Cell-Free Scaled Production and Adjuvant Addition to a Recombinant Major Outer Membrane Protein from Chlamydia muridarum for Vaccine Development
Summary
This protocol describes using commercial, cell-free protein expression kits to produce membrane proteins supported in nanodisc that can be used as antigens in subunit vaccines.
Abstract
Subunit vaccines offer advantages over more traditional inactivated or attenuated whole-cell-derived vaccines in safety, stability, and standard manufacturing. To achieve an effective protein-based subunit vaccine, the protein antigen often needs to adopt a native-like conformation. This is particularly important for pathogen-surface antigens that are membrane-bound proteins. Cell-free methods have been successfully used to produce correctly folded functional membrane protein through the co-translation of nanolipoprotein particles (NLPs), commonly known as nanodiscs.
This strategy can be used to produce subunit vaccines consisting of membrane proteins in a lipid-bound environment. However, cell-free protein production is often limited to small scale (<1 mL). The amount of protein produced in small-scale production runs is usually sufficient for biochemical and biophysical studies. However, the cell-free process needs to be scaled up, optimized, and carefully tested to obtain enough protein for vaccine studies in animal models. Other processes involved in vaccine production, such as purification, adjuvant addition, and lyophilization, need to be optimized in parallel. This paper reports the development of a scaled-up protocol to express, purify, and formulate a membrane-bound protein subunit vaccine.
Scaled-up cell-free reactions require optimization of plasmid concentrations and ratios when using multiple plasmid expression vectors, lipid selection, and adjuvant addition for high-level production of formulated nanolipoprotein particles. The method is demonstrated here with the expression of a chlamydial major outer membrane protein (MOMP) but may be widely applied to other membrane protein antigens. Antigen effectiveness can be evaluated in vivo through immunization studies to measure antibody production, as demonstrated here.
Introduction
Prokaryotic or eukaryotic lysates for cell-free expression of proteins are readily available as commercial products for synthesizing proteins of interest (for a complete review, see 1). These expression systems are available at various scales and utilize lysates from various organisms, including E. coli, tobacco plants, and mammalian cultures. Cell-free lysates offer multiple benefits over traditional recombinant protein production approaches, including ease of use and robust, rapid protein production. While these approaches are primarily used to produce soluble proteins, this group has pioneered an approach for their use to express membrane proteins.
This novel approach makes minor modifications to existing cell-free expression systems by including DNA encoding two protein products for expression, an apolipoprotein and the membrane protein of interest. The expressed apolipoprotein (derivatives of either ApoA1 or ApoE4) interacts with lipids added to the cell-free lysate to spontaneously assemble (~20 nm) NLPs. When co-translated with a membrane protein of interest, the NLP and membrane protein form a soluble nanoparticle complex wherein the membrane protein is embedded within the NLP lipid bilayer. Thus, the membrane protein is more accessible for downstream applications, as it is contained within soluble, discrete particles. This approach can produce functional oligomeric protein complexes within the NLP bilayer2 and can produce the antigen component of a subunit vaccine, which is subsequently mixed with lipophilic adjuvants to form a nanoparticle vaccine featuring co-localized antigen and adjuvant suitable for in vivo assessment.
This current method is modified from a previously published protocol3. Key modifications are focused on the scale-up of the cell-free reaction and subsequent purification of the protein-NLP complex. A further modification includes the addition of an amphiphilic polymer known as a telodendrimer, which is first mixed with the lipids before addition to the cell-free reaction. Co-translation of the plasmids in the presence of the telodendrimer and the lipids produces a telodendrimer NLP (tNLP). The addition of the telodendrimer also helps modulate the size and monodispersity of the resulting tNLP nanoparticles4. This protocol is specifically optimized for large-scale vaccine studies to produce a membrane-bound subunit antigen protein, chlamydial MOMP5,6. The method produces recombinant MOMP associated with tNLP to form a highly soluble MOMP-tNLP complex that retains MOMP oligomerization. A typical 3 mL scale-up production yields >1.5 mg of purified MOMP. The cell-free produced MOMP-tNLP is amenable to rapid adjuvant addition for in vivo immunogenicity testing.
Protocol
All animal studies were performed at the University of California, Irvine, in Public Health Service (PHS)-assured facilities in accordance with the guidelines set by the Institutional Animal Care and Use Committee.
1. Glassware preparation
NOTE: All materials used in producing vaccine-grade formulations for animals are endotoxin-free.
- To destroy contaminating endotoxin, bake cleaned glassware that will hold buffers in an oven at 180 °C for 4 h.
2. Buffer preparation
- Prepare 250 mL of the Ni affinity purification buffers listed in Table 1. Store them at 4 °C for up to 6 months.
3. Reaction preparation
- Weigh out 20 mg of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) into an endotoxin-free, 1.5 mL centrifuge tube. Dissolve it in 1 mL of endotoxin-free water, probe-sonicate at least four times at 6 A for 1 min, with 1 min pauses in between, until clear. Remove any contaminant metal from the probe by centrifugation at 13,000 × g for 2 min at 22 °C and then transfer the solubilized lipid to a new 1.5 mL endotoxin-free tube.
- Weigh out 1 mg of PEG5k-CA8 telodendrimer into a 1.5 mL endotoxin-free tube. Dissolve in endotoxin-free water to a concentration of 20 mg/mL. Vortex until fully dissolved and dilute to 2 mg/mL.
- In a new endotoxin-free tube, combine 210 µL of 20 mg/mL DMPC solution with 210 µL of 2 mg/mL telodendrimer solution.
4. Cell-free production of MOMP-tNLPs for subunit vaccine formulations
- Prepare MOMP-tNLPs using cell-free methods modified from a previously published protocol5.
- Two hours before setting up the cell-free reaction, open the prokaryotic cell-free protein expression kit and thaw one of the reconstitution buffers. Once thawed, add one tablet of EDTA-free protease inhibitor cocktail and let it dissolve fully.
- Follow this protocol using a kit designed to run 5 x 1 mL reactions.
NOTE: A typical scale-up production is 3 x 1 mL.- For each 1 mL reaction, add 525 µL of reconstitution buffer to the E. coli lysate bottle and gently roll to dissolve. Add 250 µL of reconstitution buffer to the bottle containing reaction additives (e.g., ATP, GTP) and gently roll to dissolve.
- Add 8.1 mL of reconstitution buffer to the reaction feed bottle, recap with a rubber stopper (take care not to touch the inside of the rubber stopper), and invert/roll gently to dissolve.
- Add 3 mL of reconstitution buffer to the amino acid mixture bottle, recap with a rubber stopper, and invert/roll gently to dissolve.
NOTE: Take care not to touch the inside of the rubber stopper as this can lead to contamination. - Add 1.8 mL of reconstitution buffer to the methionine bottle, roll gently to dissolve, and then store on ice until use.
- Prepare the reaction solution.
- To the E. coli lysate bottle, add 225 μL of reconstituted Reaction Mix, 270 μL of reconstituted amino acid mix without methionine, and 30 μL of reconstituted methionine. Additionally, add 400 μL of the DMPC/telodendrimer mixture, 15 μg of MOMP plasmid, and 0.6 μg of Δ49ApoA1 plasmid. Roll/gently shake to mix.
NOTE: Ensure that both plasmids are constructed from the same plasmid backbone. Do not vortex. - Take 20 μL of the total solution and set it aside in a 1.5 mL tube for the GFP-expressing control reaction (see below).
- To the E. coli lysate bottle, add 225 μL of reconstituted Reaction Mix, 270 μL of reconstituted amino acid mix without methionine, and 30 μL of reconstituted methionine. Additionally, add 400 μL of the DMPC/telodendrimer mixture, 15 μg of MOMP plasmid, and 0.6 μg of Δ49ApoA1 plasmid. Roll/gently shake to mix.
- Prepare the feed solution. To the feed mix bottle, add 2.65 mL of reconstituted amino acid mix without methionine and 300 μL of reconstituted methionine. Roll/gently shake to dissolve.
NOTE: At this time, the unused reconstitution buffer and Methionine can be returned to the freezer for storage. - Transfer 1 mL of the reaction solution to the inner reaction chamber provided in the cell-free reaction kit and seal when filled. Transfer 10 mL of the feed solution to the outer chamber of the reaction vessel and seal.
NOTE: Do not overfill the chambers! The presence of air bubbles at the top of both the inner reaction chamber and the inner feed chamber will adversely affect the reaction. Any remaining reaction solution can be placed in a 1.5 mL tube and allowed to mix alongside the main vessel. - Add 0.5 μL of the GFP control plasmid (0.5 mg/mL) to the previously aliquoted 20 µL reaction mixture.
NOTE: Many kits are supplied with a control plasmid for quality-control purposes. Most GFP expressing plasmid with a T7 promoter and E.coli ribosome binding site (RBS) can also be used as the control plasmid. - Place the reaction in a shaker at 300 rpm, 30 °C for up to 18 h. To verify that the reaction was successful, use a UV light source to check for fluorescence due to the synthesis of the control GFP (Figure 1A) after as little as 15 min of incubation.
NOTE: These conditions, particularly temperature, may need to be optimized for expression of other membrane proteins.
5. MOMP-tNLP purification
- Use immobilized nickel affinity chromatography to purify the MOMP-tNLP nanoparticle complex from the cell-free reaction mixture using the His-tag on the Δ49ApoA1 protein.
- Transfer 1 mL of a 50% slurry of His-Tag Purification Resin to a disposable 10 mL chromatography column and equilibrate it with 3 mL of Binding buffer.
- Let the buffer drain, cap the outlet, and add 250 μL of Binding buffer to the resin.
- Before adding the cell-free reaction to the column, save 20 μL for later analysis by SDS-PAGE. Mix the cell-free reaction with the equilibrated resin and incubate it on a laboratory rocker at 4 °C for 1 h.
- Uncap the column, wash the cap with 500 μL of additional Binding buffer, and add this liquid to the rest of the column.
- Collect the liquid flow-through from the column for later analysis by SDS-PAGE.
- Wash the column with 1 mL of wash buffer containing 20 mM imidazole six times and collect fractions. Take care not to let the resin dry out between washes. On the second wash, vigorously agitate the resin by pipetting up and down using a 1 mL pipette.
- Elute the MOMP-tNLPs in six 300 μL fractions of Elution buffer 1 (containing 250 mM imidazole), followed by one final elution with 300 μL of Elution buffer 2 (containing 500 mM imidazole). On the second elution, vigorously agitate the resin by pipetting up and down using a 1 mL pipette.
6. Analysis by SDS-PAGE
NOTE: All elution fractions should be analyzed by SDS-PAGE to screen for quantity and purity of the protein of interest.
- Load 1 µL each of the total lysate and flow-through and then 5 µL for all collected washes and elution fractions.
- Mix aliquots of the eluted MOMP–tNLPs, washes, flow-through, and total lysate with 4x SDS-PAGE sample loading buffer. Mix and heat-denature the samples with 10x sample reducing agent unless otherwise indicated.
- Analyze the fractions by gel electrophoresis using 1.0 mm, 4 to 12%, Bis-Tris SDS-PAGE gels with 1x MES-SDS running buffer, along with an appropriate molecular weight standard. Run the gels for 35 min at 200 V.
- Stain the gels according to the manufacturer’s instructions.
- Remove the gel from the cassette and place it in 60 mL of gel stain. Microwave the gel in the gel stain for 30 s, and gently rock the container for 30 s to distribute heat evenly. Microwave the gel in the stain to 80–85 °C for another 30 s, and place the gel on an orbital shaker to rock for 5 min.
- Microwave the gel a third time for 30 s, and then return to the orbital shaker to rock for another 23 min.
- Transfer the gel to a clean container and wash in 100 mL of wash solution (10% methanol, 7% acetic acid) for 30 min.
NOTE: This is a critical step as it is essential to avoid heating the wash solution. Failure to do so can result in background staining and irregularities in the final gel image. - After washing, rinse the gel in ultrapure water twice for 5 min each.
- Image the gels using a gel imager at 600 nm (Figure 2). Use SDS-PAGE to quantify the amount of individual protein in the nanoparticle solution if there is a protein standard for comparison.
NOTE: In this example, serial dilutions of recombinantly expressed MOMP are resolved by SDS-PAGE and the densities of the bands are quantified using instrument software. - Generate a standard curve using the densities of the MOMP bands. Resolve the MOMP-tNLP samples on the same SDS-PAGE gel and calculate the MOMP component of the particles using the MOMP standard curve (Figure 3).
7. Western and dot blots and storage
- For western blotting, resolve the samples by SDS-PAGE and transfer the gels using a commercial dry blotting system with standard settings according to the manufacturer’s protocol.
- Remove the blots from the stack after the transfer is complete, and incubate each blot overnight at 4 °C in a suitable blocking buffer containing 0.2% Tween 20 and either 0.5 mg/mL MAb40 or 0.2 mg/mL MAbHIS anti-His-tag antibody directed against the His-tag from Δ49ApoA1 protein.
NOTE: The antibody dilutions used for blotting are 1:1,000 for MAb40 and 1:500–1,000 for MAbHIS antibody. - Wash each blot 3 times for 5 min with PBS-T (1x PBS, 0.2% Tween 20, pH 7.4).
- Incubate the blots for 1 h in blocking buffer containing secondary antibody conjugated to a fluorophore (e.g., IRDye) at a 1:10,000 dilution.
- Rewash the blots 3 times for 5 min with PBS-T. Use a fluorescence imager to image the blots after the final wash.
- Remove the blots from the stack after the transfer is complete, and incubate each blot overnight at 4 °C in a suitable blocking buffer containing 0.2% Tween 20 and either 0.5 mg/mL MAb40 or 0.2 mg/mL MAbHIS anti-His-tag antibody directed against the His-tag from Δ49ApoA1 protein.
- For dot blots, blot 3 μg of purified MOMP-tNLP and empty tNLP using a dot blot apparatus. Block and develop the blots using the same methods described above for western blotting.
8. Endotoxin assessment
- Quantify endotoxin levels using an endotoxin testing system based on the Limulus Amebocyte Lysate (LAL) assay. Prepare endotoxin-free 25 mM Tris, pH 7.4, sample buffer using 1 M Tris hydrochloride solution and endotoxin-free water.
NOTE: Typically, samples need to be diluted using this sample buffer, and the dilutions adjusted to find the suitable range for individual samples. Here, MOMP-tNLP samples are diluted 500-fold in sample buffer and 25 µL are loaded into each well of a device cartridge with 0.05 EU/mL sensitivity. The endotoxin levels of MOMP-tNLP and empty tNLP used in the mouse studies described below are between 0.4 to 12 EU/µg protein depending on the sample.
9. Lyophilization
- Lyophilize and store the MOMP-tNLP nanoparticles for long-term (up to years) use at -20 °C. To prepare tNLP and MOMP-tNLP suspensions for lyophilization, add trehalose as a protectant during the freezing and lyophilization process.
NOTE: This process has been extensively validated for a variety of tNLP formulations7,8. - Divide the current volume of the MOMP-tNLP solution by 9 to obtain the volume of 1 M trehalose in sterile, endotoxin-free, deionized water required to reach a final concentration of 0.1 M trehalose. Make note of the final volume and aliquot into endotoxin-free 15 mL or 50 mL polypropylene tubes as desired.
- Freeze the mixed solution on dry ice and lyophilize it overnight using a lyophilizer. Store the dried formulations at -20 °C until needed.
- Reconstitute lyophilized tNLPs using endotoxin-free water. Gently roll until the lyophilized cake is fully dissolved and rehydrated. To remove trehalose, dialyze the solution against PBS using a 3.5 kDa cutoff dialysis membrane.
10. Adjuvant addition
NOTE: These and other similar NLP-based sub-unit vaccine formulations can readily incorporate lipophilic adjuvants such as CpG-ODN1826 and FSL-1. CpG-ODN1826 is a modified Class B CpG oligonucleotide (5'-tccatgacgttcctgacgtt-3') with a full phosphorothioate backbone featuring a 5' cholesterol moiety (5'-chol-C6). The conjugation of CpG-ODN1826 to tNLPs is mediated by the hydrophobic interactions between the cholesterol moiety and the phospholipid bilayer of the tNLP and has been demonstrated and well-characterized, as previously reported9,10.
- Prior to incorporation into these formulations, purify the cholesterol-modified CpG by reversed-phase chromatography to remove contaminating endotoxin as well as any unmodified CpG molecules.
- Upon receipt from the vendor, rehydrate the lyophilized CpG material in endotoxin-free water and purify it on a preparative C4 RP-HPLC column using a separation gradient consisting of 10 mM triethylammonium acetate (TEAA) (mobile phase A) and acetonitrile (mobile phase B).
NOTE: Additional details are available in Table 2. - Pool and lyophilize the fractions containing cholesterol-modified CpG. To ensure complete removal of residual TEAA, reconstitute the CpG with 15 mL of endotoxin-free water and re-lyophilize it three times.
- After the final lyophilization, reconstitute CpG in endotoxin-free water (>20 mg/mL final CpG concentration), aliquot, and store it at -80 °C until needed. For addition to formulations, dilute the CpG to a concentration of 1–2.5 mg/mL.
NOTE: FSL-1 is available as a vaccine-grade, lyophilized powder. This is reconstituted using sterile and endotoxin-free water at a concentration of 1 mg/mL. The vaccine is administered intramuscularly (i.m.), with each dose containing 10 μg of MOMP in a total volume of 50 μL.
- Upon receipt from the vendor, rehydrate the lyophilized CpG material in endotoxin-free water and purify it on a preparative C4 RP-HPLC column using a separation gradient consisting of 10 mM triethylammonium acetate (TEAA) (mobile phase A) and acetonitrile (mobile phase B).
- To achieve the desired formulation dose, dialyze the nanoparticles into PBS and concentrate them using a centrifugal vacuum concentrator before adjuvant addition. Take care when doing this to prevent complete drying of the sample—check the sample volume every 20–30 min during centrifugation.
- Add the adjuvant under sterile conditions in a biosafety cabinet. To assess successful incorporation, analyze the final formulations and their components by analytical size-exclusion chromatography (SEC).
NOTE: For these preparations, an SEC column was used in PBS buffer (0.5 mL/m in flow rate), and elution was detected using a UV-vis diode array detector. Incorporation was assessed by comparing the absorption of the adjuvanted particles to that of the unadjuvanted particles at 214 and 280 nm. - Store the adjuvanted MOMP-tNLP and empty tNLP at 4 °C prior to animal use for a period of up to 14 days. To fully assess the stability of a new tNLP formulation, periodically analyze the stored tNLPs by SEC.
NOTE: Stability will vary from formulation to formulation.
11. Serum testing
- Obtain female 3-week-old mice (BALB/c, n = 6).
- Vaccinate the mice intramuscularly (i.m.) in each hindlimb with 10 µg of MOMP in the form of MOMP-tNLP adjuvanted with 5 µg of CpG and 1 µg FSL-1 (total volume per injection = 50 mL).
- After vaccination, observe the mice until they are able to maintain sternal recumbency.
- Four weeks after the initial vaccination (prime), vaccinate the animals a second time (boost) with 10 µg of MOMP in the form of MOMP-tNLP adjuvanted with 5 µg of CpG and 1 µg FSL-1 (total volume per injection = 50 mL).
- On day 56 after the initial vaccination, collect blood to assess antibody titers. Begin by anesthetizing the mice by injecting i.p. a solution of xylazine (0.3 mg/20 g body weight) and ketamine (3.0 mg/20 g body weight). Pinch the front and hind legs to make sure no jerking occurs. Apply petroleum jelly around the eyes to prevent eye dryness during anesthesia.
- Using a micro-hematocrit capillary tube, puncture the retro-orbital plexus. Collect 100 mL of blood in a microcentrifuge tube.
- After blood collection, observe the mice until they recover from anesthesia and can maintain sternal recumbency.
- Let the blood clot at room temperature for 30 min and then spin down at 2,000 × g for 10 min. Collect the serum and freeze at -80 °C.
- At this time, challenge the animals with Chlamydia muridarum or euthanize them. Euthanize the mice by first injecting i.p. a solution of xylazine (0.3 mg/20 g body weight) and ketamine (3.0 mg/20 g body weight) followed by cervical dislocation.
- Test serum antibodies specific for MOMP using western blotting techniques as described above. Pool mouse sera from all immunized mice and use the pooled serum in place of a primary antibody at 1:5,000 dilution.
Representative Results
The SDS-PAGE profile of the Ni affinity purification of MOMP-tNLP from a 1 mL cell-free reaction is shown in Figure 1B. The reaction resulted in high levels of expression for both the MOMP and the Δ49ApoA1 protein. Previous results showed that the cell-free expression of Δ49ApoA1 in the presence of DMPC and telodendrimer resulted in the formation of telodendrimer nanolipoprotein particles (tNLPs)4. The co-elution of MOMP with Δ49ApoA1 indicated that MOMP is associated with tNLPs, as the His-tag is only present on the tNLP scaffold Δ49ApoA1 and not on MOMP. MOMP is a highly insoluble protein that can only be eluted through complexing with tNLPs, which have been shown to facilitate the solubilization of membrane proteins.
The elution fractions containing MOMP-tNLPs were pooled and the total protein concentration determined using a fluorescence-based quantitation device, or a device that measures concentration through absorbance at 280 nm, following the manufacturer's instructions for protein quantitation. To allow for precise dosing of the MOMP vaccine, it is also important to determine the concentration of MOMP in the purified complexes. We developed a method to quantify MOMP based on gel densitometry (Figure 2) wherein a purified recombinant MOMP with known concentration was used as the standard. By establishing the standard curve and comparing it to the MOMP-tNLP sample, the MOMP concentration can be quantified accurately. The determination of MOMP concentration in the purified sample enabled the estimation of the yield of MOMP in cell-free reactions at various scales, which is important for planning the reaction setup appropriate to downstream studies (Table 3).
MOMP needs to form oligomers to elicit a robust immune response11. To test the oligomeric state of MOMP, MOMP-tNLP was analyzed in the presence and absence of both heat and the reducing agent dithiothreitol (DTT, 50 mM, Figure 3A). Higher-order oligomers of MOMP were identified through SDS-PAGE when samples were not treated with heat and DTT. In comparison, samples treated with heat in the presence of DTT showed primarily two distinct bands on the gel, corresponding to MOMP and Δ49ApoA1 (approximately 40 kDa and 22 kDa, respectively). These results closely resemble the gel banding pattern attributed to oligomer formation of MOMP, which is critical to its effectiveness.
Further western blot analysis using MAb40, an antibody against the linear epitope on the variable domain of MOMP protein, showed a similar banding pattern, confirming the oligomer formation by MOMP protein in its non-denatured state (Figure 3B). An important factor impacting MOMP oligomer formation is the ratio between the MOMP plasmid and the Δ49ApoA1 plasmid during the cell-free reaction setup. Table 4 lists the ratio of plasmids and the resulting insertion rate of MOMP into tNLPs. Previous studies indicated that chlamydial MOMP and other outer membrane proteins may exist primarily as trimers12. To maximize the trimer formation in the cell-free reaction, it is desirable to have the insertion rate close to three MOMP proteins per NLP, which corresponds to a ~25:1 MOMP-to-Δ49ApoA1 plasmid ratio.
A dot blot assay was used as a more streamlined method to detect the presence of MOMP and tNLP. The MAb40 antibody was used to detect total MOMP. The MAbHIS antibody targeted to the His-tag on the Δ49ApoA1 scaffold of the tNLP was used to assess the presence of tNLP. The co-signaling of MAb40 and MAbHIS antibodies indicated MOMP-tNLP formation. The control reaction produced empty tNLP, which only showed a positive signal from MAbHIS (Figure 3C). To test the immunogenicity of MOMP-tNLPs produced in the cell-free reaction, we adjuvanted MOMP-tNLP with CpG + FSL-1 and injected intramuscularly (i.m.) into mice in a prime-boost regimen as described above. Sera were collected from the immunized mice, and MOMP-specific IgG antibody was measured using a western blot assay (Figure 4). The sera from mice injected with adjuvanted MOMP-tNLP showed strong MOMP binding, indicating that MOMP-tNLP could elicit an immune response in vivo.
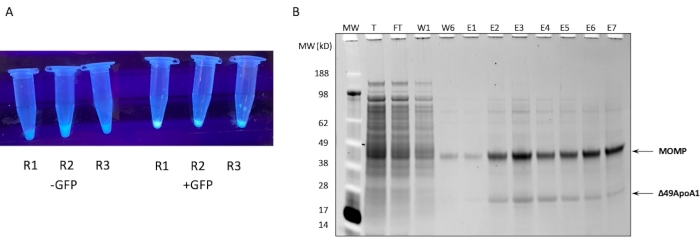
Figure 1: Expression and purification of MOMP-tNLP. (A) Image of tubes containing small aliquots of a cell-free reaction that successfully expressed GFP controls luminating under UV light source (right) compared to lysates without GFP plasmid (left). (B) Protein Gel stained with SYPRO Ruby after SDS-PAGE shows the purification profile of MOMP-tNLP. MOMP migrates at 40 kDa and the 49ApoA1 migrates at 22 kDa. Abbreviations: MOMP = chlamydial major outer membrane protein; tNLP = telodendrimer nanolipoprotein particle; MOMP-tNLP = MOMP-tNLP complex; GFP = green fluorescent protein-encoding plasmid; MW = Molecular weight marker; T = total cell-free lysate; FT = flow-through; R1-R3 = cell-free reaction aliquots; W1, W6 = Washes 1 and 6; E1-E7 = Elutions 1 through 7; Δ49ApoA1 = His-tagged mouse ApoA1 derivative. Please click here to view a larger version of this figure.
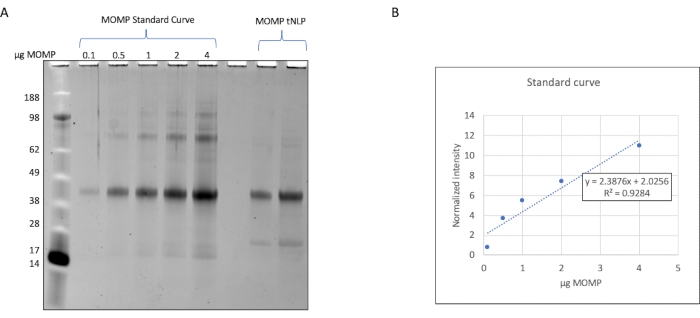
Figure 2: Quantification of MOMP in MOMP-tNLP samples. (A) SDS-PAGE gel stained with SYPRO Ruby for the quantification of MOMP. Recombinant MOMP with known concentration was loaded onto the gel to obtain the standard curve. Each lane contained 0.1 µg, 0.5 µg, 1.0 µg, 2.0 µg, and 4.0 µg of MOMP. MOMP-tNLP samples that were being quantified were loaded on the same gel. (B) The MOMP concentration standard curve was generated using densitometry. An equation relating normalized band density and the amount of MOMP was established. The equation was used to calculate the MOMP content in the unknown samples. Abbreviations: MOMP = chlamydial major outer membrane protein; tNLP = telodendrimer nanolipoprotein particle; MOMP-tNLP = MOMP-tNLP complex; SDS-PAGE = sodium dodecylsulfate polyacrylamide gel electrophoresis. Please click here to view a larger version of this figure.
Figure 3: Cell-free-produced MOMP-tNLP allow MOMP to form higher-order structures. (A) SDS-PAGE gel of MOMP-tNLP with and without treatment of heat and reducing agent DTT, stained with SYPRO Ruby. With heat and DTT, MOMP primarily appeared as a monomer band at ~40 kDa, as heat and the reducing agent broke down the majority of higher-order MOMP structure. In the absence of heat and DTT, the higher-order bands were present, indicating MOMP oligomer conformation. (B) Western blot of MOMP-tNLP and MOMP alone, untreated and treated with heat and DTT. After transfer, the membrane was probed with MAb40 (1:1,000 dilution). A banding pattern similar to the SYPRO Ruby-stained gel was observed, confirming that the higher molecular weight bands were indeed MOMP oligomers. (C) Dot blot of MOMP-tNLP and empty tNLP samples (in duplicate) probed with MAb40 and MAbHIS. Abbreviations: MOMP = chlamydial major outer membrane protein; tNLP = telodendrimer nanolipoprotein particle; MOMP-tNLP = MOMP-tNLP complex; SDS-PAGE = sodium dodecylsulfate polyacrylamide gel electrophoresis; DTT = dithiothreitol. Please click here to view a larger version of this figure.
Figure 4: Cell-free-produced MOMP-tNLP is highly immunogenic. Serum from immunized mice showed strong anti-MOMP IgG signal. MOMP-tNLP adjuvanted with CpG + FSL-1 was used to immunize mice. Sera from six immunized mice were collected, pooled, and used to probe MOMP-tNLP. The serum was able to bind to MOMP in a western blotting assay and showed strong IgG signal (left). The western blot using MAb40 as primary antibody (right) showed similar bands, indicating that the serum contained MOMP-specific IgG. Abbreviations: MOMP = chlamydial major outer membrane protein; tNLP = telodendrimer nanolipoprotein particle; MOMP-tNLP = MOMP-tNLP complex; CpG = cholesterol-modified CpG adjuvant; FSL-1 = lipophilic adjuvant. Please click here to view a larger version of this figure.
| Buffer name | NaH2PO4 | NaCl | Imidazole | pH |
| Binding Buffer | 50 mM | 300 mM | 10 mM | 8.0 |
| Wash Buffer | 50 mM | 300 mM | 20 mM | 8.0 |
| Elution Buffer 1 | 50 mM | 300 mM | 250 mM | 8.0 |
| Elution Buffer 2 | 50 mM | 300 mM | 500 mM | 8.0 |
Table 1: List of buffers needed for nickel affinity purification detailing concentrations of each component and pH.
| Runtime | 50 min |
| Flow rate | 6.0 mL/min |
| Gradient type | Binary |
| Buffer A | 10 mM TEAA in H20 |
| Buffer B | MeCN |
| Gradient | % Buffer B |
| 0 min | 25% |
| 30 min | 60% |
| 30.5 min | 100% |
| 40 min | 100% |
| 40.5 min | 25% |
| 50 min | 25% |
Table 2: Conditions for reversed phase HPLC purification of cholesterol-modified CpG. Abbreviations: TEAA = triethylammonium acetate; MeCN = acetonitrile.
| Cell-free lysate (mL) | DMPC lipid (mg) | Telodendrimer (mg) | MOMP plasmid (µg) | Purified MOMP yield (mg) |
| 1 | 4 | 0.4 | 15 | 0.5 |
| 2 | 8 | 0.8 | 30 | 1.1 |
| 3 | 12 | 1.2 | 45 | 1.6 |
| 5 | 20 | 2 | 75 | 2.7 |
Table 3: The quantity of lipids, telodendrimer, and plasmids used for differently scaled cell-free reactions and the corresponding yields. Abbreviations: MOMP = chlamydial major outer membrane protein; DMPC = 1,2-dimyristoyl-sn-glycero-3-phosphocholine.
| Ratios of plasmid input, MOMP : Δ49ApoA1 | 1:1 | 5:1 | 10:1 | 25:1 | 50:1 | 100:1 | |
| Ratios of the amount of protein produced, MOMP : Δ49ApoA1 | 0.02 | 0.32 | 0.64 | 3.46 | 6.55 | 20.04 | |
| Estimated number of MOMP insertion per tNLP | 0.03 | 0.37 | 0.75 | 4.04 | 7.65 | 23.39 | |
Table 4: The plasmid ratios in a cell-free reaction and the resulting MOMP insertion rates. Abbreviations: MOMP = chlamydial major outer membrane protein; tNLP = telodendrimer nanolipoprotein particle; Δ49ApoA1 = His-tagged mouse ApoA1 derivative.
Discussion
Chlamydia is the most common sexually transmitted infection that affects both men and women. Although vaccine research on Chlamydia spans decades, a safe and effective vaccine that can be scaled to mass production has remained elusive13. The chlamydial MOMP is considered the lead candidate as a protective vaccine antigen; however, MOMP is highly hydrophobic and prone to incorrect folding14,15. Further study has revealed that MOMP exists in oligomeric states that are essential for its immunogenicity11. Detailed here is a validated, cell-free co-expression method that produces oligomeric MOMP formed within tNLP nanoparticle as a vaccine, with yields of approximately 1.5 mg of purified MOMP per 3 mL of lysate. This fully collated procedure can be further scaled for industrial production, increasing its prospects as a useful approach for generating vaccines.
We have previously published on using cell-free expression to produce membrane proteins embedded within NLPs3,16, as well as expression into telodendrimer-stabilized discs. However, this latter technique produced membrane-protein particles with greater heterogeneity and lower solubility.4 Additionally, the immunogenicity of MOMP-telodendrimer particles is unclear compared to MOMP-tNLP particles6.
This procedure can be adapted to scale up the expression of bacterial membrane proteins that are promising candidates as antigens for use in subunit vaccines. Not only does this procedure produce solubilized bacterial membrane protein, but the overall nanoparticle structure is amenable to further modification using a variety of lipophilic vaccine adjuvants including, but not limited to, CpG conjugated to a cholesterol moiety or FSL-1. Expression of other candidate antigens from bacteria is feasible, although parameters such as expression temperature, lipid choice, and type of expression system may need to be explored to achieve optimal yields.
Additionally, plasmid choice and ratio are critical in this process. Both plasmids used should be constructed from the same backbone. If the inserts are approximately the same length, the ratios can be based on the mass of the plasmid added, as described here. However, ratioing based on moles will give more reproducible results, particularly when scaling the reactions. Ratios that work well in screen-scale reactions (< 0.5 mL) may not be applicable to larger reactions and may require additional optimization. Non-membrane proteins can still be expressed using cell-free kits but may not require the lipid nanoparticle (co-expression) to produce a soluble product. Additionally, while this protocol describes adjuvanting with CpG and FSL-1, this system is amenable to formulation with other lipophilic adjuvants or admixing with soluble adjuvants as desired.
It is essential to avoid contamination when setting up the cell-free expression reaction as this can affect yields. Any additives to the reaction, including the plasmids themselves, should be highly pure. Additionally, the expressed proteins should only be in contact with materials and solutions that are free of endotoxin contamination. Endotoxin contamination in candidate formulations can lead to inconsistent and spurious results of immunological assays and can be harmful in sufficient quantities. While not described here, additional purification following nickel affinity chromatography may be necessary if many contaminants are observed in subsequent analysis steps such as through SDS-PAGE. This could be accomplished with SEC, although conditions may require optimization on a formulation by formulation basis.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by Public Health Service grant R21 AI20925 and U19 AI144184 from the National Institute of Allergy and Infectious Diseases. This work was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344 [LLNL-JRNL-822525, LLNL-VIDEO-832788].
Materials
| 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) as powder | Avanti Polar Lipids | 850345 | |
| 1.5 mL endotoxin-free centrifuge tubes | Eppendorf | 2600028 | |
| 1 M Trizma hydrochloride solution | Millipore Sigma | T2194 | |
| Acetic acid, glacial, ACS reagent, ≥99.7% | Millipore Sigma | 695092 | |
| Bio-Dot apparatus | Bio-Rad | 1706545 | |
| Buffer Dam for XCell SureLock | Life Technologies | EI0012 | |
| C24 Incubator shaker | New Brunswick Scientific | ||
| Cell-Free Expression System: RTS 500 ProteoMaster E. coli HY Kit | BiotechRabbit | BR1400201 | |
| cOmplete His-Tag Purification Resin | Roche Molecular Diagnostics | 5893682001 | |
| cOmplete, EDTA-free Protease Inhibitor Cocktail | Roche Molecular Diagnostics | 4693132001 | |
| CpG-ODN1826 | Biosearch Technologies | T9449 | |
| D-(+)-Trehalose dihydrate | Millipore Sigma | 71509 | |
| Dialysis tubes D-Tube Dialyzer Maxi | Millipore Sigma | 71508-3 | |
| Disposable, polypropylene fritted columns 10 mL capacity | Bio-Rad | 7311550EDU | |
| Dulbecco’s Phosphate-buffered Saline (PBS) | Millipore Sigma | D8537 | |
| Electrophoresis Power Supply | |||
| Endosafe PTS cartridge | Thermo Fisher Scientific | NC9594798 | |
| Endosafe-PTS Testing System | Charles River | ||
| Gel wash solution: 10% methanol, 7% acetic acid | |||
| HCl and NaOH solutions for pH adjustment | |||
| HPLC with UV-vis diode array detector | Shimadzu | ||
| HyClone HyPure culture-grade water | VWR | 82007-328 | |
| iBlot 2 Dry Blotting System | Life Technologies | ||
| iBlot 2 Transfer Stacks, PVDF | Life Technologies | IB24001 | |
| Image Studio V2.0 software | Li-COR Biiosciences | ||
| Imidazole | Millipore Sigma | I5513 | |
| Immun-Blot PVDF Membrane | Bio-Rad | 1620177 | |
| LI-COR Odyssey Fc imager | Li-COR Biiosciences | ||
| Lyophilizer | Labconco | ||
| Methanol (≥99.9%) | Millipore Sigma | 34860 | |
| Microcentrifuge | |||
| Microwave oven | |||
| NanoDrop One/OneC Microvolume UV-Vis Spectrophotometer | Thermo Fisher Scientific | ND-ONE-W | |
| NuPAGE 4 to 12%, Bis-Tris, 1.0 mm | Life Technologies | NP0321 | |
| NuPAGE LDS Sample Buffer (4x) | Life Technologies | NP0007 | |
| NuPAGE MES SDS Running Buffer (20x) | Life Technologies | NP000202 | |
| NuPAGE Sample Reducing Agent (10x) | Life Technologies | NP0009 | |
| Odyssey Blocking Buffer in TBS containing 0.2% Tween 20 | Li-COR Biosciences | 927-50000 | |
| Orbital Shaker | |||
| PBS-T (1x PBS, 0.2% Tween 20, pH 7.4) | |||
| PEG5K-CA8 Telodendrimer (custom synthesis product) | |||
| pIVEX2.4d vector | Roche Molecular Diagnostics | ||
| Plasmid Maxi Kit | Qiagen | 12162 | |
| Primary antibody: MAb40 (monoclonal antibody to the variable domain 1 (VD1) of C. muridarum MOMP, de la Maza laboratory)4 | |||
| Primary antibody: MAbHIS, Penta-His antibody | Qiagen | 34660 | |
| Probe sonicator | |||
| Qubit 3.0 Fluorometer | Life Technologies | Q33216 | |
| Qubit Protein Assay Kit | Life Technologies | Q33212 | |
| Rainin Pipette tips: LTS 1000 µL | Rainin | 17002428 | |
| Rainin Pipette tips: LTS 20 µL | Rainin | 17002429 | |
| Rainin Pipette tips: LTS 200 µL | Rainin | 17002426 | |
| Rainin Pipettes | Rainin | ||
| Secondary antibody: IRDye 800CW goat (polyclonal) anti-mouse IgG (heavy and light) | Li-COR Biosciences | 926-32210 | |
| SeeBlue Plus2 Pre-stained Protein Standard | Life Technologies | LC5925 | |
| Sodium chloride NaCl | Millipore Sigma | S7653 | |
| Sodium phosphate monobasic NaH2PO4 | Millipore Sigma | S0751 | |
| Superdex 200, 5/150 GL column | Cytiva | GE28-9909-45 | |
| Synthetic diacylated lipoprotein-TLR2/6 FSL-1 | Invivogen | tlrl-fsl | |
| SYPRO Ruby Protein Gel Stain | Life Technologies | S12001 | |
| TWEEN 20 | Millipore Sigma | P1379 | |
| UV light source | |||
| Vacufuge Bench Top Centrifuge | Eppendorf | ||
| Vortexer | |||
| VWR 15 mL conicals (89039-666) | VWR | ||
| VWR 50 mL conicals (89039-656) | VWR | ||
| XCell SureLock Mini-Cell (Life Technologies ) | Life Technologies | EI0001 |
References
- Carlson, E. D., Gan, R., Hodgman, C. E., Jewett, M. C. Cell-free protein synthesis: applications come of age. Biotechnology Advances. 30 (5), 1185-1194 (2012).
- Coleman, M. A., et al. Expression and association of the Yersinia pestis translocon proteins, YopB and YopD, are facilitated by nanolipoprotein particles. PLoS One. 11 (3), 0150166 (2016).
- Cappuccio, J. A., et al. Cell-free co-expression of functional membrane proteins and apolipoprotein, forming soluble nanolipoprotein particles. Molecular & Cellular Proteomics: MCP. 7 (11), 2246-2253 (2008).
- He, W., et al. Controlling the diameter, monodispersity, and solubility of ApoA1 nanolipoprotein particles using telodendrimer chemistry. Protein Science. 22 (8), 1078-1086 (2013).
- He, W., et al. Cell-free production of a functional oligomeric form of a Chlamydia major outer-membrane protein (MOMP) for vaccine development. Journal of Biological Chemistry. 292 (36), 15121-15132 (2017).
- Tifrea, D. F., et al. Induction of protection in mice against a Chlamydia muridarum respiratory challenge by a vaccine formulated with the major outer membrane protein in nanolipoprotein particles. Vaccines. 9 (7), 755 (2021).
- Cleveland, T. E., et al. Small-angle X-ray and neutron scattering demonstrates that cell-free expression produces properly formed disc-shaped nanolipoprotein particles. Protein Science. 27 (3), 780-789 (2018).
- Fischer, N. O., et al. Conjugation to nickel-chelating nanolipoprotein particles increases the potency and efficacy of subunit vaccines to prevent West Nile encephalitis. Bioconjugate Chemistry. 21 (6), 1018-1022 (2010).
- Fischer, N. O., et al. Colocalized delivery of adjuvant and antigen using nanolipoprotein particles enhances the immune response to recombinant antigens. Journal of the American Chemical Society. 135 (6), 2044-2047 (2013).
- Fischer, N. O., et al. Evaluation of nanolipoprotein particles (NLPs) as an in vivo delivery platform. PLoS One. 9 (3), 93342 (2014).
- Pal, S., Peterson, E. M., de la Maza, L. M. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infection and Immunity. 73 (12), 8153-8160 (2005).
- Sun, G., et al. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. Journal of Bacteriology. 189 (17), 6222-6235 (2007).
- Hafner, L. M., Wilson, D. P., Timms, P. Development status and future prospects for a vaccine against Chlamydia trachomatis infection. Vaccine. 32 (14), 1563-1571 (2014).
- Findlay, H. E., McClafferty, H., Ashley, R. H. Surface expression, single-channel analysis and membrane topology of recombinant Chlamydia trachomatis Major Outer Membrane Protein. BMC Microbiology. 5, 5 (2005).
- Sun, G., Pal, S., Weiland, J., Peterson, E. M., de la Maza, L. M. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine. 27 (36), 5020-5025 (2009).
- He, W., et al. Cell-free expression of functional receptor tyrosine kinases. Scientific Reports. 5 (1), 12896 (2015).

