Development of Organoids from Mouse Pituitary as In Vitro Model to Explore Pituitary Stem Cell Biology
Summary
The pituitary gland is the key regulator of the body’s endocrine system. This article describes the development of organoids from the mouse pituitary as a novel 3D in vitro model to study the gland’s stem cell population of which the biology and function remain poorly understood.
Abstract
The pituitary is the master endocrine gland regulating key physiological processes, including body growth, metabolism, sexual maturation, reproduction, and stress response. More than a decade ago, stem cells were identified in the pituitary gland. However, despite the application of transgenic in vivo approaches, their phenotype, biology, and role remain unclear. To tackle this enigma, a new and innovative organoid in vitro model is developed to deeply unravel pituitary stem cell biology. Organoids represent 3D cell structures that, under defined culture conditions, self-develop from a tissue’s (epithelial) stem cells and recapitulate multiple hallmarks of those stem cells and their tissue. It is shown here that mouse pituitary-derived organoids develop from the gland’s stem cells and faithfully recapitulate their in vivo phenotypic and functional characteristics. Among others, they reproduce the activation state of the stem cells as in vivo occurring in response to transgenically inflicted local damage. The organoids are long-term expandable while robustly retaining their stemness phenotype. The new research model is highly valuable to decipher the stem cells’ phenotype and behavior during key conditions of pituitary remodeling, ranging from neonatal maturation to aging-associated fading, and from healthy to diseased glands. Here, a detailed protocol is presented to establish mouse pituitary-derived organoids, which provide a powerful tool to dive into the yet enigmatic world of pituitary stem cells.
Introduction
The pituitary is a tiny endocrine gland located at the base of the brain, where it is connected to the hypothalamus. The gland integrates peripheral and central (hypothalamic) inputs to generate a tuned and coordinated hormone release, thereby regulating downstream target endocrine organs (such as adrenal glands and gonads) for producing appropriate hormones at the proper time. The pituitary is the key regulator of the endocrine system and is therefore rightfully termed the master gland1.
The mouse pituitary consists of three lobes (Figure 1), i.e., the anterior lobe (AL), the intermediate lobe (IL), and the posterior lobe (PL). The major endocrine AL contains five hormonal cell types, including somatotropes that produce growth hormone (GH); lactotropes generating prolactin (PRL); corticotropes that secrete adrenocorticotropic hormone (ACTH); thyrotropes responsible for thyroid-stimulating hormone (TSH) production; and gonadotropes that make luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The PL consists of axonal projections from the hypothalamus in which the hormones oxytocin and vasopressin (antidiuretic hormone) are stored. The IL is located in-between the AL and PL and houses melanotropes that produce melanocyte-stimulating hormone (MSH). In the human pituitary, the IL regresses during development, and melanotropes are spread within the AL1. In addition to the endocrine cells, the pituitary gland also contains a pool of stem cells, essentially marked by the transcription factor SOX22,3,4,5,6. These SOX2+ cells are located in the marginal zone (MZ), the epithelial lining of the cleft (an embryonic remnant lumen between the AL and IL), or are spread as clusters throughout the parenchyma of the AL, thereby proposing two stem cell niches in the gland (Figure 1)2,3,4,5,6.
Given the indispensable nature of the pituitary, malfunctioning of the gland is associated with serious morbidity. Hyperpituitarism (characterized by over-secretion of one or more hormones) and hypopituitarism (defective or missing production of one or more hormones) can be caused by pituitary neuroendocrine tumors (PitNETs; e.g., ACTH-producing tumors leading to Cushing's disease) or by genetic defects (e.g., GH deficiency resulting in dwarfism)7. In addition, pituitary surgery (e.g., to remove tumors), infections (e.g., hypothalamic-pituitary tuberculosis, or infections following bacterial meningitis or encephalitis), Sheehan's syndrome (necrosis because of insufficient blood flow due to heavy blood loss at birth-giving), pituitary apoplexy and traumatic brain injury are other important causes of pituitary hypofunction8. It has been shown that the mouse pituitary possesses the regenerative capacity, being able to repair local damage introduced by transgenic ablation of endocrine cells9,10. The SOX2+ stem cells acutely react to the inflicted injury showing an activated phenotype, marked by enhanced proliferation (resulting in stem cell expansion) and increased expression of stemness-related factors and pathways (e.g., WNT/NOTCH). Moreover, the stem cells start to express the ablated hormone, finally resulting in substantial restoration of the depleted cell population over the following (5 to 6) months9,10. Also, during the neonatal maturation phase of the gland (the first 3 weeks after birth), the pituitary stem cells are thriving in an activated state6,11,12,13, whereas organismal aging is associated with declined in situ stem cell functionality, due to an increasing inflammatory (micro-) environment at aging (or 'inflammaging')10,14. In addition, tumorigenesis in the gland is also associated with stem cell activation7,15. Although stem cell activation has been detected in several situations of pituitary remodeling (reviewed in7,16), underlying mechanisms remain unclear. Since in vivo approaches (such as lineage tracing in transgenic mice) have not delivered a clear or comprehensive picture of pituitary stem cells, the development of reliable in vitro models to explore stem cell biology in normal and diseased pituitary is essential. Standard in vitro culture of primary pituitary stem cells remains inadequate because of very limited growth capacity and non-physiological (2D) conditions with rapid loss of phenotype (for a more detailed overview, see16). 3D sphere cultures (pituispheres) have been established from pituitary stem cells as identified by side population and SOX2+ phenotype2,3,4. The pituispheres clonally grow from the stem cells, express stemness markers and show differentiation capacity into the endocrine cell types. However, they do not considerably expand while showing only limited passageability (2-3 passages)3,4. Sphere-like structures were also obtained from non-dissociated pituitary stem cell clusters when cultured in 50% diluted Matrigel for 1 week, but expandability was not shown17. The pituisphere approach is mostly used as a read-out tool for stem cell numbers, but further applications are limited by inferior expansion capacity16.
To address and overcome these shortcomings, a new 3D model has recently been established, i.e., organoids, starting from the major endocrine AL of mice containing the MZ and parenchymal stem cells. It has been shown that the organoids are indeed derived from the pituitary's stem cells and faithfully recapitulate their phenotype18. Moreover, the organoids are long-term expandable, while robustly maintaining their stemness nature. Therefore, they provide a reliable method to expand primary pituitary stem cells for profound exploration. Such exploration is not achievable with the limited number of stem cells that can be isolated from a pituitary, which are also not expandable in 2D conditions16. It has been shown that the organoids are valuable and reliable tools to uncover new pituitary stem cell features (translatable to in vivo)14,18. Importantly, the organoid model faithfully mirrors the pituitary stem cell activation status as occurring during local tissue damage and neonatal maturation, showing enhanced formation efficiency and replicating upregulated molecular pathways14,18. Hence, the pituitary-derived organoid model is an innovative and powerful pituitary stem cell biology research model as well as a stem cell activation readout tool.
This protocol describes in detail the establishment of mouse pituitary-derived organoids. To this aim, the AL is isolated and dissociated into single cells, which are embedded in extracellular matrix-mimicking Matrigel (hereon referred to as ECM). The cell-ECM assembly is then cultured in a defined medium, essentially containing stem cell growth factors and pituitary embryonic regulators (further referred to as 'pituitary organoid medium' (PitOM)18; Table 1). Once the organoids are fully developed (after 10-14 days), they can be further expanded trough sequential passaging and subjected to extensive downstream exploration (e.g., immunofluorescence, RT-qPCR, and bulk or single-cell transcriptomics; Figure 1). In the longer run, it is expected that the pituitary stem cell organoids will pave the way to tissue repair approaches and regenerative medicine.
Protocol
Animal experiments for this study were approved by the KU Leuven Ethical Committee for Animal Experimentation (P153/2018). All mice were housed at the university's animal facility under standardized conditions (constant temperature of 23 ± 1.5 °C, relative humidity 40%-60%, and a day/night cycle of 12 h), with access to water and food ad libitum.
1. Mice
- Use commercially available mouse strains, such as C57BL/6J mice, of young-adult age (8-12 weeks old). In general, 2-3 mice provide a sufficient number of AL cells for the protocol.
2. Isolation and dissociation of mouse AL
NOTE: Medium A, B, and C are prepared in advance19,20. Compositions are shown in Table 2.
- Isolation of mouse AL
- Euthanize the mice by CO2 asphyxiation, followed by decapitation (Figure 2A). Wash mice heads with deionized water to remove the blood and spray them with 70% EtOH to generate a sterile environment.
- Using sterile surgical tools, remove the skin of the head between the ears (Figure 2B).
- Open the cranium and remove the brain.
- Break the 'nose bridge' (i.e., anterior part of the frontal bone; Figure 2B) with sterile scissors.
- Open the cranium further with scissors, starting from the broken nose bridge toward the ears, on both sides (Figure 2C).
- Remove the cranium and the brain with sterile tweezers, without touching the pituitary gland (Figure 2D).
- Remove the diaphragma sellae with blunt tweezers, without damaging the pituitary. Discard the PL and the IL from the AL under a stereomicroscope.
NOTE: The PL and IL are linked and thus removed simultaneously. These parts appear as white tissue, as compared to the pink-colored AL (Figure 2D). - Carefully isolate the AL with blunt tweezers and collect it in a 10 mL Erlenmeyer flask, filled with 3 mL of medium A (see Table 2). Place the flask on ice until further processing.
- Dissociation of mouse AL
- Remove the supernatant (SN) medium A from the Erlenmeyer flask containing the isolated AL. Add 2 mL of prewarmed (37 °C) 2.5% trypsin solution and incubate at 37 °C for 15 min.
- Without removing the trypsin solution, add 2 mL of prewarmed (37 °C) DNase solution (2 µg/mL in medium A; sterile-filtered through a 0.22 µm mesh), and swirl the Erlenmeyer flask 10 times. Let the pituitary sink to the bottom (~1 min) and remove the SN.
- Add 2 mL of prewarmed (37 °C) trypsin inhibitor solution (0.1 mg/mL in medium A; sterile-filtered through a 0.22 µm mesh) and incubate at 37 °C for 10 min. Let the pituitary sediment to the bottom and remove the SN.
- Add 2 mL of prewarmed (37 °C) medium B (see Table 2) and incubate at 37 °C for 5 min. Without removing the SN, add 2 mL of prewarmed (37 °C) medium C (see Table 2) and incubate at 37 °C for 15 min.
- Let the pituitary sink to the bottom and remove the SN. Rinse the pituitary three times with prewarmed (37 °C) medium C.
- Dissociate the pituitary into single cells.
- Add 2 mL of prewarmed (37 °C) medium C. Aspirate and expel the pituitary gland with a sterile, flame-polished Pasteur pipette multiple times, until fragments are not visible anymore.
- Transfer the suspension to a 15 mL tube with 4.5 mL of prewarmed (37 °C) DNase solution (2 µg/mL in medium A; sterile-filtered through a 0.22 µm mesh). Rinse the Erlenmeyer three times with 2 mL of prewarmed (37 °C) medium C and transfer the suspension to the 15 mL tube.
- Mix the collected cell suspension and filter it through a 40 µm cell strainer into a 30 mL tube. Rinse the 15 mL tube and the cell strainer three times with 2 mL of medium C and transfer the suspension to the 30 mL tube.
- Position the tip of a glass Pasteur pipette, filled with 2 mL of 3% bovine serum albumin (BSA) solution (in medium A; sterile-filtered through a 0.22 µm mesh), at the bottom of the tube and gently pipette out to form a visible density layer. Centrifuge at 190 x g for 10 min at 4 °C.
- Remove the SN by inverting the tube in one fluent movement and remove the remaining SN droplets with a P1000 tip. Resuspend the cell pellet in 1 mL of ice-cold Advanced DMEM/F12 (Adv DMEM/F12) and quantify the cells with a cell counter.
3. Establishment and culturing of AL-derived organoids
NOTE: Thaw ECM on ice in advance (2-3 h for 1 mL) and keep it on ice for the duration of the protocol.
- Organoid seeding and culturing
- Centrifuge the AL cell suspension at 190 x g for 10 min at 4 °C and remove the SN. Resuspend the cell pellet in Adv DMEM/F12 using the specific volume calculated to reach a cell density of 1.1 x 106 cells/mL.
NOTE: For instance, if the cell suspension contains 500,000 cells/mL, one must resuspend the cell pellet in 454.54 µL of Adv DMEM/F12 to reach the desired density of 1.1 x 106 cells/mL. - Take out the volume of cell suspension needed for plating (according to the desired number of wells to seed for organoid development) and add ECM in a 30:70 ratio (30% cell suspension (in Adv DMEM/F12 ) and 70% ECM). Mix well by pipetting up and down.
NOTE: For instance, for one droplet of 30 µL (see step 3.1.3), one should (gently) mix 9 µL of cell suspension (containing ~10,000 cells when taken from the 1.1 x 106 cells/mL suspension) with 21 µL of ECM. - Per well, deposit a 30 µL drop of the cell suspension/ECM mixture (see step 3.1.2) on a pre-warmed (37 °C) 48-well plate. Turn the plate upside down and let the ECM solidify at 37 °C for 20 min.
NOTE: Pre-warm the culture plates for at least 24 h at 37 °C. - Return the plate to its proper orientation and carefully add 250 µL of prewarmed (37 °C) PitOM (see Table 1) supplemented with 10 µM Rock Inhibitor (Y-27632).
- Continue to culture the organoids by changing the medium (devoid of Y-27632) every 2-3 days until the organoids are fully grown, which takes between 10-14 days (Figure 3A). Then, passage the organoids.
NOTE: When aspirating the medium, make sure not to disrupt the ECM dome. Tilt the culture plate slightly and remove the medium from the bottom rim of the well. Fresh (prewarmed at 37 °C) medium should be added gently to the side of the well. If gel droplets de-attach, collect the organoids and resuspend and culture them again in a new ECM droplet.
- Centrifuge the AL cell suspension at 190 x g for 10 min at 4 °C and remove the SN. Resuspend the cell pellet in Adv DMEM/F12 using the specific volume calculated to reach a cell density of 1.1 x 106 cells/mL.
- Organoid passaging
- Aspirate the medium gently and add 400 µL of ice-cold Adv DMEM/F12 to disintegrate the ECM and collect the organoids in a microcentrifuge tube. Wash once with 400 µL of ice-cold Adv DMEM/F12 EM. Centrifuge at 200 x g for 5 min at 4 °C.
- Remove the SN carefully and add 400 µL of prewarmed (37 °C) TrypLE Express Enzyme (1X). Mix by inverting the tube several times, and incubate at 37 °C for 5 min.
- Add 400 µL of ice-cold Adv DMEM/F12 and centrifuge at 200 x g for 5 min at 4 °C. Remove the SN.
- Resuspend the pellet with 100 µL of ice-cold Adv DMEM/F12 and subsequently break up the organoids by vigorously pipetting up and down with a narrowed P200 tip (i.e., push down the empty tip against the bottom of the microcentrifuge tube, to reduce its opening diameter) until organoid fragments (with a diameter around 50 µm) are obtained (Figure 3B).
NOTE: The dissociation mixture should contain predominantly organoid fragments and only a few single cells. Harsh dissociation of the organoids into single cells negatively impacts the re-growth of the organoids. - Add 800 µL of Adv DMEM/F12 and centrifuge at 190 x g for 10 min at 4 °C. Remove the SN.
- Passage the organoids in a 1:2 to 1:4 ratio. Resuspend the pellet in an adequate volume of Adv DMEM/F12 as needed for plating and add ECM in a 30:70 ratio (30% cell suspension and 70% ECM). Mix well by pipetting up and down.
- Seed and culture the organoids as described above in steps 3.1.3-3.1.5.
NOTE: On average, 20 organoids develop per well from the 10,000 whole-AL cells seeded (0.2%). These passage 0 organoids can be split in a 1:2 ratio, resulting in >50 organoids developing per well (passage 1). Organoids can then be split in a 1:2 to 1:4 ratio during subsequent passages. Re-growth of the organoids slows down after ~10 passages (corresponding to 3 months of culture), concretized in gradually fewer and smaller organoids.
4. Cryopreservation of AL-derived organoids and thawing
- Cryopreservation of organoids
- Follow the passaging protocol from step 3.2.1 until step 3.2.5.
- Resuspend the organoid pellet (containing fragments and cells) with 1 mL of cryopreservation medium (Table 3). Transfer the suspension into a cryovial and place it on ice.
NOTE: Organoids (i.e., resultant fragments and cells) from up to four wells of the 48-well plate can be combined in one cryovial. - Place the cryovials in a freezing container and transfer them to -80 °C.
- After 24 h, transfer the samples to a cryobox and store them in liquid nitrogen (-196 °C) for long-term storage.
- Thawing of cryopreserved organoids
- Remove the cryovial from the liquid nitrogen tank and place it on ice. Immediately proceed with the thawing protocol.
- Thaw the solution with the cryopreserved organoid fragments and single cells at 37 °C (water bath).
NOTE: Do not keep the solution for more than 2 min at 37 °C to avoid cell toxicity by DMSO. - Transfer the content to a 15 mL tube containing 10 mL of ice-cold Adv DMEM/F12 with 30% fetal bovine serum (FBS). Rinse the cryovial with 1 mL of Adv DMEM/F12 with 30% FBS.
- Centrifuge at 190 x g for 10 min at 4 °C. Resuspend the pellet with 1 mL of ice-cold Adv DMEM/F12 and transfer the suspension to a microcentrifuge tube.
- Centrifuge at 190 x g for 10 min at 4 °C. Resuspend the pellet in an adequate volume of Adv DMEM/F12 as needed for plating and add ECM in a 30:70 ratio. Mix well by pipetting up and down.
- Seed and culture the organoids as described above in steps 3.1.3-3.1.5.
5. Validation of AL-derived organoids
- Collection and lysis of organoids for RNA isolation
- Collect and centrifuge the organoids as described above (step 3.2.1).
- Remove the SN and add 350 µL of lysis buffer with 1% 2-mercapto-ethanol. Vortex for 30 s and store at -80 °C or proceed immediately to RNA isolation.
CAUTION: Beware that 2-mercapto-ethanol is a toxic compound. All work must be done in a chemical fume hood while wearing nitrile gloves, a dust mask, and safety glasses. 2-Mercapto-ethanol can cause irreversible damage to the eyes and skin.
- Fixation and embedding of organoids for immuno-histochemistry/-fluorescence staining
- Collect and centrifuge the organoids as described above (step 3.2.1).
- Remove the SN, add 1 mL of 4% paraformaldehyde (PFA) and incubate for 30 min at room temperature (RT) on an orbital shaker (100 rpm).
CAUTION: PFA is a known human carcinogen that can cause irreversible damage to the cornea. All work must be done in a chemical fume hood. Nitrile gloves and safety glasses must always be worn. - Centrifuge at 200 x g for 5 min and remove the SN. Add 1 mL of PBS, incubate 10 min at RT on an orbital shaker (100 rpm), and centrifuge at 90 x g for 3 min at 4 °C. Repeat the washing step twice. Store in PBS at 4 °C.
- For tissue processing and dehydration, remove the SN and add 150 µL of 2% agarose gel (in PBS) to the organoid pellet using a prewarmed widened p200 tip (made by cutting a small piece of the tip). Immediately pipet the entire volume up and eject in the lid of the microcentrifuge tube.
NOTE: It is important to work swiftly, as the gel containing the organoids will quickly solidify. - Let the gel firmly solidify for 30 min and move the gel disc to a histology cassette. Immerse and store in 50% EtOH, until dehydration in the tissue processor.
- For paraffin embedding, place the gel disc (using forceps) in an embedding mold and fill with warm paraffin (60 °C). Place the molds at 4 °C until the paraffin is solid (approximately 45 min). These samples can either be stored at 4 °C or can immediately be subjected to sectioning.
- Microtome the paraffin blocks containing organoids at 5 µm thickness and collect the samples on glass slides. Add one drop of deionized water underneath each section to allow proper stretching of the section and place the slides on a flat heating plate at 37 °C overnight. Store the slides with sections at 4 °C or directly continue with immunohistochemical or immunofluorescence staining.
Representative Results
After isolation and dissociation of the AL, the obtained single cells are seeded in ECM and grown in PitOM (Figure 1, Table 1). Figure 3A displays the cell culture and density at seeding (Day 0). Some small debris may be present (Figure 3A, white arrowheads), but will disappear at passaging. Fourteen days after seeding, the AL-derived organoids are fully developed (Figure 3A). The organoids exhibit a cystic morphology, with an epithelial layer that encloses a lumen. At this stage, the organoids reach a diameter of 500 µm and have to be passaged. Figure 3B shows the AL-derived organoid culture after passaging at the indicated time following re-seeding of the dissociated organoid fragments.
Occasionally, one or more dense structures may appear in the organoid culture (Figure 3A, Unfavorable). When passaging, dense organoids tend to take over, ending up in cultures with only dense structures after a couple of passages (Figure 3B, Unfavorable). Therefore, it is recommended not to proceed with wells that contain dense organoids (passage 0). Alternatively, dense organoids can be discarded by sedimentation, which leaves the cystic organoids to continue with. The origin of these dense organoids is at present unclear, but they show a less pronounced pituitary nature18. If organoids do not, or less efficiently regrow after passaging, dissociation procedures need to be optimized. In particular, one must pay attention not to dissociate too harsh; the organoids must be split up to fragments, not to single cells (Figure 3B, Day 0, inset).
Immunofluorescence staining analysis confirms the epithelial character of the AL-derived organoids, as they express the epithelial markers E-cadherin (E-Cad) and cytokeratin 8/18 (CK8/18; Figure 3C), which, moreover, have been described as stem cell markers in the pituitary18. The stemness nature of the organoids is additionally demonstrated by SOX2 and TROP2 expression, both of which were also identified as pituitary stem cell markers (Figure 3C)14,18. LHX3, a transcription factor specifically expressed in the (early-developing) pituitary, validates the organoids' pituitary phenotype (Figure 3C). Some of the organoid-constituting cells are in a proliferative state, expressing the proliferation marker Ki67 (Figure 3C).
Further exploration and validation of the pituitary (stemness) phenotype of the AL-derived organoids is performed with reverse transcription-quantitative PCR (RT-qPCR). High expression of the stemness markers Sox2, Cdh1 (encoding E-Cad), Krt8, Krt18 and Trop2 is present in the organoids, clearly higher than in primary AL, indicating that the organoids enrich for the stem cells and thus represent the AL stem cell compartment, as previously described (Figure 3D)18. Notably, the developmental transcription factors Pitx1 and Pitx2 remain expressed after development in several hormonal cell types in the AL, and hence their high expression in the AL as well. The cultures robustly retain their stemness phenotype, as demonstrated by the sustained (high) expression of these markers after multiple passages (Figure 3D).
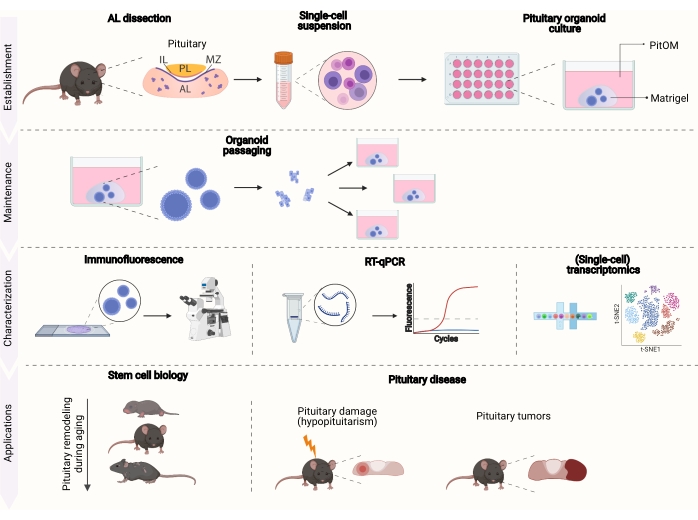
Figure 1: Overview of the establishment, maintenance, characterization, and application potential of organoids from healthy and diseased pituitary. AL, anterior lobe; IL, intermediate lobe; PL, posterior lobe; MZ, marginal zone; PitOM, pituitary organoid medium (created with BioRender.com). Stem cell niches in the AL are indicated in purple. Please click here to view a larger version of this figure.
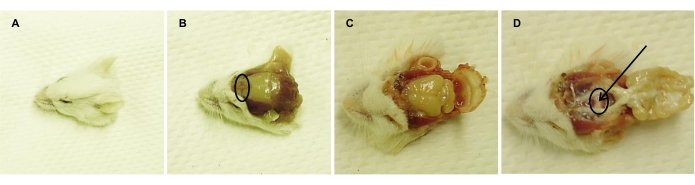
Figure 2: Isolation of the pituitary gland from adult euthanized mouse. Representative images consecutively taken following (A) decapitation, (B) removal of head skin (nose bridge is encircled), (C) opening of the cranium, and (D) removal of the brain, exposing the pituitary gland (encircled). Arrow points to the PL, which is discarded (together with the associated IL), leaving the AL for isolation and dissociation. Please click here to view a larger version of this figure.
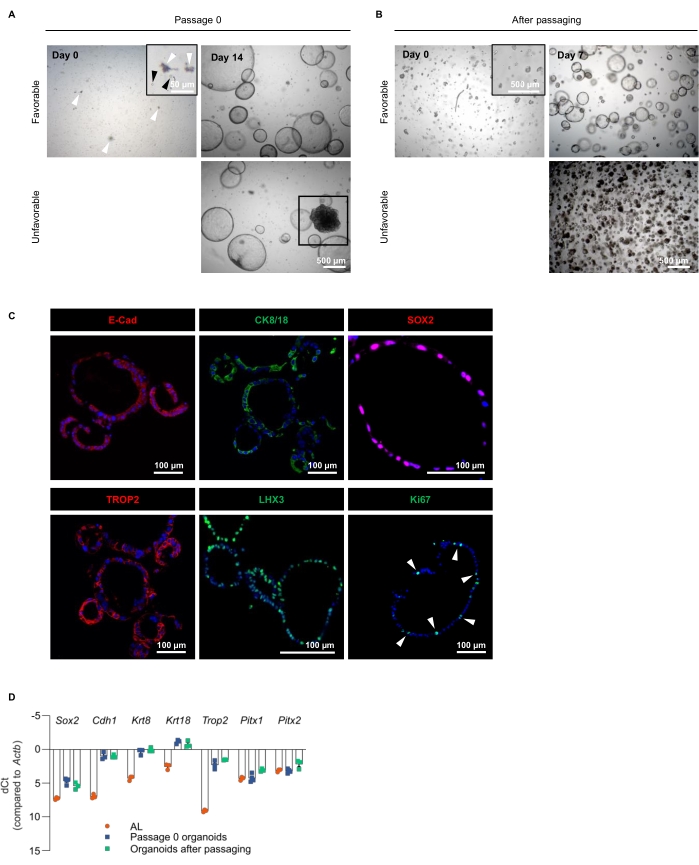
Figure 3: Establishment and validation of AL-derived organoids. (A) AL cell seeding and organoid development in PitOM at indicated days (passage 0). The top row shows favorable organoid growth, with only cystic structures developing. The bottom row shows unfavorable growth with a large dense structure appearing (boxed). White arrowheads indicate debris, black arrowheads indicate single cells (magnified in inset). (B) Organoid fragments (magnified in inset) seeded at passaging (Day 0) and regrowth of organoids as observed 7 days later. The top row shows favorable organoid regrowth, with only cystic structures growing. The bottom row shows unfavorable regrowth with dense organoids taking over the culture. (C) Immunofluorescence staining of E-Cad, SOX2, TROP2 (all red), CK8/18, LHX3 and Ki67 (all green) in AL-derived organoids. Nuclei are labeled with Hoechst33342 (blue). Arrowheads indicate Ki67+ cells. Scale bars are indicated. (D) Gene expression analysis of stemness markers (Sox2, Cdh1, Krt8, Krt18, Trop2), and developmental transcription factors (Pitx1, Pitx2) in primary AL and AL-derived organoids (Passage 0 means 14 days after cell seeding) determined by RT-qPCR (mean ± SEM). Data points represent biological replicates. Delta cycle threshold (dCT) values are shown, calculated using the formula: CT(gene of interest) – CT(housekeeping gene Actb). The more positive the dCT value (which is presented on the Y-axis below the zero X-axis), the lower the expression level of the gene of interest. The lower (or more negative) the dCT value, the higher the expression level14,18,21,22. Please click here to view a larger version of this figure.
| Pituitary organoid medium (PitOM) | |
| Component | Concentration |
| Advanced DMEM/F12 | |
| Hepes | 1% |
| Penicillin-Streptomycin | 1% |
| Glutamax | 1% |
| B-27 Supplement (50X), minus vitamin A | 1X |
| L-Glutamine (200 mM) | 2 mM |
| Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein | 20 ng/mL |
| Recombinant Human IGF-1 | 100 ng/mL |
| N-2 Supplement (100X) | 1X |
| N-acetyl-cysteine | 1.25 mM |
| Recombinant Human/Murine FGF-8b | 200 ng/mL |
| Recombinant Human FGF-10 | 100 ng/mL |
| A83-01 (activin receptor-like kinase 4/5/7 inhibitor) | 0.50 µM |
| Recombinant Mouse Sonic Hedgehog/Shh (C25II) N-Terminus | 100 ng/mL |
| Recombinant Human EGF Protein, CF | 50 ng/mL |
| SB202190 (p38 mitogen-activated protein kinase inhibitor) | 10 µM |
| Recombinant Human Noggin | 100 ng/mL |
| Cholera Toxin from Vibrio cholerae | 100 ng/mL |
| Recombinant Human R-Spondin-1 | 200 ng/mL |
| Recombinant Human IL-6 | 20 ng/mL |
Table 1. Composition of PitOM. PitOM is filtered through a 0.22 µm mesh filter and stored at 4 °C for a maximum of 2 weeks.
| Medium A | |
| Component | Quantity |
| DMEM, powder, high glucose | 13.38 g |
| HEPES | 5.96 g |
| Sodium-Pyruvate (C3H3NaO3) | 0.11 g |
| Penicillin G sodium salt | 35.00 mg |
| Streptomycin sulfate salt | 50.00 mg |
| Sodium Chloride (NaCl) | 0.50 g |
| Sodium Hydrogen Carbonate (NaHCO3) | 1.00 g |
| Albumin Bovine (cell culture grade) | 3.00 g |
| Sterile water | 1.00 L |
| Medium C | |
| Component | Quantity |
| Sodium Chloride (NaCl) | 7.50 g |
| Potassium Chloride (KCl) | 0.40 g |
| Sodium di-Hydrogen Phosphate 1-hydrate | 0.14 g |
| D-glucose | 1.00 g |
| HEPES | 4.76 g |
| Streptomycin sulfate salt | 50.00 mg |
| Penicillin G sodium salt | 35.00 mg |
| Phenol red | 10.00 mg |
| Albumin Bovine (cell culture grade) | 3.00 g |
| Sodium Hydrogen Carbonate (NaHCO3) | 1.00 g |
| Sterile water | 1.00 L |
| Medium B | |
| Component | Quantity |
| Titriplex III (Edetate disodium salt dihydrate) | 0.74 g |
| Medium C | 100 mL |
Table 2. Composition of medium A, B, and C. All media are filtered through a 0.22 µm mesh filter and stored at 4 °C for a maximum of 4 months. The pH of medium A and C must be adjusted to 7.3.
| Cryopreservation medium | |
| Component | Concentration |
| Advanced DMEM/F12 | 60% |
| FBS | 30% |
| DMSO | 10% |
Table 3. Composition of cryopreservation medium.
Discussion
The AL-derived organoids, as described here, represent a powerful research model to study pituitary stem cells in vitro. At present, this organoid approach is the only available tool to reliably and robustly grow and expand primary pituitary stem cells. A pituitary organoid model derived from embryonic stem cells (ESC) or induced pluripotent stem cells (iPSC) has been reported previously, which closely recapitulates pituitary embryonic organogenesis23; however, although highly useful to study pituitary development or model pituitary disease23,24,25, the reported protocol, starting from ESC/iPSC, is very time-consuming compared to the protocol described here, and the resulting organoids are also not expandable.
Successful culturing of pituitary stem cell organoids depends on some critical steps in the protocol. It is important to plate an appropriate number of cells at initial cell seeding. A very high number will give rise to overcrowded cultures, which deteriorates the viability of the organoids and obstructs full organoid expansion, whereas a very low number of cells will result in limited organoid formation. Furthermore, it is important not to disturb the integrity of the ECM dome once in culture. Adding and removing medium should be done very carefully, without touching the gel droplet. In addition, prewarming the culture medium reduces the risk of depolymerization of the gel. Finally, passaging the organoids correctly (i.e., dissociating to fragments and not to single cells) is crucial for efficient expansion of the cultures.
These pituitary stem cell organoids can be harnessed to answer questions regarding the stem cells' phenotype, biology, and function. They have already been proven valuable in uncovering novel stem cell features as well as markers of pituitary damage-associated stem cell activation and as a read-out tool for stem cell activity (Figure 1)14,18. Current efforts include their derivation from diseased pituitary, such as hypopituitarism and PitNETs (Figure 1). Eventually, organoids can also be engaged into a platform for drug screening, as successfully established for other diseases26,27. Therefore, further upscaling of the organoid cultures to reach high throughput analysis will be necessary. It has been noticed already that AL-derived organoids can be efficiently grown in a 96-well format, also resulting in more homogenous cultures.
It has been observed that after ~10 passages (corresponding to 3 months of culture), organoid growth efficiency gradually decreased with organoids regrowing at lower numbers and smaller size. This growth decline may be inherent to the intrinsic nature of pituitary stem cells, which may not need to self-renew many times in the gland in vivo, which is only slowly turning over, thus becoming exhausted after a couple of division rounds16,28. Although this eventual growth decline might be considered as a limitation, the model is highly useful since organoid expansion during the preceding passages is more than sufficient for extensive downstream analyses.
Another aspect that might be regarded as a limitation is that the pituitary stem cell organoids do not show prominent differentiation capacity toward the endocrine cell types of the AL, even after xenografting under the kidney capsule of immunodeficient mice (which resulted in a limited number of GH+ and PRL+ cells as described in detail in reference18). Either the right in vitro conditions to drive the stem cells into differentiation are not identified yet, or the major role of the stem cells (especially in the adult gland) is not situated in generating new endocrine cells (since likely not needed in the lazy gland but only in perturbed or challenged conditions)9,10,14,18. Instead, the major function may be situated in other biological aspects (e.g., paracrine signaling to the hormonal progenitor/precursor or mature cells in basic, but likely more in active (developmental, repair, disease) conditions)13,16. Indeed, although pituitary stem cells have been shown to possess multipotent differentiation capacity especially in the embryonic and neonatal period, it is conceivable that stem cells in the adult gland do not (need to) maintain this capacity, given the very low turnover of the adult gland16,28. It is possible that the adult pituitary stem cells act more as a paracrine signaling hub, involved in stimulating or regulating the surrounding progenitor/precursor/endocrine cells13,16. Hence, robust differentiation of the pituitary stem cell organoids culminating in hormone secretion may be an erroneous expectation that will never be reached.
Taken together, the protocol presented here offers a swiftly applicable and reliable tool to robustly expand primary pituitary stem cells in a 3D setting in vitro. The protocol gives rise to organoids that faithfully capture the pituitary stem cell phenotype. The system has already been successfully applied to study pituitary stem cell biology and activation14,18, and the findings are highly translatable to the in vivo situation.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the KU Leuven Research Fund and the Fund for Scientific Research (FWO) – Flanders. E.L. (11A3320N), and C.N. (1S14218N) are supported by a Ph.D. Fellowship from the FWO/FWO-SB.
Materials
| 2-Mercaptoethanol | Sigma-Aldrich | M6250 | |
| 48-well plates, TC treated, individually wrapped | Costar | 734-1607 | |
| A83-01 | Sigma-Aldrich | SML0788 | |
| Advanced DMEM | Gibco | 12491023 | |
| Albumin Bovine (cell culture grade) | Serva | 47330 | |
| B-27 Supplement (50X), minus vitamin A | Gibco | 12587010 | |
| Base moulds | VWR | 720-1918 | |
| Buffer RLT | Qiagen | 79216 | |
| Cassettes, Q Path Microtwin | VWR | 720-2191 | |
| Cell strainer, 40 µm mesh, disposable | Falcon | 352340 | |
| Cholera Toxin from Vibrio cholerae | Sigma-Aldrich | C8052 | |
| Deoxyribonuclease I from bovine pancreas | Sigma-Aldrich | D5025 | |
| D-glucose | Merck | 108342 | |
| Dimethylsulfoxide (DMSO) | Sigma-Aldrich | D2650 | |
| DMEM, powder, high glucose | Gibco | 52100039 | |
| Eppendorf Safe-Lock Tubes, 1.5 mL | Eppendorf | 30120086 | |
| Epredia SuperFrost Plus Adhesion slides | Thermo Fisher Scientific | J1800AMNZ | |
| Epredia HistoStar Embedding Workstation, 220 to 240Vac | Thermo Fisher Scientific | 12587976 | |
| Ethanol Absolute 99.8+% | Thermo Fisher Scientific | 10342652 | |
| Fetal bovine serum (FBS) | Sigma-Aldrich | F7524 | |
| GlutaMAX Supplement | Gibco | 35050061 | |
| HEPES | Sigma-Aldrich | H4034 | |
| HEPES Buffer Solution | Gibco | 15630056 | |
| InSolution Y-27632 | Sigma-Aldrich | 688001 | |
| L-Glutamine (200 mM) | Gibco | 25030081 | |
| Matrigel Growth Factor Reduced (GFR) Basement Membrane Matrix, LDEV-Free | Corning | 15505739 | |
| Mr. Frosty Freezing Container | Thermo Fisher Scientific | 5100-0001 | |
| N-2 Supplement (100X) | Thermo Fisher Scientific | 17502048 | |
| N-Acetyl-L-cysteine | Sigma-Aldrich | A7250 | |
| Nunc Biobanking and Cell Culture Cryogenic Tubes | Thermo Fisher Scientific | 375353 | |
| Paraformaldehyde for synthesis (PFA) | Merck | 818715 | |
| PBS, pH 7.4 | Gibco | 10010023 | |
| Penicillin G sodium salt | Sigma-Aldrich | P3032 | |
| Penicillin-Streptomycin (10,000 U/mL) | Gibco | 15140122 | |
| Phenol red | Merck | 107241 | |
| Potassium Chloride (KCl) | Merck | 104936 | |
| Recombinant Human EGF Protein, CF | R&D systems | 236-EG | |
| Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein | R&D systems | 234-FSE | |
| Recombinant Human FGF-10 | Peprotech | 100-26 | |
| Recombinant Human IGF-1 | Peprotech | 100-11 | |
| Recombinant Human IL-6 | Peprotech | 200-06 | |
| Recombinant Human Noggin | Peprotech | 120-10C | |
| Recombinant Human R-Spondin-1 | Peprotech | 120-38 | |
| Recombinant Human/Murine FGF-8b | Peprotech | 100-25 | |
| Recombinant Mouse Sonic Hedgehog/Shh (C25II) N-Terminus | R&D systems | 464-SH | |
| RNeasy micro kit | Qiagen | 74004 | |
| SB202190 | Sigma-Aldrich | S7067 | |
| SeaKem LE Agarose | Lonza | 50004 | |
| Sodium Chloride (NaCl) | BDH | 102415K | |
| Sodium di-Hydrogen Phosphate 1-hydrate | PanReac-AppliChem | A1047 | |
| Sodium Hydrogen Carbonate (NaHCO3) | Merck | 106329 | |
| Sodium-Pyruvate (C3H3NaO3) | Sigma-Aldrich | P5280 | |
| Stericup-GP, 0.22 µm | Millipore | SCGPU02RE | |
| Steriflip-GP Sterile Centrifuge Tube Top Filter Unit, 0.22 μm | Millipore | SCGP00525 | |
| Sterile water | Fresenius | B230531 | |
| Streptomycin sulfate salt | Sigma-Aldrich | S6501 | |
| Syringe, with BD Microlance needle with intradermal bevel, 26G | BD Plastipak | BDAM303176 | |
| Thermo Scientific Excelsior ES Tissue Processor | Thermo Scientific | 12505356 | |
| Titriplex III | Merck | 108418 | |
| TrypL Express Enzyme (1X), phenol red | Thermo Fisher Scientific | 12605028 | |
| Trypsin inhibitor from Glycine max (soybean) | Sigma-Aldrich | T9003 | |
| Trypsin solution 2.5 % | Thermo Fisher Scientific | 15090046 |
References
- Melmed, S. . The pituitary. 3rd ed. , 1 (2011).
- Chen, J., et al. The adult pituitary contains a cell population displaying stem/progenitor cell and early-embryonic characteristics. Endocrinology. 146 (9), 3985-3998 (2005).
- Chen, J., et al. Pituitary progenitor cells tracked down by side population dissection. Stem Cells. 27 (5), 1182-1195 (2009).
- Fauquier, T., Rizzoti, K., Dattani, M., Lovell-Badge, R., Robinson, I. C. A. F. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proceedings of the National Academy of Sciences of the United States of America. 105 (8), 2907-2912 (2008).
- Rizzoti, K., Akiyama, H., Lovell-Badge, R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell. 13 (4), 419-432 (2013).
- Andoniadou, C. L., et al. Sox2+ stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 13 (4), 433-445 (2013).
- Nys, C., Vankelecom, H. Pituitary disease and recovery: How are stem cells involved. Molecular and Cellular Endocrinology. 525 (4), 111176 (2021).
- Schneider, H. J., Aimaretti, G., Kreitschmann-Andermahr, I., Stalla, G. K., Ghigo, E. Hypopituitarism. Lancet. 369 (9571), 1461-1470 (2007).
- Fu, Q., et al. The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration. Endocrinology. 153 (7), 3224-3235 (2012).
- Willems, C., et al. Regeneration in the pituitary after cell-ablation injury: time-related aspects and molecular analysis. Endocrinology. 157 (2), 705-721 (2016).
- Gremeaux, L., Fu, Q., Chen, J., Vankelecom, H. Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells and Development. 21 (5), 801-813 (2012).
- Zhu, X., Tollkuhn, J., Taylor, H., Rosenfeld, M. G. Notch-dependent pituitary SOX2+ stem cells exhibit a timed functional extinction in regulation of the postnatal gland. Stem Cell Reports. 5 (6), 1196-1209 (2015).
- Russell, J. P., et al. Pituitary stem cells produce paracrine WNT signals to control the expansion of their descendant progenitor cells. eLife. 10 (1), 59142 (2021).
- Vennekens, A., et al. Interleukin-6 is an activator of pituitary stem cells upon local damage, a competence quenched in the aging gland. Proceedings of the National Academy of Sciences of the United States of America. 118 (25), 2100052118 (2021).
- Mertens, F., et al. Pituitary tumors contain a side population with tumor stem cell-associated characteristics. Endocrine-Related Cancer. 22 (4), 481-504 (2015).
- Laporte, E., Vennekens, A., Vankelecom, H. Pituitary remodeling throughout life: are resident stem cells involved. Frontiers in Endocrinology. 11 (1), 604519 (2021).
- Yoshida, S., et al. Isolation of adult pituitary stem/progenitor cell clusters located in the parenchyma of the rat anterior lobe. Stem Cell Research. 17 (2), 318-329 (2016).
- Cox, B., et al. Organoids from pituitary as novel research model to study pituitary stem cell biology. Journal of Endocrinology. 240 (2), 287-308 (2019).
- Denef, C., Hautekeete, E., De Wolf, A., Vanderschueren, B. Pituitary basophils from immature male and female rats: distribution of gonadotrophs and thyrotrophs as studied by unit gravity sedimentation. Endocrinology. 130 (3), 724-735 (1978).
- Vander Schueren, B., Denef, C., Cassiman, J. J. Ultrastructural and functional characteristics of rat pituitary cell aggregates. Endocrinology. 110 (2), 513-523 (1982).
- Claes, C., et al. Human stem cell-derived monocytes and microglia-like cells reveal impaired amyloid plaque clearance upon heterozygous or homozygous loss of TREM2. Alzheimer’s and Dementia. 15 (3), 453-464 (2019).
- Trompeter, H. -. I., et al. MicroRNAs miR-26a, miR-26b, and miR-29b accelerate osteogenic differentiation of unrestricted somatic stem cells from human cord blood. BMC Genomics. 14, 111 (2013).
- Suga, H., et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 480 (7375), 57-62 (2011).
- Matsumoto, R., et al. Congenital pituitary hypoplasia model demonstrates hypothalamic OTX2 regulation of pituitary progenitor cells. Journal of Clinical Investigation. 130 (2), 641-654 (2019).
- Kanie, K., et al. Pathogenesis of anti-PIT-1 antibody syndrome: PIT-1 presentation by HLA class I on anterior pituitary cells. Journal of the Endocrine Society. 3 (11), 1969-1978 (2019).
- Lee, S. H., et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 173 (2), 515-528 (2018).
- Yan, H. H. N., et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 23 (6), 882-897 (2018).
- Nolan, L. A., Kavanagh, E., Lightman, S. L., Levy, A. Anterior pituitary cell population control: basal cell turnover and the effects of adrenalectomy and dexamethasone treatment. Journal of Neuroendocrinology. 10 (3), 207-215 (1998).

