Drosophila Passive Avoidance Behavior as a New Paradigm to Study Associative Aversive Learning
Summary
This work describes a simple behavioral paradigm that allows the analysis of aversive associative learning in adult fruit flies. The method is based on suppressing the innate negative geotaxis behavior due to the association formed between a specific environmental context and an electric shock.
Abstract
This protocol describes a new paradigm for analyzing aversive associative learning in adult flies (Drosophila melanogaster). The paradigm is analogous to passive avoidance behavior in laboratory rodents in which animals learn to avoid a compartment where they have previously received an electric shock. The assay takes advantage of negative geotaxis in flies, which manifests as an urge to climb up when they are placed on a vertical surface. The setup consists of vertically oriented upper and lower compartments. On the first trial, a fly is placed into a lower compartment from where it usually exits within 3-15 s, and steps into the upper compartment where it receives an electric shock. During the second trial, 24 h later, the latency is significantly increased. At the same time, the number of shocks is decreased compared to the first trial, indicating that flies formed long-term memory about the upper compartment. The recordings of latencies and number of shocks could be performed with a tally counter and a stopwatch or with an Arduino-based simple device. To illustrate how the assay can be used, the passive avoidance behavior of D. melanogaster and D. simulans male and female were characterized here. Comparison of latencies and number of shocks revealed that both D. melanogaster and D. simulans flies efficiently learned the passive avoidance behavior. No statistical differences were observed between male and female flies. However, males were a little faster while entering the upper compartment on the first trial, while females received a slightly higher number of shocks in every retention trial. The Western diet (WD) significantly impaired learning and memory in male flies while flight exercise counterbalanced this effect. Taken together, the passive avoidance behavior in flies offers a simple and reproducible assay that could be used for studying basic mechanisms of learning and memory.
Introduction
Learning and memory is an evolutionarily ancient adaptation mechanism to the environment, conserved from Drosophila (D.) to human1. The fruit fly is a robust model organism to study fundamental principles of learning and memory as it offers a wide range of powerful genetic tools to dissect intrinsic molecular mechanisms2. The pioneering genetic screening studies, which identified rutabaga3, amnesiac4, and dunce5 genes critical for learning and memory2, took advantage of olfactory conditioning as the fruit flies rely on their keen sense of smell to find food, potential mates, and to avoid predators6.
Olfactory conditioning has become a popular paradigm to study the mechanism of learning and memory, thanks to the introduction of olfactory T-maze by Tully and Quinn7,8. Subsequently, other methods to measure various types of learning and memory have been proposed, including visual conditioning9, courtship conditioning10, aversive phototaxis suppression assay11, and wasp-exposure conditioning12. However, most of these assays have a complex setup that must be custom-built at a university workshop or purchased through a vendor. The paradigm described here is based on a simple behavioral assay to study aversive associative learning in flies that can be easily assembled with a few available supplies.
The described paradigm is equivalent to passive (or inhibitory) avoidance behavior in laboratory mice and rats in which animals learn to avoid a compartment where they have previously received electric foot shock13. In murids, the procedure is based on their innate avoidance of bright light and preference for darker areas14. On the first trial, the animal is placed into the bright compartment, from where the animal quickly exits, stepping into a dark compartment, where an electric foot shock is delivered. Usually, a single trial is sufficient to form a solid long-term memory, resulting in significantly increased latency 24 h later. The latency is then used as an index of the ability of the animal to remember the association between the aversive stimulus and the specific environment15.
This work describes an analogous procedure using D. as a model system which offers several advantages over rodent models including cost-effectiveness, larger sample size, the absence of regulatory oversight, and access to powerful genetic tools16,17. The procedure is based on negative geotaxis behavior, which manifests in flies' urge to climb up when they are placed on a vertical surface18. The setup consists of two vertical chambers. On the first trial, a fruit fly is placed into a lower compartment. From there, it usually exits within 3-15 s, stepping into the upper compartment where it receives an electric shock. During a 1 min trial, some flies may occasionally re-enter the upper compartment, which results in an additional electric shock. During the testing phase, 24 h later, the latency is significantly increased. At the same time, the number of shocks is decreased compared to the first day indicating that flies formed aversive associative memory about the upper compartment. The latency, number of shocks, and the duration and frequency of grooming bouts are then used to analyze the animal behavior and the ability to form and remember the association between the aversive stimulus and the specific environment. The representative results reveal that exposure to the Western diet (WD) significantly impairs passive avoidance behavior in male flies, suggesting that the WD profoundly impacts the fly's behavior and cognition. Conversely, flight exercise alleviated the negative effect of the WD, improving passive avoidance behavior.
Protocol
1. Preparation of passive avoidance apparatus
- Drill a 4 mm hole perpendicular to the wall surface of the 14 mL polypropylene culture tube and 8 mm away from the tube bottom.
NOTE: Use an electric drill and 5/32 drill bit for best results. - Using a steel utility knife, cut off the upper part of the 14 mL polypropylene culture tube to create a 45 mm long tube bottom fragment. The bottom fragment serves as the lower compartment.
- Cut off the tip of 1,000 µL blue pipette tip using a single edge razor blade to make the opening wide enough for a passage of a single fly. Cut off the narrowing part of the blue tip to create a 12 mm fragment. Insert this fragment firmly into a 4 mm hole of the lower compartment. This is used as a loading dock for transferring the flies.
- Cut a 15 mm piece of transparent vinyl tubing 5/8" ID (see Table of Materials) to create a coupling. Insert upper and lower compartment into the coupling from opposite ends to securely attach the lower compartment to the upper compartment.
- Using a 2-prong adjustable clamp, attach the assembly to a vertical stand. Orient the assembly vertically with the shock tube as an upper compartment.
- Connect the shock tube wires to an electrical stimulator (see Table of Materials) to deliver electric shocks. The duration of the training period is 1 min.
NOTE: To facilitate observation, position a piece of white paper behind the shock tube to serve as a white background of the apparatus. Put a lamp with a 75 W equivalent soft light bulb above the shock compartment. Place an adjustable arm magnifier lamp in front of the setup. A representation of the passive avoidance apparatus is shown in Figure 1.
2. Preparation of the flies for the passive avoidance procedure
- Immobilize 3-4 day old D. melanogaster or D. simulans flies using ice-cold block and transfer them into individual vials with food 24 h before the experiment (1 fly per vial) following the procedure described previously19.
NOTE: The experiments described here compared 3-4 day-old male vs. female flies in D. melanogaster and D. simulans. - Before the behavioral experiments, code all the vials. For this, assign each group a letter, for example, "A", "B", "C", etc., and each fly a number. Reveal this code only after all data have been recorded and analyzed. Use at least 20 flies per genotype or treatment to counter individual variations.
NOTE: Performing the experiments and analyses "blind" allows excluding a bias in assessing the performance of the fly and data analysis.
3. Performing the first trial
- Using a fly mouth aspirator (see Table of Materials) described previously20, gently transfer a fly from the individual vial into the lower compartment via the loading dock. Gently suck one fly into the mouth aspirator by sucking air. Deposit the fly by lightly blowing into the loading dock.
NOTE: Avoid stressing the animal during catching and loading. - Immediately after the fly is loaded into the lower compartment, start a 1 min timer and stopwatch.
NOTE: The stopwatch is used to measure latencies and tally counter to count the number of shocks. - Press the stopwatch to record the first latency when the fly enters the shock tube by placing all paws on the grid. Turn on the stimulator to deliver an electric shock to the fly. The stimulation parameters are 120 volts, 1000 ms duration, 1 pulse/s (PPS), train duration 2000 ms.
- Deliver additional shocks if fly re-enters shock tube. Record the number of received shocks during a 1 min trial with a tally counter or an Arduino-based counter (see Table of Materials). If using the Arduino-based counter, please follow the steps below.
NOTE: An optional Arduino-based device AKM-007 (see Table of Materials) can be used to measure time, latency, the number of shocks, and the frequency and duration of grooming bouts for each animal by pressing and releasing the corresponding buttons on the device. The buttons on the device are assigned to measure latency, administer and record the number of shocks, and measure the frequency and duration of grooming bouts.- Press the Start button at step 3.2., and press the Shock button at step 3.3.
- To record the duration of a grooming bout, press the Grooming button at the beginning of a grooming bout on the device and release this button at the end of the grooming bout.
NOTE: The grooming bouts were measured throughout 1 min trial. Extensive grooming could be indicative of animal stress21,22. The Arduino-based device saves all data as CSV file to a memory card.
- At the end of a 1 min trial, gently transfer the fly back to an individual vial. Write down the latency, the number of received shocks, and any notable changes in the behavior.
- Clean the lower and shock compartment with 70% ethanol, wipe with a lint-free cleaning tissue (see Table of Materials), and dry with the hairdryer. Repeat the trial with the next fly.
- After the behavioral experiments, clean the lower compartment with water and odorless detergent. Wipe the lower compartment and the shock compartment with 70% ethanol, and air-dry overnight.
4. Performing the second trial
- Perform the second trial by repeating the procedure described above (step 3) 24 h later. Test the flies in the same sequence as in the previous day.
5. Analysis of the results
- Calculate the average latency, the average number of shocks, and duration of grooming bouts for trial 1 and trial 2 for each experimental group of animals. Perform student t-test for two-group comparison or ANOVA for multiple comparisons with post-hoc analyses using Tukey's test23.
Representative Results
The passive avoidance was studied in D. melanogaster (Canton-S) and D. simulans. The experiments compared the latencies and number of received shocks between consecutive trials. Initially, the experiments were performed with 3-4 day old male D. melanogaster flies. Flies were maintained on the standard Bloomington Formulation diet in a climate-controlled environment at 24 °C under a 12 h light-dark cycle, 70% humidity, and controlled population density. The density was controlled by keeping breeding conditions constant for all groups. 15 males and 15 females were bred for 48 h in a bottle at 24 °C, 70% humidity, and a 12 h light cycle to generate the offspring. Passive avoidance behavior in a fruit fly was studied in four 2 min trials spaced 24 h apart. The trials were performed at the same time of the day. A fly was gently aspirated from an individual vial during a trial and transferred into the lower compartment via a loading dock (Figure 1). The experiments revealed that D. melanogaster could successfully learn and memorize passive avoidance behavior. In the first trial, the naive fly would enter the upper compartment on average within 16 s (16.15±2.64) and would often re-enter into it, receiving on average 2 shocks (2.18±0.19). During consecutive trials, the latency would significantly increase while the number of shocks would decrease, indicating that flies learned association between the upper compartment and an electric shock (Table 1, Figure 2 A,B). Analysis of frequency distribution showed that negative geotaxis is a powerful motivation. During the first trial, most naive flies would enter the upper compartment within 5-15 s (bin 10, Figure 2C) and receive 1-3 shocks (Figure 2D). In contrast, most flies would not enter the upper compartment in the fourth trial and would correspondingly not receive any shocks (Figure 2E,F).
To verify that the passive avoidance assay works in other fruit fly species, the experiments were repeated in D.simulans male and female flies kept under the same housing conditions as melanogaster flies described above. The trial duration was reduced to 1 min. The frequency distribution for latencies clearly showed that if the fly does not enter the upper compartment within 60 s in the first trial, it usually does not enter at all (Figure 3C). The number of trials was reduced to three as the above-described experiments reliably demonstrated that even a single trial was sufficient for a fly to form aversive associations. D. simulans males and females flies were both effective at learning the passive avoidance behavior, as evident from the data in the graphs and tables (Figure 3, Figure 4; and Table 2, Table 3).
Comparison of latencies and number of shocks between males and females did not reveal any statistically significant differences (Figure 5 A,B). However, male flies were a little faster entering the upper compartment on the first trial, while females received a slightly higher number of shocks in every trial (Figure 4A,B). The difference was particularly evident in the graphs illustrating frequency distributions (Figure 4C-F). The frequency distribution of latencies in the third trial indicated that most flies entered the upper compartment within 7.5-12.5 s (Figure 4E). The frequency distribution for the number of shocks in the third trial shows that female flies received on average 1-2 shocks in the last trial (Figure 4F).
The total duration of grooming behavior was also recorded during trials. Fly grooming behavior consists of repeated coordinated movement of forelegs and hindlegs sweeping over the wings, head, and body24. Self-grooming, also known as auto grooming, has been previously linked to behavioral stress in flies24, laboratory rodents25, and humans22. Therefore, studies of self-grooming behavior -can provide insights into the anxiety-related behaviors26.The analysis of the total duration of grooming bouts during passive avoidance trials revealed that self-grooming was significantly increased in female flies in trials two and three (Figure 5C, Table 4).
Growing evidence suggests an association between high-calorie WD and impaired cognitive performance27. Experimentally, the WD produces adverse effects on mitochondrial brain function, neurogenesis, and synaptic plasticity28,29,30. A recent study documented that the WD increases triglycerides and shortens lifespan in D., while flight exercise counterbalances the detrimental effect of the WD31. To begin to explore the effects of the WD and flight exercise on passive avoidance behavior, D. simulans male flies were subjected to 5 days WD, flight exercise, or a combination of WD and flight exercise according to protocols described previously31. The WD, containing 15% Nutiva USDA Certified Organic, non-GMO, Red Palm Oil, 15% Sucrose, and 0.1M NaCl, was prepared as published elsewhere31. Flight exercise was performed on groups of sixty male flies housed in 1-gallon clear plastic drums strapped to a horizontal platform attached to an electrical motor and operated by a timer. The exercise was performed daily for 7 h for 5 days as published previously31. At the end of the 5-day diet and exercise regimen, flies were sorted into individual vials under cold anesthesia and subjected to two trials of passive avoidance behavior. The experiments showed that the WD increases the number of shocks received in trials one and two and decreases latency in trial two, indicating that the WD significantly impairs aversive associative learning in flies (Figure 6, Table 5). There was also a downward trend in the number of shocks in trial two in a group with the WD and exercise combination, suggesting that exercise may mitigate the impact of caloric overload.
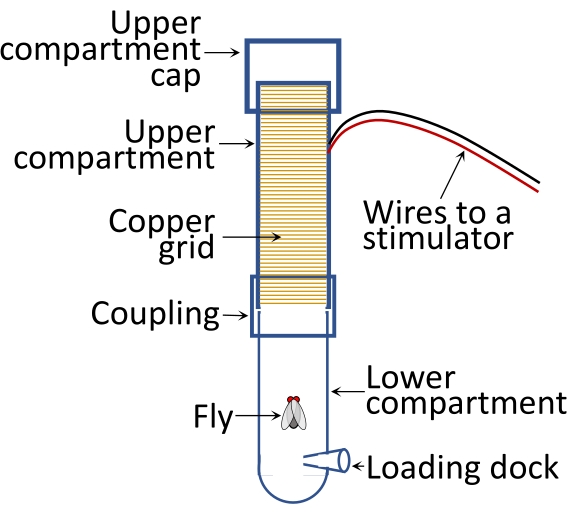
Figure 1: Schematic illustrating passive avoidance assay. The lower compartment dimensions are 45 mm in length and 17 mm in outer diameter. The upper compartment dimensions are 80 mm in length and 18 mm in outer diameter. Please click here to view a larger version of this figure.
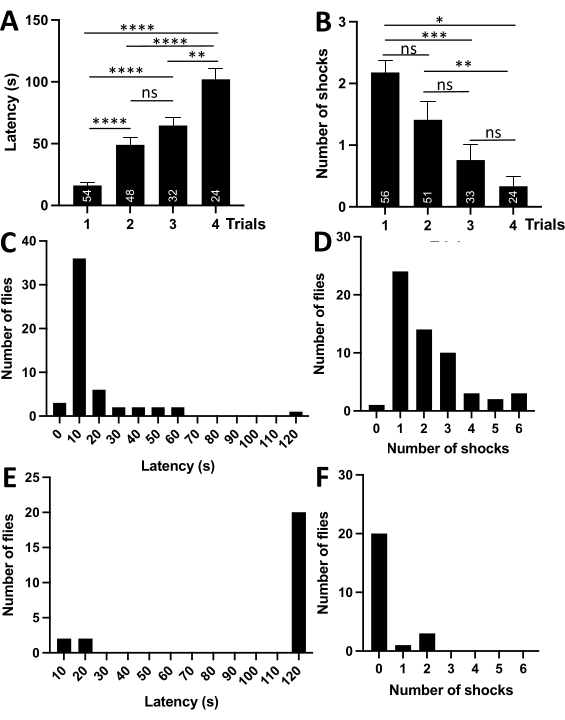
Figure 2: Passive avoidance behavior in D. melanogaster males. (A) Average latency (s) per trial. The graph shows that the latencies increase significantly with the number of trials. Number of flies is indicated on each bar. (B) An average number of received shocks per trial. The graph shows that the number of shocks decreases significantly with the number of trials. (C) Frequency distribution displaying the number of flies within a bin of latencies in the first trial. Numbers on the x-axis are bin centers. The bins are intervals (0-5 s, 5-15 s, 15-25 s, etc.). The graph indicates that most flies enter the upper compartment within 5-15 s. (D) Frequency distribution displaying the number of flies within a bin of shocks in the first trial. Numbers on the x-axis are bin centers. The graph indicates that most flies receive 1-3 shocks in the first trial. Few flies did not enter the shock compartment on the first trial and received zero shocks. They were excluded from the subsequent trials. (E) Frequency distribution displaying the number of flies within a bin of latencies in the fourth trial. The graph indicates that most flies do not enter the upper compartment. (F) Frequency distribution displaying the number of flies within a bin of shocks in the fourth trial. The graph shows that most flies receive 0 shocks in the last trial. Abbreviations: ns- nonsignificant, *- P<0.05, **- P<0.01, ***-P<0.001, ****- P<0.0001. One-way ANOVA with Tukey's multiple comparisons test. Please click here to view a larger version of this figure.
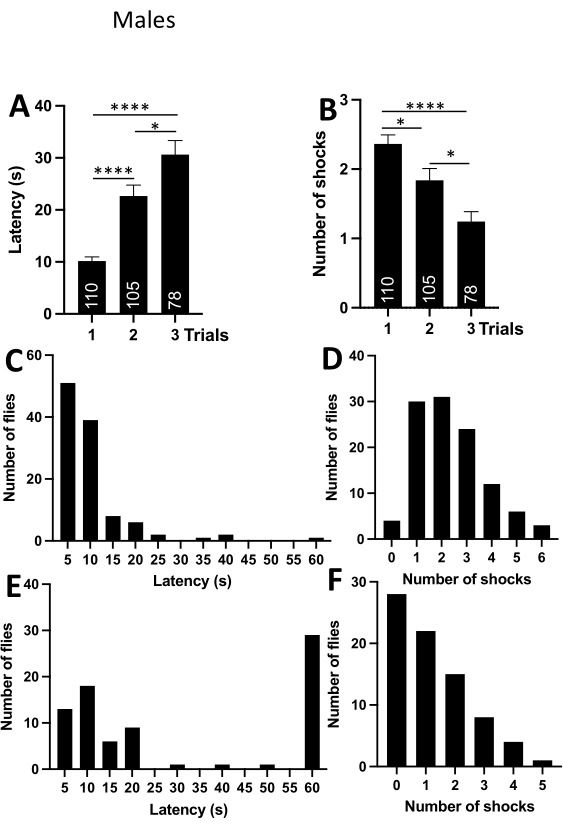
Figure 3: Passive avoidance behavior in D. simulans males. (A) Average latency (s) per trial. The graph shows that the latencies increase significantly with the number of trials. (B) An average number of received shocks per trial. The graph shows that the number of shocks decreases significantly with the number of trials. (C) Frequency distribution displaying the number of flies within a bin of latencies in the first trial. The graph indicates that most flies enter the upper compartment within 2.5-12.5 s. Numbers on the x-axis are the bin centers. (D) Frequency distribution displaying the number of flies within a bin of shocks in the first trial. The graph indicates that most flies receive 1-3 shocks in the first trial. (E) Frequency distribution displaying the number of flies within a bin of latencies in the third trial. The graph indicates that most flies do not enter the upper compartment. (F) Frequency distribution displaying the number of flies within a bin of shocks in the third trial. The graph shows that most flies receive 0 shocks in the last trial. Abbreviations: *- P<0.05, ****- P<0.0001. One-way ANOVA with Tukey's multiple comparisons test. Please click here to view a larger version of this figure.
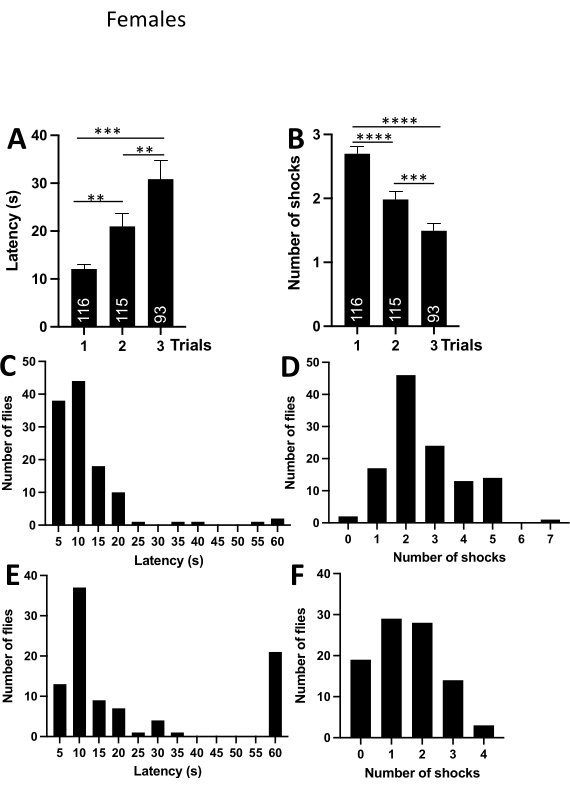
Figure 4: Passive avoidance behavior in D. simulans females. (A) Average latency (s) per trial. The graph shows that the latencies increase significantly with the number of trials. (B) An average number of received shocks per trial. The graph shows that the number of shocks decreases significantly with the number of trials. (C) Frequency distribution displaying the number of flies within a bin of latencies in the first trial. The graph indicates that most flies enter the upper compartment within 2.5-12.5 s. Numbers on the x-axis are the bin centers. (D) Frequency distribution displaying the number of flies within a bin of shocks in the first trial. The graph indicates that most flies receive 2 shocks in the first trial. (E) Frequency distribution displaying the number of flies within a bin of latencies in the third trial. The graph indicates that most flies enter the upper compartment within 7.5-12.5 s. (F) Frequency distribution displaying the number of flies within a bin of shocks in the third trial. The graph shows that most female flies received 1-2 shocks in the last trial. Abbreviations: **- P<0.01, ***-P<0.001, ****- P<0.0001. One-way ANOVA with Tukey's multiple comparisons test. Please click here to view a larger version of this figure.
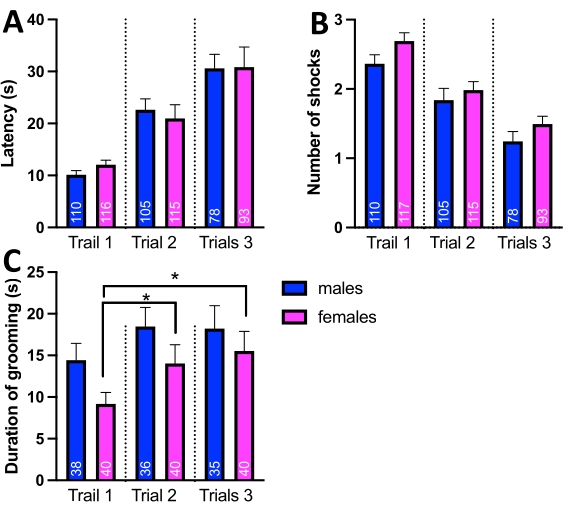
Figure 5: Comparison of passive avoidance and grooming behavior in D. simulans males and females. (A) Average latency (s) per trial. The graph shows no statistically significant differences between males and females in the latencies. (B) An average number of received shocks per trial. The graph shows no statistically significant differences between males and females in the number of received shocks. (C) The total duration of grooming bouts in trials 1-3. While there were no statistically significant differences between males and females, the female flies showed a considerable decrease in grooming behavior during trials 2 and 3 compared to trial 1. Abbreviations: *- P<0.05. One-way ANOVA with Tukey's multiple comparisons test. Please click here to view a larger version of this figure.
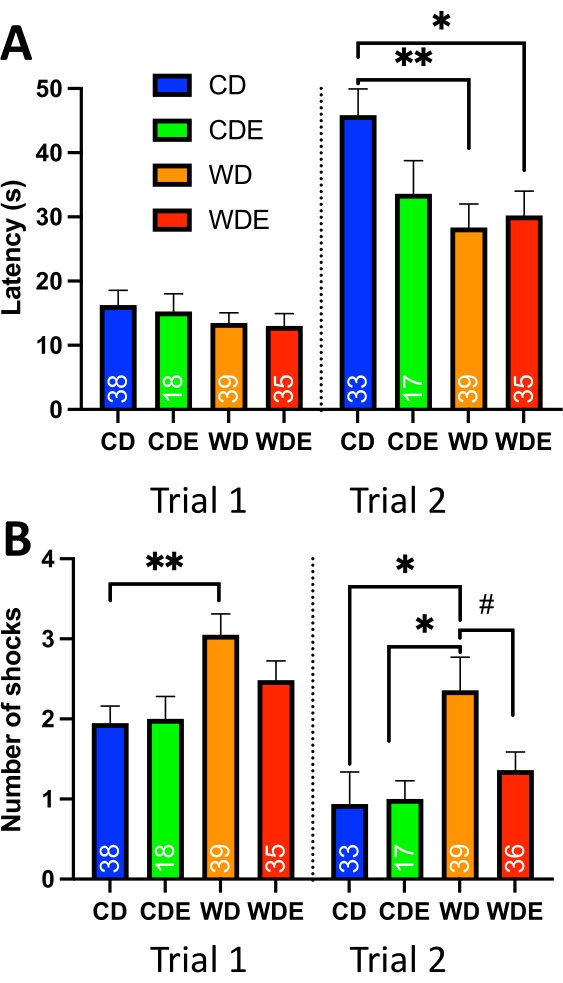
Figure 6: Effect of the WD on D. simulans male's passive avoidance behavior. (A) Average latency (s) per trial. The graph shows that the WD significantly increased latency in WD and WDE groups. (B) An average number of received shocks per trial. The graph shows a statistically significant increase in the number of shocks received by flies on the WD in trials 1 and 2. Interestingly, flight exercise significantly negated the effect of the WD according to the Student t-test. Abbreviations: CD- control diet, CDE- control diet with exercise, WD- western diet, WDE- western diet with exercise. *- P<0.05, **- P<0.01. One-way ANOVA with Tukey's multiple comparisons test. #- P<0.05, Student t-test. Please click here to view a larger version of this figure.
| Trials | Trial 1 | Trial 2 | Trial 3 | Trial 4 |
| Latency (s) | 16.15±2.64 | 49.08±6.2 | 64.72±6.8 | 102.1±12.1 |
| Shocks | 2.18±0.19 | 1.41±0.29 | 0.76±0.25 | 0.33±0.22 |
| Latency Tukey's multiple comparisons between trials | Summary | Adjusted P Value | ||
| 1 vs. 2 | **** | <0.0001 | ||
| 1 vs. 3 | **** | <0.0001 | ||
| 1 vs. 4 | **** | <0.0001 | ||
| 2 vs. 3 | ns | 0.2043 | ||
| 2 vs. 4 | **** | <0.0001 | ||
| 3 vs. 4 | ** | 0.0099 | ||
| Shocks Tukey's multiple comparisons between trials | Summary | Adjusted P Value | ||
| 1 vs. 2 | ns | 0.0646 | ||
| 1 vs. 3 | *** | 0.0004 | ||
| 1 vs. 4 | **** | <0.0001 | ||
| 2 vs. 3 | ns | 0.2569 | ||
| 2 vs. 4 | * | 0.0253 | ||
| 3 vs. 4 | ns | 0.694 | ||
Table 1: Effect of training on avoidance of electric shock in D. melanogaster males. The data for latencies and received shocks are presented as means ± SEM. One-way ANOVA with Tukey's multiple comparisons test.
| Trials | Trial 1 | Trial 2 | Trial 3 |
| Latency (s) | 10.16±0.78 | 22.64±2.1 | 30.63±2.69 |
| Shocks | 2.36±0.13 | 1.84±0.17 | 1.24±0.14 |
| Latency Tukey's multiple comparisons between trials | Summary | Adjusted P Value | |
| 1 vs. 2 | **** | <0.0001 | |
| 1 vs. 3 | **** | <0.0001 | |
| 2 vs. 3 | * | 0.0229 | |
| Shocks Tukey's multiple comparisons between trials | Summary | Adjusted P Value | |
| 1 vs. 2 | * | 0.0166 | |
| 1 vs. 3 | **** | <0.0001 | |
| 2 vs. 3 | * | 0.0172 | |
Table 2: Effect of training on avoidance of electric shock in D. simulans males. The data for latencies and received shocks are presented as means ± SEM. One-way ANOVA with Tukey's multiple comparisons test.
| Trials | Trial 1 | Trial 2 | Trial 3 |
| Latency (s) | 12.07±0.89 | 20.98±2.63 | 23.20±2.16 |
| Shocks | 2.65±0.12 | 1.98±0.12 | 1.49±0.11 |
| Latency Tukey's multiple comparisons between trials | Summary | Adjusted P Value | |
| 1 vs. 2 | ** | 0.0023 | |
| 1 vs. 3 | **** | <0.0001 | |
| 2 vs. 3 | ** | 0.0028 | |
| Shocks Tukey's multiple comparisons between trials | Summary | Adjusted P Value | |
| 1 vs. 2 | **** | <0.0001 | |
| 1 vs. 3 | **** | <0.0001 | |
| 2 vs. 3 | *** | 0.0003 | |
Table 3: Effect of training on avoidance of electric shock in D. simulans females. The data for latencies and received shocks are presented as means ± SEM. One-way ANOVA with Tukey's multiple comparisons test.
| Trials | Trial 1 | Trial 2 | Trial 3 |
| Male’s grooming (s) | 14.42±1.02 | 18.47±2.82 | 18.23±2.75 |
| Female’s grooming (s) | 9.15±1.39 | 14.03±2.26 | 15.50±2.38 |
| Tukey's multiple comparisons between trials males | Summary | Adjusted P Value | |
| 1 vs. 2 | ns | 0.4384 | |
| 1 vs. 3 | ns | 0.4873 | |
| 2 vs. 3 | ns | 0.9971 | |
| Tukey's multiple comparisons between trials females | Summary | Adjusted P Value | |
| 1 vs. 2 | * | 0.0299 | |
| 1 vs. 3 | * | 0.0426 | |
| 2 vs.3 | ns | 0.8481 | |
Table 4: Effect of passive avoidance on D. simulans grooming behavior. The data are presented as means ± SEM. One-way ANOVA with Tukey's multiple comparisons tests.
| Latencies | Trial 1 | Trial 2 |
| CD | 16.26±2.28 | 45.85±4.09 |
| CDE | 15.28±2.75 | 33.59±5.19 |
| WD | 13.49±1.58 | 28.33±3.7 |
| WDE | 13.00±1.95 | 30.23±3.79 |
| Latency Tukey's multiple comparisons between groups Trial 1 | Summary | Adjusted P Value |
| CD vs. CDE | ns | 0.9916 |
| CD vs. WD | ns | 0.7369 |
| CD vs. WDE | ns | 0.6475 |
| CDE vs. WD | ns | 0.9523 |
| CDE vs. WDE | ns | 0.9122 |
| WD vs. WDE | ns | 0.9981 |
| Latency Tukey's multiple comparisons between groups Trial 2 | Summary | Adjusted P Value |
| CD vs. CDE | ns | 0.2785 |
| CD vs. WD | ** | 0.0082 |
| CD vs. WDE | * | 0.0283 |
| CDE vs. WD | ns | 0.8578 |
| CDE vs. WDE | ns | 0.9594 |
| WD vs. WDE | ns | 0.9844 |
| Shocks | Trial 1 | Trial 2 |
| CD | 1.95±0.21 | 0.94±0.4 |
| CDE | 2.0±0.28 | 1.0±0.23 |
| WD | 3.05±0.26 | 2.36±0.41 |
| WDE | 2.49±0.24 | 1.36±0.23 |
| Shocks Tukey's multiple comparisons between groups Trial 1 | Summary | Adjusted P Value |
| CD vs. CDE | ns | 0.9992 |
| CD vs. WD | ** | 0.005 |
| CD vs. WDE | ns | 0.3765 |
| CDE vs. WD | ns | 0.0523 |
| CDE vs. WDE | ns | 0.6446 |
| WD vs. WDE | ns | 0.3268 |
| Shocks Tukey's multiple comparisons between groups Trial 2 | Summary | Adjusted P Value |
| CD vs. CDE | ns | 0.9996 |
| CD vs. WD | * | 0.0194 |
| CD vs. WDE | ns | 0.8246 |
| CDE vs. WD | * | 0.0122 |
| CDE vs. WDE | ns | 0.9306 |
| WD vs. WDE | ns | 0.1508 |
Table 5: Effect of the WD and exercise on avoidance of electric shock in D. simulans males.The data for latencies and received shocks are presented as means ± SEM. One-way ANOVA with Tukey's multiple comparisons test. Abbreviations: CD- control diet, CDE- control diet with exercise, WD- western diet, WDE- western diet with exercise.
Discussion
Avoidance of threatening stimuli is a crucial characteristic of adaptive behavior in various species from C. elegance to human32. Avoidance learning procedures which typically entail the escaping of an aversive event, are commonly used behavioral tasks to investigate learning and memory processes in laboratory rodents13 since the 1970's32. In active avoidance procedures, an indifferent stimulus or conditioned signal (CS) is followed by an aversive event or unconditioned signal (US), which animals learn to avoid by performing a specific behavioral task. In passive avoidance procedures, an animal needs to avoid the aversive US by associating a previously punished behavior with a specific environmental context33. Longer retention test latencies indicating better memory suggest that the animal developed a detailed representation of the training experience13. Passive avoidance training consists of a single trial; however, the brain mechanisms underlying the acquisition of this task are complex as the animal learns to associate various pieces of information, including environmental, spatial-positional, and aversive stimuli13. Altering these stimuli allows studying of episodic and contextual types of memory13.
The paradigm described here adopted passive avoidance behavior in rodents to adult fly as a model system. While several procedures have been developed to study learning and memory in flies, including olfactory7,8, visual9, and courtship conditioning10, these assays are rather time-consuming and require a complex setup. The protocol described here is a simple behavioral assay to study associative aversive learning in flies, which consists of 1 min trials and can be easily set up with a few available supplies. The protocol potentially allows for additional manipulation of the environmental cues, with the addition of olfactory, visual, and other contextual stimuli, by changing colors or geometrical patterns for the lower compartment or adding olfactory cues into the cap of the upper chamber. In addition, this assay can be easily adapted to study short-term memory, middle-term memory, and anesthesia-resistant memory by manipulating intervals between trials and subjecting flies to anesthesia.
The assay worked equally well in D. melanogaster and D. simulans male and female flies, demonstrating that the paradigm could be adapted to different D. species. The changes in fly behavior characterized by increased latencies and decreased number of shocks were statistically significant in the second trial and would strengthen with subsequent trials. Interestingly, if naïve flies were habituated to the apparatus without electric shock, they would enter the upper compartment a little faster on the second and the third trials. However, the decrease in latencies was not statistically significant (data not shown). No statistically significant differences were observed between sexes, although female flies had somewhat longer latencies and received slightly more shocks. This difference could be due to a combination of factors, including females' failure to associate the shock with the upper compartment, a stronger geotaxis, or possibly because females are slightly larger and slower than males. The total duration of grooming bouts was significantly lower in the second and third trials in female flies, which draws a parallel between D. and rodent anxiety-like behaviors26.
Interestingly, as shown in Figures 3E and Figure 4E, the latencies in the third trial were split into two extremes (less than 30 s and 60 s). These differences, however, were not observed in the 4th trial indicating that the majority of the flies eventually acquired the task (Figure 2E). While the exact reasons for the individual differences in developing passive avoidance tasks are unclear, natural populations may contain polymorphism and mutations influencing learning and memory3,5,34,35. A recent study showed that polymorphism in the foraging (for) locus encoding a cGMP-dependent protein kinase (PKG) might mediate alternative strategies in learning foraging behavior34. Flies with the "rover" allele and higher activity have better short-term memory, while flies with the "sitter" allele and sedentary behavior show better long-term memory34.
The assay revealed that the WD significantly impaired learning and memory in flies, which was evident by shorter latencies and receiving more shocks in the second trial. The higher number of shocks in the first trial after the WD could indicate deficits in short-term memory and a failure to make associations between specific environmental context and an electric shock. Interestingly, flight exercises partially mitigated the negative effect of the WD as the WDE flies had received significantly fewer shocks in the second trial. This suggests that flight exercise could reverse the impact of metabolic overload and improve cognitive performance. These data are supported by our previous observation on the beneficial effects of flight exercise on fly physiology, reproduction, and behavior31.
One of the critical steps in the described protocol is transferring the flies from the individual vial into the lower chamber. Stressed flies would either enter the upper compartment too quickly or won't enter at all. The flies that did not enter the upper compartment on the first trial could still enter the upper compartment after being transferred back to an individual vial and given a 5 min break. However, it is better to exclude these flies from the experiment. Fly age is another crucial factor as the negative geotaxis became significantly weaker in 30-day old flies (data not shown). Thus more flies would not enter the upper chamber on the first trial and would be excluded from the experiment.
The advantage of the described assay is that it is a simple behavioral task that produces reproducible data in both D. melanogaster and D. simulans male and female flies. The assay could help the study of basic mechanisms underlying learning and memory impairments resulting from various genetic, pharmacological, and dietary manipulations. Moreover, since the retention trials can be performed at different times after initial training, short-term, intermediate, and long-term memory could be potentially interrogated separately.
There are also potential limitations with this one-trial task, including inter-subject variability and age differences (discussed above). The learning and memory could also be influenced by various factors, including the behavioral state at the time of learning, experimental manipulations, and responses to stress36. Since the US is given in the compartment with a copper grid, the entry of animals is sometimes difficult to assess unless the photo beams and infrared cameras could be installed for video tracking in the future.
Taken together, the assay presented here is a simple, reliable, and reproducible procedure that allows for studying memory mechanisms. It could potentially open new avenues for detailed analysis of memory consolidation in flies, including gene-drug interactions, in both contextual and episodic-like aspects of memory.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This study was supported in parts by NIH R15ES029673 (AKM).
Materials
| Bloomington Formulation diet | Nutri-Fly | 66-112 | Available from Genesee Scientific Inc., San Diego, CA |
| 1000 µL Blue tip | Fisher | NC9546243 | |
| 17 x 100 mm 14 mL polypropylene culture tube | VWR | 60818-689 | |
| Aduino-based Automatic Kontrol Module | In-house | AKM-007 | This unit is optional. Complete description, schematics, wiring diagram and a code are provided at the ECU Digital Market – https://digitalmarket.ecu.edu/akmmodule |
| Dual-Display 2-Channel Digital Clock/Timer | Digi-Sense | AO-94440-10 | https://www.amazon.com/Cole-Parmer-AO-94440-10-Dual-Display-2-Channel-Jumbo-Digit/dp/B00PR0809G/ref=sr_1_5?dchild=1&keywords=Dual-Display+timer+jumbo&qid=1627660660&sr= 8-5#customerReviews |
| Electronic Finger Counter | N/A | N/A | https://www.amazon.com/gp/product/B01M8IRK6F/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&psc=1 |
| Fisherbrand Sparkleen 1 Detergent | Fisher Scientific | 04-320-4 | |
| Fly mouth aspirator | In-house | Prepared as described in reference 19. | |
| Grass S88 stimulator | N/A | N/A | Could be replaced with any stimulator which can provide described parameters |
| Kim-wipes | Fisher Scientific | 06-666 | Kimberly-Clark Professional 34120 |
| Metal block for fly immobilization | In-house | 4 x 13 x 23.5cm aluminum block | |
| Nutiva USDA Certified Organic, non-GMO, Red Palm Oil | Nutiva | N/A | https://www.amazon.com/Nutiva-Certified-Cold-Filtered-Unrefined-Ecuadorian/dp/B00JJ1E83G/ref=sxts_rp_s1_0?cv_ct_cx=Nutiva+USDA+Certified+Organic%2C+non-GMO%2C+Red+Palm+Oil&dchild=1&keywords=Nutiva+USDA+Certified+Organic%2C+non-GMO%2C+Red+Palm+Oil&pd_rd_i=B00JJ1E83G&pd_ rd_r=f35e9d2f-afe4-44b6-afc2-1c9cd705be18&pd_rd_w= R3Zb4&pd_rd_wg=eUv1m&pf_rd_ p=c6bde456-f877-4246-800f-44405f638777&pf _rd_r=M94N11RC7NH333EMJ66Y &psc=1&qid=1627661533&sr=1-1-f0029781-b79b-4b60-9cb0-eeda4dea34d6 |
| Shock tube | CelExplorer | TMA-201 | https://www.celexplorer.com/product_detail.asp?id=217&MainType=110&SubType=8 |
| Stopwatch | Accusplit | A601XLN | https://www.amazon.com/gp/product/B0007ZGZYI/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&psc=1 |
| Transparent vinyl tubing (3/4” OD, 5/8” ID) | Lowes | Avaiable from Lowes |
References
- Kandel, E. R., Dudai, Y., Mayford, M. R. The molecular and systems biology of memory. Cell. 157 (1), 163-186 (2014).
- McGuire, S. E., Deshazer, M., Davis, R. L. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Progress in Neurobiology. 76 (5), 328-347 (2005).
- Livingstone, M. S., Sziber, P. P., Quinn, W. G. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 37 (1), 205-215 (1984).
- Quinn, W. G., Sziber, P. P., Booker, R. The Drosophila memory mutant amnesiac. Nature. 277 (5693), 212-214 (1979).
- Dudai, Y., Jan, Y. N., Byers, D., Quinn, W. G., Benzer, S. dunce, a mutant of Drosophila deficient in learning. Proceedings of the National Academy of Sciences of the United States of America. 73 (5), 1684-1688 (1976).
- Busto, G. U., Cervantes-Sandoval, I., Davis, R. L. Olfactory learning in Drosophila. Physiology. 25 (6), 338-346 (2010).
- Tully, T., Quinn, W. G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. Journal of Comparative Physiology. A: Sensory, Neural, and Behavioral Physiology. 157 (2), 263-277 (1985).
- Wright, N. J. Evolution of the techniques used in studying associative olfactory learning and memory in adult Drosophila in vivo: A historical and technical perspective. Invertebrate Neuroscience. 14 (1), 1-11 (2014).
- Vogt, K., Yarali, A., Tanimoto, H. Reversing stimulus timing in visual conditioning leads to memories with opposite valence in Drosophila. PloS One. 10 (10), 0139797 (2015).
- Koemans, T. S., et al. Drosophila courtship conditioning as a measure of learning and memory. Journal of Visualized Experiments. (124), e55808 (2017).
- Ali, Y. O., Escala, W., Ruan, K., Zhai, R. G. Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. Journal of Visualized Experiments. (49), e2504 (2011).
- Bozler, J., et al. A systems level approach to temporal expression dynamics in Drosophila reveals clusters of long term memory genes. Plos Genetics. 13 (10), 1007054 (2017).
- Atucha, E., Roozendaal, B. The inhibitory avoidance discrimination task to investigate accuracy of memory. Frontiers in Behavioral Neuroscience. 9, 60 (2015).
- Thiels, E., Hoffman, E. K., Gorin, M. B. A reliable behavioral assay for the assessment of sustained photophobia in mice. Current Eye Research. 33 (5), 483-491 (2008).
- Detrait, E. R., Hanon, E., Dardenne, B., Lamberty, Y. The inhibitory avoidance test optimized for discovery of cognitive enhancers. Behavior Research Methods. 41 (3), 805-811 (2009).
- Piper, M. D. W., Partridge, L. Drosophila as a model for ageing. Biochimica et Biophysica Acta – Molecular Basis of Disease. 1864 (9), 2707-2717 (2018).
- Chalmers, J., et al. A multicomponent screen for feeding behaviour and nutritional status in Drosophila to interrogate mammalian appetite-related genes. Molecular Metabolism. 43, 101127 (2021).
- Gargano, J. W., Martin, I., Bhandari, P., Grotewiel, M. S. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Experimental Gerontology. 40 (5), 386-395 (2005).
- Yang, D. Simple homemade tools to handle fruit flies-Drosophila melanogaster. Journal of Visualized Experiments. (149), e59613 (2019).
- Barradale, F., Sinha, K., Lebestky, T. Quantification of Drosophila grooming behavior. Journal of Visualized Experiments. (125), e55231 (2017).
- Denmark, A., et al. The effects of chronic social defeat stress on mouse self-grooming behavior and its patterning. Behavioural Brain Research. 208 (2), 553-559 (2010).
- Kalueff, A. V., et al. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nature Reviews: Neuroscience. 17 (1), 45-59 (2016).
- Motulsky, H. . Intuitive biostatistics: A nonmathematical guide to statistical thinking. Fourth edition. , (2018).
- Qiao, B., Li, C., Allen, V. W., Shirasu-Hiza, M., Syed, S. Automated analysis of long-term grooming behavior in Drosophila using a k-nearest neighbors classifier. Elife. 7, 34497 (2018).
- Mu, M. D., et al. A limbic circuitry involved in emotional stress-induced grooming. Nature Communications. 11 (1), 2261 (2020).
- Song, C., Berridge, K. C., Kalueff, A. V. Stressing’ rodent self-grooming for neuroscience research. Nature Reviews: Neuroscience. 17 (9), 591 (2016).
- Wang, C., Chan, J. S., Ren, L., Yan, J. H. Obesity reduces cognitive and motor functions across the lifespan. Neural Plasticity. 2016, 2473081 (2016).
- Lewis, A. R., Singh, S., Youssef, F. F. Cafeteria-diet induced obesity results in impaired cognitive functioning in a rodent model. Heliyon. 5 (3), 01412 (2019).
- Yohn, S. E., Galbraith, J., Calipari, E. S., Conn, P. J. Shared behavioral and neurocircuitry disruptions in drug addiction, obesity, and binge eating disorder: Focus on Group I mGluRs in the mesolimbic dopamine pathway. ACS Chemical Neuroscience. 10 (5), 2125-2143 (2019).
- Lopez-Taboada, I., Gonzalez-Pardo, H., Conejo, N. M. Western Diet: Implications for brain function and behavior. Frontiers in Psychololgy. 11, 564413 (2020).
- Murashov, A. K., et al. Preference and detrimental effects of high fat, sugar, and salt diet in wild-caught Drosophila simulans are reversed by flight exercise. FASEB Bioadvances. 3 (1), 49-64 (2021).
- Krypotos, A. M., Effting, M., Kindt, M., Beckers, T. Avoidance learning: A review of theoretical models and recent developments. Frontiers in Behavioral Neuroscience. 9, 189 (2015).
- Binder, M. D., Hirokawa, N., Windhorst, U. . Encyclopedia of Neuroscience. , 3093 (2009).
- Mery, F., Belay, A. T., So, A. K., Sokolowski, M. B., Kawecki, T. J. Natural polymorphism affecting learning and memory in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 104 (32), 13051-13055 (2007).
- Tan, Y., Yu, D., Pletting, J., Davis, R. L. Gilgamesh is required for rutabaga-independent olfactory learning in Drosophila. Neuron. 67 (5), 810-820 (2010).
- Ögren, S. O., Stiedl, O., Stolerman, I. P. . Encyclopedia of Psychopharmacology. , 960-967 (2010).

