cues, the QST operator and handler can manually lay the dog in lateral recumbency. - Apply minimal to moderate restraint as needed to maintain the dog in lateral recumbency and relatively still.
NOTE: The handler performs this step. - Apply the stimulus once the dog is calm and relaxed and the limb being tested is at approximately 70% extension. Provide gentle manual support to the limb being tested to keep the limb off the floor and provide stable backing to apply force against, while not preventing the dog from withdrawing the limb.
NOTE: The QST operator performs this step. - Apply the blunt probe perpendicular to the skin of the area being tested (Figure 2C). If the dog exhibits reflex movements (e.g., twitching of the paw or withdrawal of the limb before force is being applied) from the sensation of the blunt probe on the skin, allow the dog to relax the limb again before reapplying the blunt probe. Take a measurement when the application of the blunt probe to the skin does not cause reflex movements.
- Apply steadily increasing force with the probe (~20 g/s) until the dog withdraws the limb, vocalizes, turns to look at the stimulus, or exhibits other movements or behavioral responses that indicate the conscious perception of the stimulus. Remove the stimulus when the dog withdraws the limb or the maximum force is reached.
NOTE: Do not exceed 2,500 g of force. - Record the maximum force applied that is displayed on the recording device.
NOTE: If the safety cut-off of 2,500 g of force is reached, 2,500 g is recorded as the sensory threshold, and it is noted that there was no response before the safety cut-off. - Repeat the measurements for a total of five trials, allowing 1 min between each measure (inter-trial interval). Zero the instrument between each step by pressing the ZERO button.
- Allow the dog to remain in lateral recumbency during the inter-trial interval if they remain relatively calm and relaxed with no or minimal restraint. Otherwise, allow the dog to sit, stand, or move about the QST room to maintain their comfort. Place the dog back in lateral recumbency before the subsequent measurement.
- Record a feasibility score of 0-5 to indicate the ease with which the data was collected.
NOTE: Feasibility scores are as follows: 0 = no problem, 1 = mild difficulty, 2 = moderate difficulty, 3 = significant difficulty, 4 = extremely difficult, 5 = impossible. - Give the dog a 5 min break before starting measurements with the hot thermal probe.
- Place the dog in lateral recumbency for measuring thresholds.
4. Hot thermal probe
- Instrument set-up
- Connect the thermosensory analyzer to the computer via the USB cable and ensure that the 16 x 16 mm thermode is connected to the analyzer. Turn on the analyzer.
- Open the thermosensory analyzer software on the computer and select the TSA II analyzer from the startup menu. Click on OK on the pop-up warning for the analyzer self-test. Ensure that the thermode is not connected to the study subject during the self-test.
- In the TEST tab (upper right-hand corner), under the Select Patient prompt (left-hand side of the screen), select the appropriate patient by double-clicking on the name in the list.
- To create a new patient, click on the PATIENTS tab to the right of the TEST tab. Click on the New Patient icon in the lower left-hand corner and fill in the patient details (department, patient first and last name, ID, gender, and date of birth).
- Under the Select Program prompt in the TEST tab, select the appropriate program by double-clicking on the program in the list.
- To create a new program, single-click on the PROGRAMS tab to the right of the PATIENTS tab. Click on the New Program icon in the lower left-hand corner and fill in the program details.
NOTE: For this protocol, the program details are given in Table 2. A body site does not need to be selected under the Select Body Site prompt in the TEST tab.
- To create a new program, single-click on the PROGRAMS tab to the right of the PATIENTS tab. Click on the New Program icon in the lower left-hand corner and fill in the program details.
- Once the appropriate patient and program have been selected, click on the Go to Test prompt under the TEST tab. Single click on the Pre-Test button in the lower left-hand corner to calibrate the analyzer to the specified program.
- Once the Pre-Test is complete, the Pre-Test button is replaced by the Start button, and the test window displays: Press Start button to start the test (Figure 3A).
- Unwind the thermode cable and ensure that the thermode is easily accessible.
- Data collection
- Place the dog in lateral recumbency for measuring thermal latency.
NOTE: Dogs are placed in right lateral recumbency for measuring thresholds on the left limbs or placed in left lateral recumbency for measuring thresholds on the right limbs. If the dog will not willingly lay in lateral recumbency when given verbal cues, the QST operator and handler can manually lay the dog in lateral recumbency. - Apply minimal to moderate restraint as needed to maintain the dog in lateral recumbency and relatively still.
NOTE: The handler performs this step. - Apply the stimulus once the dog is calm and relaxed and the limb being tested is in approximately 70% extension. Provide gentle manual support to the limb being tested to keep the limb off the floor while not preventing the dog from withdrawing the limb. Also, hold and operate a stopwatch with the hand supporting the limb.
NOTE: The QST operator performs this step. - Apply the thermode to the skin of the area being tested (Figure 3B). If the dog exhibits reflex movements (e.g., twitching of the paw or withdrawal of the limb before heat is being applied) from the sensation of the thermode on the skin, allow the dog to relax the limb again before reapplying the thermode. Take a measurement when the application of the thermode to the skin does not cause reflex movements.
- Click on the Start button in the lower left-hand corner of the TEST tab to start the test.
NOTE: The QST operator signals to the handler to start the test (e.g., by nodding their head), and the QST operator simultaneously starts the stopwatch. - Remove the thermode when the dog withdraws the limb, vocalizes, turns to look at the stimulus, or exhibits other movements or behavioral responses that indicate the conscious perception of the stimulus or when the maximum latency is reached while simultaneously stopping the stopwatch.
NOTE: The QST operator performs this step. Do not exceed 20 s of thermode application or 49 °C of maximum thermode temperature. - Record the latency to withdrawal. If the safety cut-off of 20 s of thermode application is reached, record 20 s as the sensory latency, and note that there was no response before the safety cut-off.
- Repeat the measurements for a total of five trials, allowing 1 min between each measure (inter-trial interval). Click on the End Test button, and then the Pre-Test button between each measurement to stop heating the thermode and recalibrate the thermode to prepare for the next application.
NOTE: The handler performs this step.- Allow the dog to remain in lateral recumbency during the inter-trial interval if they remain relatively calm and relaxed with no or minimal restraint. Otherwise, allow the dog to sit, stand, or move about the QST room to maintain their comfort. Place the dog back in lateral recumbency before the subsequent measurement.
- Record a feasibility score of 0-5 to indicate the ease with which the data was collected.
NOTE: Feasibility scores are as follows: 0 = no problem, 1 = mild difficulty, 2 = moderate difficulty, 3 = significant difficulty, 4 = extremely difficult, 5 = impossible.
- Place the dog in lateral recumbency for measuring thermal latency.
Representative Results
Mechanical and thermal QST has been performed to detect sensory thresholds in both research and client-owned dogs under various clinical conditions, including normal, healthy dogs, dogs with chronically painful conditions such as OA, dogs with acute spinal cord injury, and to assess post-operative pain and effectiveness of analgesics. Though there is a growing body of work on QST in dogs, no normal range of values has been established for any testing modalities. However, several studies have assessed the feasibility and repeatability of mechanical and thermal QST in dogs, showing QST data as accurate measurements of sensory thresholds in dogs8,9,15,16.
The values reported here are from a previously published data set of 23 normal dogs who were older than 2 years of age, weighed greater than 15 kg, had no abnormalities detected on orthopedic and neurologic examination, and had no history of impairment reported by the owner10. This group of dogs included 8 mixed breed dogs, 4 Labrador retrievers, 6 golden retrievers, and 1 each of: American Staffordshire terrier, Australian cattle dog, otterhound, Australian shepherd dog, and German shorthaired pointer. Mechanical and hot thermal QST data from these dogs, which represent typical data obtained for QST in dogs, are summarized in Table 3 and graphically represented in Figure 4, Figure 5, and Figure 6. To obtain the average QST value for each modality in each dog, based on previous work and analysis of the replicate effect16, we recommend that the dog's highest and lowest values from the five trials of the QST modality are eliminated, and the remaining three values are averaged. The original study used a repeated-measures mixed-effects models to determine the influence of covariates, including age, sex, body weight, and feasibility score. Then, the association between covariates and the QST threshold was evaluated using Wald tests10. This analysis showed no significant effect of age, sex, and feasibility score on the values of any of the QST modalities (p > 0.05) and a substantial impact of body weight on the values of hot thermal QST (p = 0.006), but neither of the other two modalities. There were not enough dogs of any breed to assess the effect of breed on the QST values.
When interpreting mechanical and thermal QST data, lower pressure thresholds and shorter latency times indicate greater sensitivity to the applied stimulus, while higher pressure thresholds and longer latency times indicate less sensitivity. A variety of clinical conditions have been shown to affect sensory thresholds in dogs. Though there is some inconsistency in the data, most studies report lower sensory thresholds (greater sensitivity, hyperalgesia) in dogs with OA both at the primary site of the joint(s) affected by OA and at secondary sites distant to the affected joint(s)8,9,10. All studies that have assessed sensory thresholds in dogs with acute thoracolumbar spinal cord injury report higher sensory thresholds (decreased sensitivity, hypoalgesia) in the pelvic limbs of these dogs3,4,5. Studies assessing post-operative pain in dogs undergoing ovariohysterectomy have indicated lower sensory thresholds at the surgical site and at a distant secondary site in the pelvic limbs (distal tibia) that were alleviated by pre-and post-operative administration of analgesic medications11,12. Thus, the population of dogs being assessed and their medical history, including the chronicity of pain and administration of analgesic medications, should be considered when determining expected results and interpreting data.
Feasibility scores are used to indicate the ease with which QST data were obtained from each subject for each testing modality. Feasibility scores are assigned based on a 6-point scale (0-5). They are determined based on the dog's level of cooperation with testing, the amount of restraint needed to accomplish testing, and the clarity of the dog's reaction to the applied stimuli (Table 1). Increasing scores on the feasibility scale indicate the increasing difficulty of data collection, with scores of 0-2 considered easy data collection and 3-5 considered difficult data collection. Mechanical and hot thermal QST is generally well-tolerated in dogs. Studies have reported feasibility scores to show that most dogs have feasibility scores indicating easy data collection8,10,15. Feasibility scores also indicate the quality of data collected, as dogs who require significant restraint, are not cooperative, are sensitive to their feet being touched, or who have unclear or inconsistent reactions to the applied stimuli decrease the QST operator's confidence that the data collected truly represent the dog's sensory thresholds (versus being an indication of the dog's reaction to these factors).
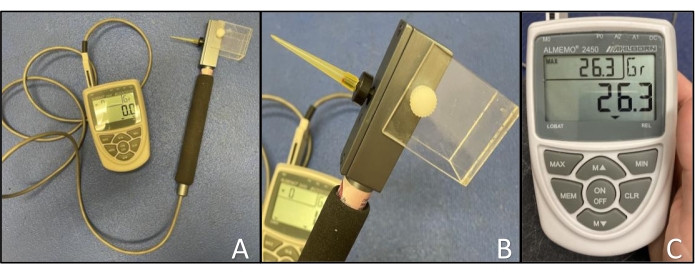
Figure 1: Electronic von Frey anesthesiometer. (A) Device set-up showing the rigid von Frey tip applied to the load cell and the cord from the handpiece connected to the recording device through the M0 channel. (B) Close-up of the von Frey tip attached to the load cell. (C) Close-up of the recording device showing the arrangement of buttons and displaying the current force (center), maximum force (upper left), and units (upper right). Please click here to view a larger version of this figure.
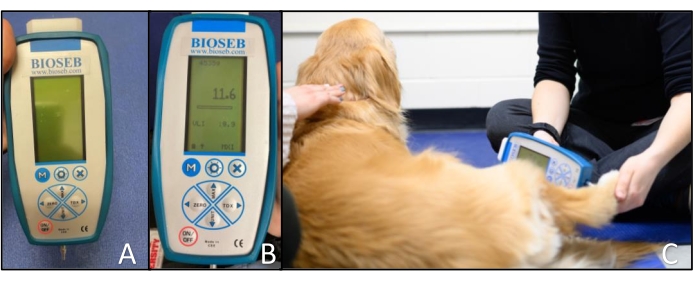
Figure 2: Blunt probed pressure algometer. (A) Device set-up showing the small blunt probe attached to the recording device. (B) Close-up of the recording device showing the arrangement of buttons and displaying the maximum force (center) and units (top). (C) Application of the blunt probed pressure algometer to the dorsal metatarsal region of a dog. The tip is applied perpendicular to the skin. Please click here to view a larger version of this figure.
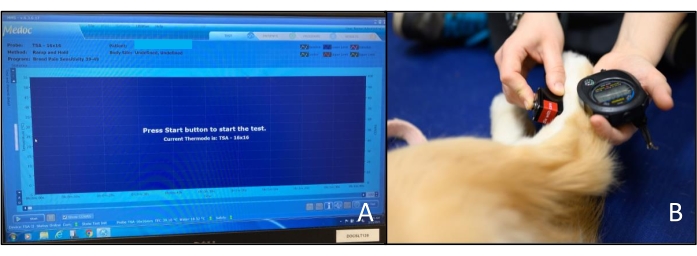
Figure 3: Hot thermosensory analyzer. (A) Computer screen display when the analyzer is ready to start a test. The Start button is in the lower-left corner of the screen. (B) Application of the thermode to the dorsal metatarsal region of a dog. The QST operator also operates the stopwatch with the hand supporting the limb. Please click here to view a larger version of this figure.
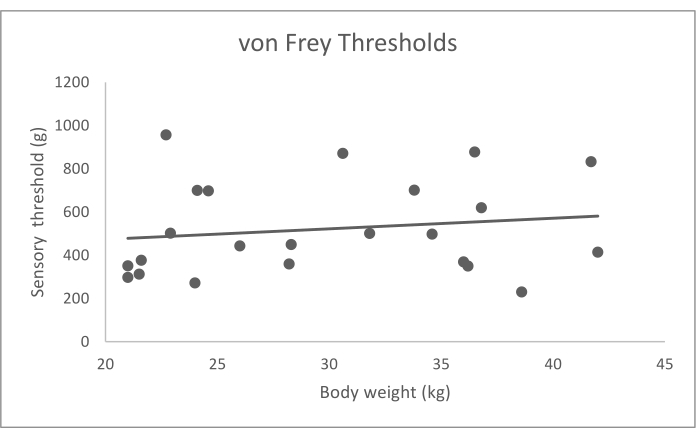
Figure 4: Electronic von Frey anesthesiometer sensory thresholds (g) data by body weight (kg). Bodyweight did not have a significant effect on sensory thresholds (p = 0.905). Please click here to view a larger version of this figure.
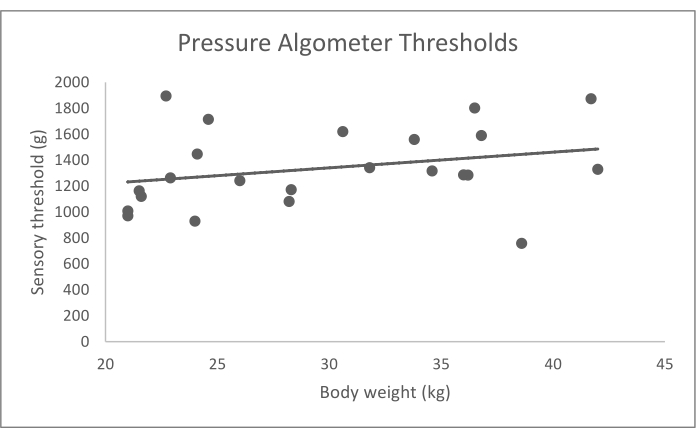
Figure 5: Data for blunt probed pressure algometer sensory thresholds (g) by body weight (kg). Bodyweight did not have a significant effect on sensory thresholds (p = 0.734). Please click here to view a larger version of this figure.
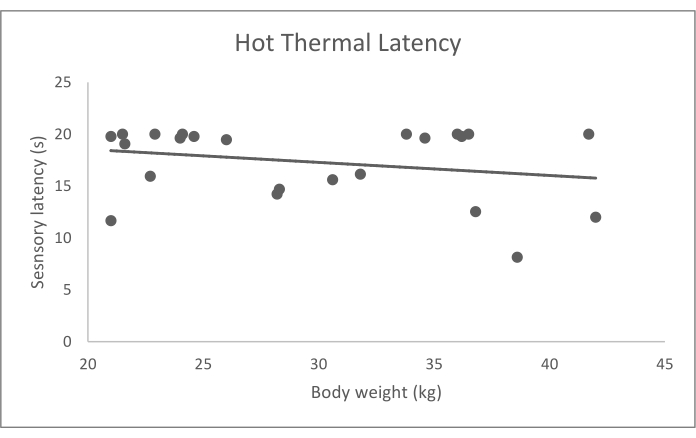
Figure 6: Hot thermal probe sensory latency (s) data by body weight (kg). Bodyweight had a significant effect on sensory latency (p = 0.006). Please click here to view a larger version of this figure.
| Feasibility score | Description | |
| 0 – No problem | Minimum restraint needed; excellent cooperation; clear reaction to stimuli | |
| 1 – Mild difficulty | Mild restraint needed; good cooperation; clear reaction to stimuli | |
| 2 – Moderate difficulty | Moderate restraint needed; good cooperation > 50% of the time; mild sensitivity of feet being touched; mild variation in reaction to stimuli | |
| 3 – Significant difficulty | Significant restraint needed and resisted lateral recumbency; good cooperation < 25% of the time; moderate sensitivity to feet being touched; moderate variation in reaction to stimuli | |
| 4 – Extreme difficulty | Constant restraint required; not cooperative; unclear reaction to stimuli, not confident in data collected |
|
| 5 – Impossible | Could not collect data due to the dog's disposition and/or lack of confidence in the reactions seen being due to the stimulus | |
Table 1: QST feasibility scoring rubric. Rubric used for evaluation of the ease with which mechanical and thermal QST data can be collected from dogs. Feasibility scores range from 0 = no problem to 5 = impossible.
| Parameters | Input |
| Method | Ramp and hold |
| Sequence | 1 |
| Baseline | 39 |
| Time Before Sequence (s) | 0 |
| Trigger | Auto |
| Destination Temperature (°C) | 49 |
| Deatinaytion Rate | 8 |
| Destination criterion | Temperature |
| Duration time (s) | 30 |
| Return option | Baseline |
| Return Rate | 1 |
| Number of Trials | 1 |
Table 2: Program details for the hot thermal probe.
| Average ± SD | Range | |
| Electronic von Frey (g) | 521.1 ± 216.8 | 230.2 – 957.1 |
| Pressure algometer (g) | 1338.0 ± 308.6 | 758.9 – 1894.0 |
| Hot thermal probe (s) | 17.31 ± 3.55 | 8.13 – 20 |
Table 3: Average and range of values of mechanical and hot thermal QST results in 23 normal dogs. The highest and lowest values of the five trials from each modality were excluded, and then the values of the remaining three trials were averaged for each dog. The overall average, standard deviation, and range were calculated from these individual averages. Thresholds for the von Frey and pressure algometer are reported in grams (g), and latency for the hot thermode is reported in seconds (s). All measurements were taken at the dorsal metatarsal region.
Discussion
It is crucial to the acquisition of accurate data – that reflects the dog's sensory thresholds – that the dog is as calm, relaxed, and positioned adequately as possible for each measurement. A previous study noted that agitation from restraint or distraction from factors within or outside the testing environment affected dogs' responses to the QST stimuli16. If the dog becomes agitated from recumbency or restraint or is distracted, the dog should be given time to settle before a measurement is taken; dogs who do not settle quickly should be given a short break from the testing procedure. Dogs who are overly anxious or become stressed from the testing procedure may exhibit stress-induced analgesia, causing a false increase in measured sensory threshold9. Anxious or stressed dogs may also become overly reactive to the stimuli or the testing procedure. They may appear to have decreased sensory thresholds. However, this is likely from the dog reacting to the stimulus's presence or the QST operator's actions instead of a noxious sensation from the stimulus. If a dog becomes anxious or stressed, the testing procedure should be terminated. For dogs who rest their limb flexibly or have muscle tension, the QST operator can gently extend the limb and hold it in extension until the dog relaxes, allowing for a more consistent withdrawal response. For dogs who exhibit reflex movements upon contact of the probes or thermode to the skin, the operator can briefly touch or very gently rub the skin of the testing area before contact or apply continuous contact of the probe or thermode without using force or heat to desensitize the skin to touch. Any touch or rubbing should be light and brief to prevent adaptation of the deeper sensory receptors being tested.
The QST operator and handler should be comfortable with handling and restraining dogs and familiar with their behavioral responses to potentially painful stimuli to acquire quality data and maintain the safety of the investigators and study subjects. Ideally, the QST operator and handler should be the same people for the duration of the collection of a data set to maintain consistency in the data. However, the effect of different handlers has not been studied. QST is a psychophysical method of sensory threshold measurement and requires observation of behavioral responses to determine the endpoint of testing in non-verbal species1. In addition to withdrawal of the limb, dogs may exhibit vocalization, turning to look directly at the stimulus, or other movements that indicate the conscious perception of the stimulus10. Each dog's behavioral response to the QST stimuli should be observed closely to determine the endpoint for measurements. In the authors' experience, a small proportion of dogs will exhibit extreme reactions to the QST stimuli, including attempting to bite, even when they appeared calm and relaxed before applying the stimulus. The operator and handler should always be aware of the dog's behavior and anxiety level to ensure safe testing. The testing procedure should be terminated if a dog exhibits potentially dangerous behavior.
The testing sites described in this protocol were selected because they are areas where differences in sensory thresholds can be detected for various clinical conditions, including spinal cord injury3,4,5, cranial cruciate ligament rupture7, and osteoarthritis10,16. In addition, several studies have shown generally good feasibility of QST testing of sites in the distal limbs8,9,15,16. Using the same testing sites also allows for a better comparison of results between studies. Though most reports of QST in dogs have the dogs positioned in lateral recumbency, several studies have been conducted with the dogs standing or in other positions dictated by the dog as needed for their clinical condition3,7,8,9. Dogs may become stressed from the restraint required to keep them in lateral recumbency, and some dogs refuse to lay in lateral recumbency altogether. These dogs can be allowed to adopt alternative positions, such as sternal recumbency with the hips in lateral recumbency or standing, to reduce stress to the dog and produce consistent responses to the stimuli. Whether or not different positioning affects the response to QST stimuli or the sensory threshold or latency has not been reported.
Performing QST in dogs presents unique challenges that give rise to some limitations in the method. When measuring sensory thresholds in dogs and other non-verbal species, determining the endpoint of testing relies on the operator's observation of a behavioral response that they judge to indicate the conscious perception of the stimulus. In humans, differentiation of the thresholds of the first detection of the stimulus, first sensation of pain, and maximum tolerable pain can be made by verbal report1. It is unknown at what intensity of the stimulus that dogs respond and it is likely that different dogs will respond to varying levels of perceived stimulus intensity. Additionally, some dogs may react to the touch or constant contact of the probe rather than the intensity of the stimulus producing a noxious sensation. In humans, cognitive factors including attention, motivation, and cognitive impairment have been found to affect QST thresholds18, and similar factors may likely affect results in dogs. The characteristics of the study population should be considered when interpreting QST results and determining factors that may alter those results.
Several studies have reported good feasibility and repeatability using various equipment and methodology for mechanical and hot thermal QST in dogs8,9,15,16,17. Results in these studies suggest that pressure algometers may produce the most consistent results of the QST modalities. Most recent studies have used an electronic von Frey anesthesiometer that measures over a continuous range of force, making more accurate and precise measurements than the graduated measurements of von Frey filaments. However, no direct comparison of the two methods has been performed in dogs1. A variety of equipment has been used to assess sensory heat thresholds in dogs. Equipment utilizing constant intensity or ramped heat stimuli, handheld thermodes, and an apparatus in which dogs stood on a glass plate heated by a light source have shown good feasibility and repeatability. However, each method has its limitations8,9,16. Some studies have found prolonged latency to respond to cold thermal stimuli in normal dogs1,15,17, who often reach the safety cut-off time without responding, and report more significant variance and lower feasibility of cold thermal QST, all findings that mirror those in human studies19. These factors may limit the usefulness of the cold thermal modality in QST testing in dogs. Therefore, a protocol for cold thermal testing was not detailed here.
Though many QST studies have been performed in dogs to establish the validity of the method and compare the sensory thresholds of normal dogs and dogs with various disease states, no study to date has aimed to establish a normative data range of QST values for dogs. Most studies have had small sample sizes of normal dogs, making it difficult to determine whether characteristics of the dogs-such as body weight, age, sex, or breed-have a significant effect on sensory thresholds. Additionally, the methodology has significantly varied, making it difficult to compare and contrast different studies and has made it impossible to combine data. Large-scale studies of diverse populations of normal dogs are warranted to establish normative ranges of QST values and to elucidate better what factors affect sensory thresholds in normal dogs. Such studies should be performed using standardized, well-described, repeatable protocols for data collection. Establishing these baseline data will help better understand how sensory thresholds are affected by different disease states in dogs.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Andrea Thomson, Jon Hash, Hope Woods, and Autumn Anthony for handling dogs for QST, Masataka Enomoto for his help screening dogs, and Sam Chiu for his contributions to establishing the protocol for hot thermal QST.
Materials
| Electronic von Frey anesthesiometer | IITC Life Science Inc. | Item # 23931 | Custom made with a 1000g max force load cell |
| Medoc Main Station software | Medoc | (supplied with TSA-II) | |
| SMALGO: SMall Animal ALGOmeter | Bioseb | Model VETALGO | |
| TSA-II NeuroSensory Analyzer | Medoc | DC 00072 TSA-II | No longer manufactured – new model is TSA-2 with same probes and same function |
References
- Hunt, J., Knazovicky, D., Lascelles, B. D. X., Murrell, J. Quantitative sensory testing in dogs with painful disease: A window to pain mechanisms. The Veterinary Journal. 243, 33-41 (2019).
- Purves, D., et al. Cutaneous and subcutaneous somatic sensory receptors. Neuroscience, 2nd edition. , (2001).
- Gorney, A. M., et al. Mechanical and thermal sensory testing in normal chondrodystrophic dogs and dogs with spinal cord injury caused by thoracolumbar intervertebral disc herniations. Journal of Veterinary Internal Medicine. 30 (2), 627-635 (2016).
- Song, R. B., et al. von Frey anesthesiometry to assess sensory impairment after acute spinal cord injury caused by thoracolumbar intervertebral disc extrusion in dogs. The Veterinary Journal. 209, 144-149 (2016).
- Moore, S. A., Hettlich, B. F., Waln, A. The use of an electronic von Frey device for evaluation of sensory threshold in neurologically normal dogs and those with acute spinal cord injury. The Veterinary Journal. 197 (2), 216-219 (2013).
- Sanchis-Mora, S., et al. Pregabalin for the treatment of syringomyelia-associated neuropathic pain in dogs: A randomized, placebo-controlled, double-masked clinical trial. The Veterinary Journal. 250, 55-62 (2019).
- Brydges, N. M., et al. Clinical assessments of increased sensory sensitivity in dogs with cranial cruciate ligament rupture. The Veterinary Journal. 193 (2), 546-550 (2012).
- Williams, M. D., et al. Feasibility and repeatability of thermal quantitative sensory testing in normal dogs and dogs with hind limb osteoarthritis-associated pain. The Veterinary Journal. 199, 63-67 (2014).
- Freire, M., Knazovicky, D., Case, B., Thomson, A., Lascelles, B. D. X. Comparison of thermal and mechanical quantitative sensory testing in client-owned dogs with chronic naturally occurring pain and normal dogs. The Veterinary Journal. 210, 95-97 (2016).
- Knazovicky, D., et al. Widespread somatosensory sensitivity in naturally occurring canine model of osteoarthritis. Pain. 157 (6), 1325-1332 (2016).
- Lascelles, B. D. X., Cripps, P. J., Jones, A., Waterman, A. E. Post-operative central hypersensitivity and pain: The pre-emptive value of pethidine for ovariohysterectomy. Pain. 73 (3), 461-471 (1997).
- Slingsby, L. S., Waterman-Pearson, A. E. The post-operative analgesic effects of ketamine after canine ovariohysterectomy – a comparison between pre- or post-operative administration. Research in Veterinary Medicine. 69 (2), 147-152 (2000).
- Sammarco, J. L., et al. Post-operative analgesia for stifle surgery: A comparison of intra-articular bupivacaine, morphine, or saline. Veterinary Surgery. 25 (1), 59-69 (1996).
- Tomas, A., Marcellin-Little, D. J., Roe, S. C., Motsinger-Reif, A., Lascelles, B. D. X. Relationship between mechanical thresholds and limb use in dogs with coxofemoral joint OA-associated pain and the modulating effects of pain alleviation from total hip replacement on mechanical thresholds. Veterinary Surgery. 43 (5), 542-548 (2014).
- Briley, J. D., Williams, M. D., Freire, M., Griffith, E. H., Lascelles, B. D. X. Feasibility and repeatability of cold and mechanical quantitative sensory testing in normal dogs. The Veterinary Journal. 199 (2), 246-250 (2014).
- Knazovicky, D., et al. Replicate effects and test-retest reliability of quantitative sensory threshold testing in dogs with and without chronic pain. Veterinary Anesthesia and Analgesia. 44 (3), 615-624 (2017).
- Sanchis-Mora, S., et al. Development and initial validation of a sensory threshold examination protocol (STEP) for phenotyping canine pain syndromes. Veterinary Anesthesia and Analgesia. 44 (3), 600-614 (2017).
- Backonja, M., et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 154 (9), 1807-1819 (2013).
- Wylde, V., Palmer, S., Learmonth, I. D., Dieppe, P. Somatosensory abnormalities in knee OA. Rheumatology. 51 (3), 535-543 (2012).

