Identification of Hemolytic and Phospholipase Activity in Crude Extracts from Sea Anemones by Straightforward Bioassays
Summary
Here, we describe a protocol to obtain crude venom extract from sea anemone and detect its hemolytic and phospholipase activity.
Abstract
Sea anemone venom composition includes polypeptide and non-proteins molecules. Cytolytic components have a high biotechnological and biomedical potential for designing new molecular tools. Sea anemone venom locates in glandular cells from ectoderm and sub-cellular structures called nematocysts, both of which are distributed throughout the sea anemone body. This characteristic implies challenges because the cells and nematocyst must be lysed to release the venom components with other non-toxic molecules. Therefore, first, the venom is derived from a crude extract (mixture of different and diverse molecules and tissue debris). The next step is to detect polypeptides with specific bioactivities. Here, we describe an efficient strategy to obtain the sea anemone crude extract and bioassay to identify the presence of cytolysins. The first step involves inexpensive and straightforward techniques (stirred and freeze-thaw cycle) to release cytolysins. We obtained the highest cytolytic activity and protein (~500 mg of protein from 20 g of dry weight). Next, the polypeptide complexity of the extract was analyzed by SDS-PAGE gel detecting proteins with molecular weights between 10 kDa and 250 kDa. In the hemolytic assay, we used sheep red blood cells and determined HU50 (11.1 ± 0.3 µg/mL). In contrast, the presence of phospholipases in the crude extract was determined using egg yolk as a substrate in a solid medium with agarose. Overall, this study uses an efficient and inexpensive protocol to prepare the crude extract and applies replicable bioassays to identify cytolysins, molecules with biotechnological and biomedical interests.
Introduction
Marine animals are a rich source of biologically active compounds. In recent decades, the composition of sea anemone venom has attracted scientific attention since it comprises a diversity of polypeptides with hemolytic, cytotoxic, enzymatic (phospholipase, protease, chitinase), and neurotoxic activity and inhibitory effects on proteolytic activity1. In addition, these polypeptides are potential sources for the development of molecular tools in biotechnological and therapeutical use2,3.
There are few reports about sea anemone venom and its molecular components due to the complexity of obtaining the venom, even isolation, and characterization of toxins. The extraction methods used in the reports involved lysis and emptying the contents of cells that are related and unrelated to the venom production1.
A particular characteristic in all cnidarians is the absence of a system for production and release of the venom centralized in a single anatomical region. Instead, the nematocysts are structures that keep the venom4,5. Other types of cells, called epidermal gland cells, also secrete toxins and are also distributed throughout the body of sea anemones6.
The first and most crucial challenge in obtaining the venom is the generation of an extract with sufficient manipulation in subsequent processes, without the inactivation or degradation of labile proteins. Next, the cells must be lysed, and the components-in this case, polypeptides must be efficiently and quickly extracted, avoiding proteolysis and hydrolysis while eliminating other cellular components7.
Different methods are used to obtain the crude extract of a sea anemone; some involve sacrificing the organism while others allow it to be kept alive. Methods that imply the use of the organism´s whole body allow for the release of most toxins from the venom8, compared to methods that keep organisms alive, which extract only some components of the venom9. The preparation of an extract requires evaluating the presence and potency of a substance of interest through a specific bioassay, which includes strategies to observe the pharmacological effects by in vivo or in vitro methods10.
Sea anemone venom contains cytolytic polypeptides, pore-forming toxins (PFTs)11, and phospholipases12; these molecules are models in the study of protein-lipid interaction, molecular tools in cancer therapy, and biosensors based on nanopore3. The classification of sea anemone PFTs is carried out according to their size or molecular weight, from 5 kDa to 80 kDa. The 20 kDa PFT, the most studied and known as actinoporins11, is of particular interest for its biomedical potential in the development of molecular tools for possible applications as anticancer, antimicrobial, and nanopore-based biosensors. Another cytolysin, including phospholipases, specifically phospholipase A2 (PLA2)13, releases a fatty acid due and hydrolyzes phospholipids, destabilizing the cell membrane. Due to this mechanism of action, PLA2 promises to be an essential model for the study and applications in inflammatory diseases. It could serve as a model for studies of lipid behavior in the cell membrane14.
Here, we describe an efficient protocol for obtaining the crude extract from sea anemone Anthopleura dowii Verrill, 1869, and detecting hemolysins and phospholipases. Both are relevant toxins that could be used as a template to design new molecular tools.
Protocol
The sea anemones were collected according to guidelines of the National Commission for Aquaculture, Fisheries, and Food of the Federal Government of Mexico (permit number PPF / DGOPTA 07332.250810.4060). Bioethics Committee of the Institute of Biotechnology, National Autonomous University of Mexico approved all the experiments with sea anemones. The sheep blood sample was purchased at the Center for Practical Teaching and Research in Animal Production and Health (CEPIPSA, National Autonomous University of Mexico).
1. Organism collection
- Collect sea anemone A. dowii.
NOTE: Here, the organisms were collected during low tide periods in the intertidal zone off the coast of Ensenada Baja, California, Mexico (Figure 1A,B). - Transport the organisms to the laboratory in containers with seawater (Figure 1C).
- In the laboratory, clean the organisms with distilled water by hand to remove the big substrate particles adhering to the body.
- Immediately freeze the organisms at -80 °C in an ultra-freezer for 72 h.
- Place the organisms in special glasses for freeze-drying, with lyophilization conditions of -20 °C, 0.015 psi, for 48 h.
- Store the lyophilized organisms at -20 °C until use.
2. Tissue hydration
- Reconstitute 20 g dry weight of lyophilized organisms with 60 mL (1/3 w/v) of 50 mM sodium phosphate buffer, pH 7.5, and 1 inhibitor cocktail tablet (Table of Materials).
- In a magnetic stirrer, continuously stir the sample at ~800 rpm, 4 °C, for 12 h.
3. Toxin release
- To induce cell lysis and nematocyst discharge, freeze the sample at -20 °C for 12 h, and then place the container in water at room temperature (RT) until it thaws up to 90%. Repeat this procedure three times.
NOTE: The sample does not thaw 100%; it is crucial to maintain the sample at ~4 °C to prevent protein degradation. - Observe the nematocyst discharged under a confocal microscope. Take 10 µL of the sample on a slide and place a coverslip over it. Observe under a confocal microscope at 100x and 60x magnification. Here a dye was not necessary.
- Process the images captured in the confocal microscope in ImageJ software (version 1.53c, Wayne Rasband, National Institutes of Health, USA, https://imagej.nih.gov/ij).
- If the tubule inside the nematocyst uncoils and is outside, the nematocysts are discharged; then, continue with the next step. Otherwise, only conduct one more freeze-thaw cycle.
- To clarify the extract, centrifuge the sample at 25,400 x g for 40 min, 4 °C, recover the supernatant, and centrifuge it again. Repeat this step 3-4 times.
- Recover the supernatant and discard the pellet. The supernatant or crude extract contains the venom components (toxins) and other cell molecules, such as lipids, carbohydrates, and nucleic acids.
4. Quantitation of total protein
- Determine the protein concentration of the crude extract using a commercial Bradford colorimetric assay15 kit (Table of Materials).
- Dilute the dye reagent (one part of dye with four parts of distilled water).
- Prepare bovine serum albumin (BSA) Fraction V (Table of Materials) solution at 1 mg/mL concentration in phosphate buffer (50 mM sodium phosphate, pH 7.5).
- Prepare the solutions listed in Table 1 in triplicate.
- Vigorously mix each solution in a shaker for 4 s.
- Incubate the samples for 5 min at RT, without shaking.
- Measure absorbance (AU) at 595 nm.
- Plot the averages of the sample BSA absorbances (AU vs. protein concentration), and determine the equation of the graph (Equation 1).
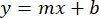 Equation 1
Equation 1
where y is the absorbance, m is the slope of the line, x is the protein concentration, and b is the y-intercept. To calculate crude extract concentration, solve for the x as follows:
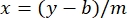 Equation 2
Equation 2
5. Determine polypeptide venom complexity
- Assemble the glass container for vertical gel electrophoresis according to the manual.
- Prepare acrylamide mix (30%): 29.2 g of acrylamide, 0.8 g of bis-acrylamide, the final volume of 100 mL. Filter the solution using a 0.45 µm membrane. Store the solution in a dark bottle at 4 °C. Then, prepare the mixture for the resolving gel (Table 2).
- The SDS-PAGE gel consists of a resolving gel and a stacking gel. Prepare the gels as per the solution volumes defined in Table 2. During preparation, ensure to maintain each mix at 4 °C to reduce polymerization time.
NOTE: The volume of each mixture varies depending on the brand of equipment used; for this protocol, the volumes correspond to the preparation of 15% acrylamide gel, 0.75 mm thick, for a vertical electrophoresis chamber (Table of Materials). - After the acrylamide has polymerized, ~10 min, add the mixture of stacking gel.
- Prepare the mixture for the stacking gel, immediately add it to the glass plates, and place a comb to form the wells for loading the samples.
- Once the stacking gel has polymerized (~15 min), remove the comb and place the glass container in a chamber for vertical electrophoresis.
- Place 200 mL of electrode buffer (0.25 M Tris, 0.192 M glycine, 0.1% SDS) in the electrophoresis chamber.
- Analyze the crude extract: Mix 30 µg of crude extract with 5 µL of protein loading buffer (25% glycerol, 15% sodium dodecyl sulfate, 25% 2-mercaptoethanol, 0.125 mM Tris pH 7, bromophenol blue 0.1 mg/mL) to denature proteins. The final volume must be <50 µL.
- Heat at 90 °C for 5 min, cool, and centrifuge for 3 s at 1,400 x g.
- Load the samples in the wells of the stacking gel. Load a standard molecular weight ladder.
- Run electrophoresis at 25 mA constant for 1 h 30 min.
- Remove the glass containers from the electrophoresis chamber to proceed with the staining of the gel proteins.
6. Protein staining
- Rinse the gel in 50 mL of distilled water to remove debris from the buffer electrode.
- Discard the water.
- To avoid the diffusion of the gel proteins, add the fixation solution (60 mL of ethanol, 20 mL of acetic acid, and 20 mL of distilled water, final volume 100 mL). Incubate at RT for 40 min with shaking at 80 rpm. Decant the solution.
- Add the protein crosslinking solution (15 mL of ethanol, 0.25 mL of glutaraldehyde, 50 mL of distilled water, final volume 65.25 mL), and incubate for 30 min at RT with shaking at 80 rpm. Remove the solution.
- Wash the gel with 100 mL of distilled water for 5 min, and remove the water. Repeat this procedure four times.
- Carry out protein staining with 50 mL of Coomassie brilliant blue R-250 filtered solution (40% methanol, 10% acetic acid, 50% distilled water, 0.1% dye, final volume 100 mL), stirring at 60 rpm in a laboratory rotary oscillator at RT for 15 min.
- Recover the solution, which can be used to stain other gels.
- To eliminate the excess dye, add a destaining solution (40 mL of methanol, 10 mL of acetic acid, 50 mL of distilled water). Keep stirring (80 rpm) at RT until the bands (stained proteins) visualize.
- Keep the gel in 50 mL of distilled water and scan the image in a Gel documentation system.
7. Prepare red blood cell solution
- Extract 1 mL of blood from the jugular vein of a sheep.
- Remove the needle from the syringe and immediately drain blood into a tube with 50 mL of Alsever solution (0.002 M citric acid, 0.07 M NaCl, 0.1 M dextrose, and 0.027 M sodium citrate as an anticoagulant, pH 7.4).
- Slowly invert the solution three times.
- Keep at 4 °C for transfer to the laboratory.
- Centrifuge at 804 x g for 5 min at 4 °C and discard the supernatant.
- Resuspend the pellet in 30 mL of Alsever solution. Add the solution by sliding it along the tube's inner walls and, using a Pasteur plastic pipette, slowly resuspend the cells. Centrifuge again at 804 x g for 5 min, 4 °C. Repeat this procedure until the supernatant is clarified.
- Add 15 mL of Alsever solution; resuspend the pellet slowly with a Pasteur plastic pipette.
- Maintain the final volume of Alsever solution to achieve a concentration of 2 x 106 cells/mL, approximately. Then, use a Neubauer chamber or hemocytometer to count the cells.
NOTE: Count the erythrocytes in the hemacytometer slide (Improved Neubauer). The slide is a 30 mm x 70 mm x 4 mm slide. The center has two silver footplates (Figure 2A) (one top and one bottom grid), each 3 mm x 3 mm, and two channels on the sides of the grid. Counting cells is in the corner and center squares (red squares in Figure 2B). The optimal concentration of cells for counting should be in the order of 106 cells. The central box has a surface area of 0.1 cm2, which is equivalent to 0.0001 mL. - Place a coverslip over the slide.
- Place 10 µL of the sample between the slide and the coverslip and wait for the sample to disburse.
- In an optical microscope (magnification 60x), perform the count in the first square box. Count only the cells in the center grid and those that touch the upper or the left margin of the box.
- Determine cell concentration using the following equation:
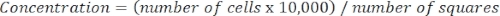 Equation 3
Equation 3
If the sample is very concentrated and dilution is required, then use the following equation:
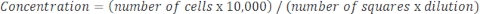 Equation 4
Equation 4
NOTE: When taking the erythrocyte solution for the hemolysis test, slowly homogenize the solution with a plastic Pasteur pipette. - Perform the steps in triplicate to reduce error.
8. Hemolysis assay
- Prepare the solution mixtures (in triplicates) indicated in Table 3 in conical 96-well plates.
NOTE: Mix the erythrocytes slowly to maintain a homogeneous suspension. - Incubate for 1 h at 37 °C, without shaking.
- Centrifuge the plate at 804 x g for 5 min at 4 °C.
- Recover the supernatant in another 96-well plate with a flat bottom.
- If the crude extract contains cytolysins, erythrocytes will lyse and release hemoglobin. Read the absorbance at 415 nm.
- Calculate hemolysis percentage, using the following equation:
% Hemolysis = ((Acrude extract-AAlsever buffer) / (AH2O-AAlsever)) x 100
NOTE: Acrude extract, AAlsever buffer, and AH2O correspond to the absorbance of erythrocytes with the crude extract, absorbance of Alsever solution, and absorbance of distilled water, respectively. - Perform a hemolysis test before and after each freezing and thawing cycle with 50 µg of total protein from the crude extract.
- Determine the number of freezing and thawing cycles required to reach 100% hemolytic activity.
- Calculate the amount of extract that produces hemolysis in 50% of the erythrocytes (HU50); follow the same protocol described in Table 3, increase the amount of sea anemone crude extract, and design the plot with sigmoidal adjustments using appropriate software (e.g., Origin, Table of Materials).
9. Phospholipase assay
- Wash one chicken egg with 1% SDS in distilled water.
- Separate the egg yolk from the egg white under sterile conditions.
- Prepare 50 mL of 0.86% NaCl solution, and filter through a 0.22 µm filter. Then, prepare Solution A: Add 12 mL of egg yolk and 36 mL of 0.86% NaCl solution.
- Prepare Solution B: Mix 300 mg of agarose in 50 mL of buffer (50 mM Tris, pH 7.5), filter through a 0.22 µm filter. Heat the solution in a microwave until boiling. Cool down in warm water to reach 43-45°C.
- Prepare Solution C: Prepare 10 mM CaCl2 and filter it with a 0.22 µm filter.
- Prepare Solution D: 10 mg of rhodamine 6G in 1 mL of distilled water.
- Under sterile conditions (laminar flow hood), add 500 µL of solutions A and C to solution B and 100 µL of solution D, mix, and pour 25 mL into each Petri dish (90 x 15 mm).
- Wait for the solution to solidify under sterile conditions (30 min).
- Make wells (~2-3 mm diameter) with a thin tube.
- Add a total of 20 µL of phosphate buffer (negative control) in one well and 20 µL of a determined phospholipase (positive control) in another well.
- Place different amounts of the crude extract protein, 5, 15, 25, 35, 45 µg, in the remaining wells, each in a final volume of 20 µL.
- Wait for the agar to adsorb all the samples (30 min).
- Incubate at 37 °C for 20 h.
- If a halo forms around the well, this indicates phospholipase activity. Measure the halo formed with a vernier caliper.
NOTE: Perform the experiment in triplicate (steps from 9.1-9.14).
Representative Results
The representative results of the protocol used to obtain the crude extract of sea anemone showed that combining two techniques (agitation and cycles of freezing and thawing) produced an efficient discharge of nematocysts, and the total amount of protein was 500 mg (8 mg/mL) (Figure 3).
The crude extract's protein complexity could be observed from 10 kDa and greater than 250 kDa through SDS-PAGE electrophoresis. In addition, cytolysins were detected in the molecular weight zone of 15 kDa and 20 kDa, a range of molecular weights that may correspond to phospholipases13 or actinoporins3 (Figure 4).
The hemolytic activity of the extract before and during the freezing and thawing cycles increased until 100% hemolysis was reached with 50 µg of the total crude extract protein in the last two cycles. The amount needed to lyse 50% sheep erythrocytes (HU50) is 11.1 ± 0.3 µg/mL (Figure 5). Phospholipase activity was detected by the formation of clear halos in the areas of the agar plate, where the crude extract sample was applied. The results show the presence of phospholipase activity from 15 µg of total protein. The diameter of the halo increased in a dose-dependent manner, i.e., if the amount of crude extract increased, the diameter of the halo increased (Figure 6A and Table 4).

Figure 1: Sea anemone collection. (A) Intertidal zone in El Sauzal, Baja California Norte, Mexico. (B) Sea anemone collected. (C) Anthoplerua dowii Verrill, 1869. Please click here to view a larger version of this figure.
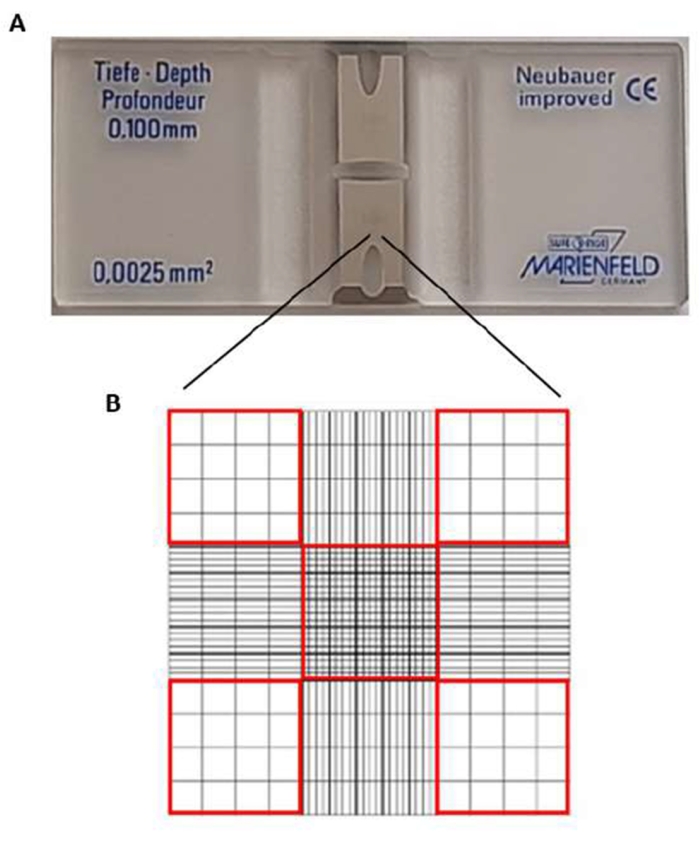
Figure 2: Hemacytometer slide. (A) In the slide center, there are two silver footplates. (B) Cells in squares with a red perimeter are counted. Please click here to view a larger version of this figure.
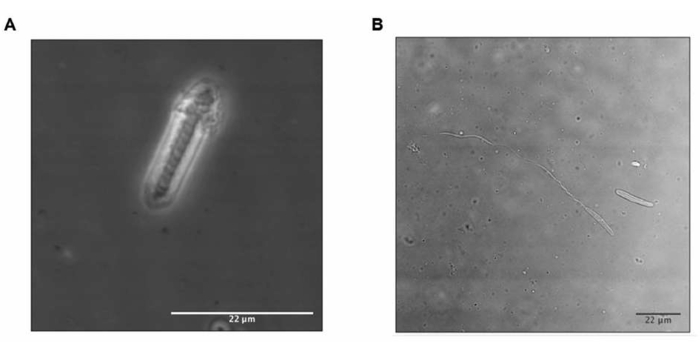
Figure 3: Nematocyst discharge. Nematocyst (A) before and (B) after stimuli. In B, the nematocyst tubule is exposed and indicates toxin release. Please click here to view a larger version of this figure.
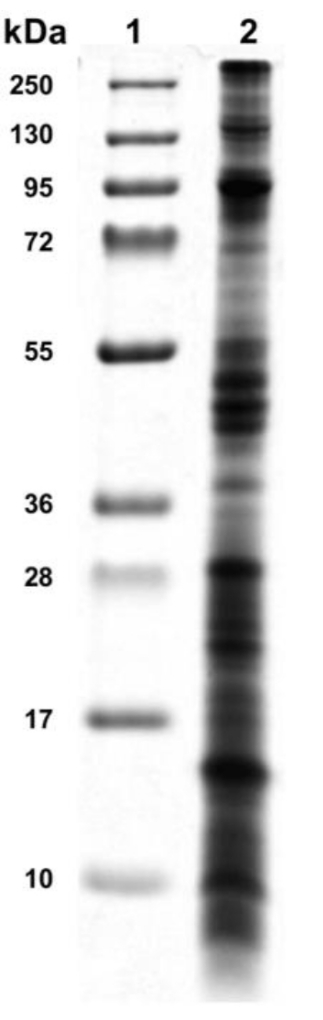
Figure 4: Electrophoretic profile of sea anemone crude extract. The sea anemone crude extract was analyzed by SDS-PAGE 15% polyacrylamide gel and showed protein from 10 kDa to 250 kDa of molecular weight. Lane 1: protein ladder, lane 2: sea anemone venom. Please click here to view a larger version of this figure.
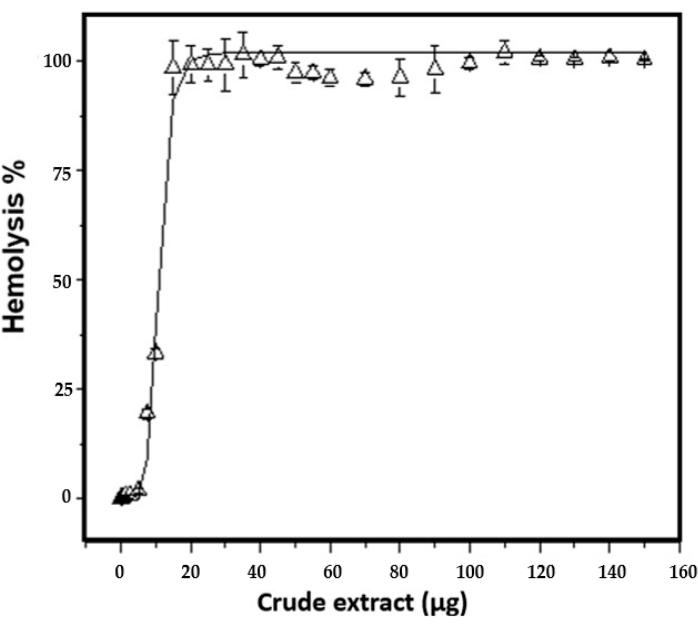
Figure 5: Hemolysis assay in erythrocytes from sheep. Hemoglobin release was detected at 415 nm. Different amount of protein was assayed to determine HU50, equal to 11.1 ± 0.3 µg/mL. Hemolysis assays were performed in triplicate. The bars represent the standard deviation. Please click here to view a larger version of this figure.
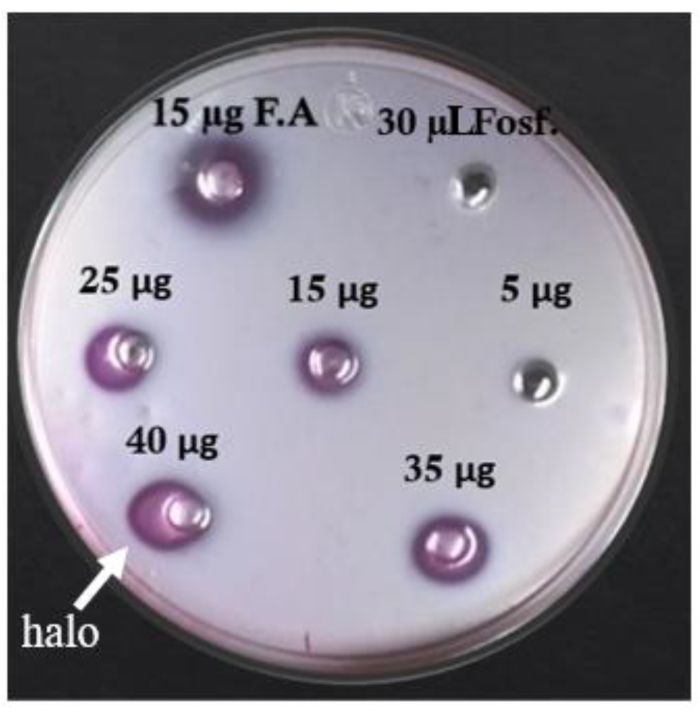
Figure 6: Phospholipase assay. Different amounts of total protein were assayed in agarose with egg yolk in the presence of Ca2+. Phospholipase activity can be observed around each well like a halo. Controls: phosphate buffer (Fosf.) and a PLA2 from a snake (F.A). Please click here to view a larger version of this figure.
| Tube | Distilled Water | BSA | Dye (µL) | Final volume | |
| (µL) | (µg) | (µL) | (µL) | ||
| 1 | 800 | – | – | 200 | 1000 |
| 2 | 799 | 1 | 1 | 200 | 1000 |
| 3 | 797 | 3 | 3 | 200 | 1000 |
| 4 | 795 | 5 | 5 | 200 | 1000 |
| 5 | 793 | 7 | 7 | 200 | 1000 |
| 6 | 790 | 10 | 10 | 200 | 1000 |
| Crude extract (µL) | |||||
| 7 | 798-790 | from 2 to 10 | 200 | 1000 | |
Table 1: Quantitation of protein by Bradford assay.
| Solution | Resolving gel | Stacking gel |
| Destilled water | 1.1 mL | 1.4 mL |
| Acrylamide mix (30%) | 2.5 mL | 0.33 mL |
| Tris (1.5 M, pH 8.8), adjust pH with HCl. | 1.3 mL | |
| Tris (0.5 M, pH 6.8), adjust pH with HCl. | 0.25 mL | |
| Sodium dodecyl sulfate (SDS) 10% (w/v) | 0.5 mL | 0.1 mL |
| Ammonium persulfate (APS) (10%) | 0.5 mL | 0.1 mL |
| N,N,Nˈ,Nˈ-tetramethylethylenediamine (TEMED) | 0.005 mL | 0.005 mL |
Table 2: Solutions for preparing a 15% acrylamide SDS-PAGE electrophoresis gel.
| Well | Alserver solution (µL) | Distilled water | Crude extract | Erythrocyte solution (µL) | Final volume | Expected absorbance |
| (µL) | (µL) | (µL) | (415 nm) | |||
| 1 | 180 | – | – | 20 | 200 | ≤0.1 |
| 2 | – | 180 | – | 20 | 200 | ≤1.0 |
| 3 | 177–170 | – | 3–10 | 20 | 200 | 0.1–1.0 |
Table 3: Hemolysis assay to calibrate erythrocytes.
| Sample | Diameter of halo (mm) |
| 15 µg of F.A | 0 |
| 30 µL of Fosf. | 6.3 ± 0.5 |
| 5 µg | 0 |
| 15 µg | 2.3 ± 0.5 |
| 25 µg | 3.5 ± 0.5 |
| 35 µg | 4.1 ± 1 |
| 45 µg | 4.6 ± 0.8 |
Table 4: Diameter of the halos at different amounts of protein from the crude extract.
Discussion
The high demand for new compounds with applications in different fields of science and industry has led to the study of venom. Venom represents a rich source of molecules that serves as a template for generating new molecular tools. However, the complexity of these venoms requires the implementation and combination of various methods to obtain and study them.
Here, we show a method for obtaining and analyzing the venom of the sea anemone Anthopleura dowii, Verrill 1869, which can be used to explore the venom of other sea anemones species starting with lyophilized specimens, and then crude extract obtention. The lyophilization step allowed preserving the organisms until use and for part of the sample to be employed according to the experiment's needs, allowing for the efficient application of organisms, avoiding the collection of high numbers of them, and maintaining protein stability7,16. Crude extract preparation with toxins from fresh organisms is more potent than those isolated from lyophilized organisms. However, proteins of crude extract conserve their function for more time when frozen at -20 °C17. Lyophilization of the organisms also has bioethical implications since this allows optimization in the use of the sample and avoids the collection of more organisms.
The freezing and thawing method allows working with the organism's complete body, with the advantage of cnida being distributed throughout the body. However, it is essential to cycle freeze and thaw efficiently (for long periods and other techniques) to obtain more venom. In other reports, venom extraction is carried out via short time cycles, reducing the amount and diversity of toxins in the crude extract18,19.
The Bradford assay used to determine the total amount of protein obtained in the crude extract is a reproducible, inexpensive, and simple method compatible with the crude extract conditions15. The total protein obtained here is higher than the amount reported previously20,21,22. The diversity and abundance of toxins in the extraction will depend on the species and collection of the organisms.
Gel electrophoresis by SDS-PAGE is an efficient technique used to analyze the complexity of crude extract. However, a good fixation of proteins with glutaraldehyde is important when analyzing the proteins of lower molecular weight and lower abundance that may diffuse from the gel during the staining process23.
One advantage of having an extract with high polypeptide complexity is the possibility of carrying out a complete proteomic study. If this extract is subjected to purification processes, it is possible to identify an even more significant number of toxins of biotechnological, ecological, and biomedical interests24.
In the study of venom, identifying a molecule or a group of polypeptides with a specific function is generally difficult. Therefore, it is essential to have reproducible, specific, fast, and inexpensive bioassays. Hemolytic and phospholipase assays are the most used to study cytolysins in venoms. As actinoporins have a high affinity for sphingomyelin, it is best to use erythrocytes with a high amount of this lipid in their membranes since fewer samples will be used in this way. In this sense, sheep erythrocytes are cells with high sphingomyelin in their membrane25. The hemolysis assay is analyzed, detecting the presence of hemoglobin at 415 nm absorbance. This bioassay only indicates that the presence of cytolysins can act on erythrocytes. However, it is not possible to determine the type of cytolysin that causes hemolysis; for this reason, we recommend conducting complementary assays.
Although the egg yolk agarose assay allows for identifying the presence of different types of phospholipases depending on the color of the halo, we cannot distinguish different phospholipases for the crude extract of A.dowii; a possibility is the high phospholipase diversity produced by A. dowii26. For future studies, we suggest performing this assay with a crude extract concentration gradient, perhaps with protein nanograms.
Although there are molecules in the marine ecosystem with high potential in medical, biotechnological, and industrial areas, there is still much to analyze and investigate in the venom of cnidarians.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), with a grant number IT200819. The authors acknowledge to Tom Musselman, Rock Paper Editing, LLC, for checking the English grammar of this manuscript; and the technical assistance of Samanta Jiménez (CICESE, Ensenada), and Juan Manuel Barbosa Castillo (Instituto de Fisiología Celular, UNAM). We also thank to Dr Augusto César Lizarazo Chaparro (CEPIPSA) for obtaining sheep blood. We especially thank Dr José Saniger Blesa, ICAT-UNAM, for the facilities in his laboratory for the video recording.
Materials
| 15 mL conical centrifuge tube | Corning | 430766 | |
| 2-Bromophenol blue | Sigma | B75808 | |
| 2-mercaptoetanol | Sigma-Aldrich | M6250-100ML | |
| 50 mL conical centrifuge tubes | Corning | 430828 | |
| Acetic Acid Glacial | J.T. Baker | 9515-03 | |
| Acrylamide | Promega | V3115 | |
| Agarose | Promega | V3125 | |
| Bisacrylamide | Promega | V3143 | |
| Bovine Serum Albumin Fraction V | Sigma | A3059-100G | |
| Bradford Protein Assays | Bio-Rad | 5000006 | |
| Calcium chloride | Sigma-Aldrich | C3306 | |
| Cell culture plates 96 well, V-bottom | Corning | 3894 | |
| Centrifuge | Eppendorf | 5804R | |
| Centrifuge tubes | Corning | CLS430829 | |
| ChemiDoc MP system | Bio-Rad | 1708280 | |
| Citric acid | Sigma-Aldrich | 251275 | |
| Clear flat.bottom 96-Well Plates | Thermo Scientific | 3855 | |
| Coomassie Brilliant Blue G-250 | Bio-Rad | #1610406 | |
| Coomassie brilliant blue R-250 | Bio-Rad | 1610400 | |
| Dextrose | J.T. Baker | 1916-01 | |
| Ductless Enclosure | Labconco | Vertical | https://imagej.nih.gov/ij ImageJ 1.53c |
| Gel Doc EZ | Bio Rad. | Gel Documentation System | |
| Glycerol | Sigma-Aldrich | G5516-4L | |
| Hemocytometer | Marienfeld | 650030 | |
| ImageJ (Software) | NIH, USA | Version 1.53c | |
| Incubator 211 | Labnet | I5211 DS | |
| Methanol | J.T. Baker | 9049-03 | |
| Mini-PROTEAN tetra cell | Bio-Rad | 1658000EDU | |
| Na2HPO4 | J.T. Baker | 3824-01 | |
| NaCl | J.T. Baker | 3624-01 | |
| NaH2PO4.H2O | J.T. Baker | 3818-05 | |
| Origin software | version 9 | To design the plot with sigmoidal adjustments | |
| Petridish | Falcon | 351007 | |
| Pipetman kit | Gilson | F167380 | |
| Precast mini gel | BioRad | 1658004 | |
| Prestained Protein Ladder | Thermo Scientific | 26620 | |
| Protease Inhibitor Cocktail | Roche | 11836153001 | |
| Protein Assay Dye Reagent Concentrate | Bio-Rad | 5000006 | |
| Rhodamine 6G | Sigma-Aldrich | 252433 | |
| SDS | Sigma-Aldrich | L4509 | |
| Sodium citrate dihydrate | JT Baker | 3646-01 | |
| Spectrophotometer | THERMO SCIENTIFIC | G10S UV-VIS | |
| Tris Base | Sigma-Aldrich | 77-86-1 | |
| Volt Power Supply | Hoefer | PS300B |
References
- Frazão, B., Vasconcelos, V., Antunes, A. Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: an overview. Marine Drugs. 10 (8), 1812-1851 (2012).
- Jayathilake, J. M. N. J., Gunathilake, K. V. K. Cnidarian toxins: recent evidences for potential therapeutic uses. The European Zoological Journal. 87 (1), 708-713 (2020).
- Ramírez-Carreto, S., Miranda-Zaragoza, B., Rodríguez-Almazán, C. Actinoporins: From the structure and function to the generation of biotechnological and therapeutic tools. Biomolecules. 10 (4), 539 (2020).
- Fautin, D. G. Structural diversity, systematics, and evolution of cnidae. Toxicon. Official Journal of the International Society on Toxinology. 54 (8), 1054-1064 (2009).
- Moran, Y., et al. Analysis of soluble protein contents from the nematocysts of a model sea anemone sheds light on venom evolution. Marine Biotechnology. 15 (3), 329-339 (2013).
- Moran, Y., et al. Neurotoxin localization to ectodermal gland cells uncovers an alternative mechanism of venom delivery in sea anemones. Proceedings of the Royal Society B: Biological Sciences. 279 (1732), 1351-1358 (2012).
- Grabski, A. C. Advances in preparation of biological extracts for protein purification. Methods in Enzymology. 463, 285-303 (2009).
- Bulati, M., et al. Partially purified extracts of sea anemone Anemonia viridis affect the growth and viability of selected tumour cell lines. BioMed Research International. 2016, 3849897 (2016).
- Orts, D. J. B., et al. Biochemical and electrophysiological characterization of two sea anemone type 1 potassium toxins from a geographically distant population of Bunodosoma caissarum. Marine Drugs. 11 (3), 655-679 (2013).
- Hader, D., Erzinger, G. . Bioassays: Advanced Methods and Applications. , (2017).
- Anderluh, G., Macek, P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria). Toxicon: Official Journal of the International Society on Toxinology. 40 (2), 111-124 (2002).
- Nevalainen, T. J., et al. Phospholipase A2 in cnidaria. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 139 (4), 731-735 (2004).
- Razpotnik, A., et al. A new phospholipase A2 isolated from the sea anemone Urticina crassicornis – its primary structure and phylogenetic classification. The FEBS Journal. 277 (12), 2641-2653 (2010).
- Dennis, E. A., Cao, J., Hsu, Y. -. H., Magrioti, V., Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chemical Reviews. 111 (10), 6130-6185 (2011).
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72, 248-254 (1976).
- Kwon, Y. -. C., Jewett, M. C. High-throughput preparation methods of crude extract for robust cell-free protein synthesis. Scientific Reports. 5, 8663 (2015).
- Eno, A. E., Konya, R. S., Ibu, J. O. Biological properties of a venom extract from the sea anemone, Bunodosoma cavernata. Toxicon: Official Journal of the International Society on Toxinology. 36 (12), 2013-2020 (1998).
- Morales-Landa, J. L., et al. Antimicrobial, antiprotozoal, and toxic activities of Cnidarian extracts from the Mexican Caribbean Sea. Pharmaceutical Biology. 45 (1), 37-43 (2007).
- Sánchez-Rodríguez, J., Cruz-Vazquez, K. Isolation and biological characterization of neurotoxic compounds from the sea anemone Lebrunia danae (Duchassaing and Michelotti, 1860). Archives of Toxicology. 80 (7), 436-441 (2006).
- de Oliveira, J. S., et al. Caissarolysin I (Bcs I), a new hemolytic toxin from the Brazilian sea anemone Bunodosoma caissarum: purification and biological characterization. Biochimica Et Biophysica Acta. 1760 (3), 453-461 (2006).
- Norton, R. S., et al. Purification and characterisation of proteins with cardiac stimulatory and haemolytic activity from the anemone Actinia tenebrosa. Toxicon: Official Journal of the International Society on Toxinology. 28 (1), 29-41 (1990).
- Gondran, M., Eckeli, A. L., Migues, P. V., Gabilan, N. H., Rodrigues, A. L. S. The crude extract from the sea anemone, Bunodosoma caissarum elicits convulsions in mice: possible involvement of the glutamatergic system. Toxicon: Official Journal of the International Society on Toxinology. 40 (12), 1667-1674 (2002).
- Dion, A. S., Pomenti, A. A. Ammoniacal silver staining of proteins: mechanism of glutaraldehyde enhancement. Analytical Biochemistry. 129 (2), 490-496 (1983).
- Ramírez-Carreto, S., et al. Identification of a pore-forming protein from sea anemone Anthopleura dowii Verrill (1869) venom by mass spectrometry. The Journal of Venomous Animals and Toxins Including Tropical Diseases. 25, 147418 (2019).
- Nelson, G. J. Studies on the lipids of sheep red blood cells. I. Lipid composition in low and high potassium red cells. Lipids. 2 (1), 64-71 (1967).
- Ramírez-Carreto, S., et al. Transcriptomic and proteomic analysis of the tentacles and mucus of Anthopleura dowii Verrill, 1869. Marine Drugs. 17 (8), 436 (2019).

