Uncovering Hidden Dynamics of Natural Photonic Structures Using Holographic Imaging
Summary
The paper is primarily focused on the combined power of optical (linear and nonlinear) and holographic methods used to reveal phenomena at the nanoscale. The results obtained from the biophotonic and oscillatory chemical reactions’ studies are given as representative examples, highlighting holography’s ability to reveal dynamics at a nanoscale.
Abstract
In this method, the potential of optics and holography to uncover hidden details of a natural system’s dynamical response at the nanoscale is exploited. In the first part, the optical and holographic studies of natural photonic structures are presented as well as conditions for the appearance of the photophoretic effect, namely, the displacement or deformation of a nanostructure due to a light-induced thermal gradient, at the nanoscale. This effect is revealed by real-time digital holographic interferometry monitoring the deformation of scales covering the wings of insects induced by temperature. The link between geometry and nanocorrugation that leads to the emergence of the photophoretic effect is experimentally demonstrated and confirmed. In the second part, it is shown how holography can be potentially used to uncover hidden details in the chemical system with nonlinear dynamics, such as the phase transition phenomenon that occurs in complex oscillatory Briggs-Rauscher (BR) reaction. The presented potential of holography at the nanoscale could open enormous possibilities for controlling and molding the photophoretic effect and pattern formation for various applications such as particle trapping and levitation, including the movement of unburnt hydrocarbons in the atmosphere and separation of different aerosols, decomposition of microplastics and fractionation of particles in general, and assessment of temperature and thermal conductivity of micron-size fuel particles.
Introduction
To fully understand and notice all the unique phenomena in the nanoworld, it is crucial to employ techniques that are capable of revealing all details regarding structures and dynamics at the nanoscale. On this account, the unique combination of linear and nonlinear methods, combined with the power of holography to reveal the system's dynamics at the nanoscale are presented.
The described holographic technique can be viewed as the triple rec method (rec is the abbreviation for recording), since at a given time the signal is simultaneously recorded by a photographic camera, a thermal camera, and an interferometer. Linear and nonlinear optical spectroscopy and holography are well-known techniques, the fundamental principles of which are extensively described in the literature1,2.
To cut a long story short, holographic interferometry allows the comparison of wavefronts recorded at different moments in time to characterize the dynamics of the system. It was previously used to measure vibrational dynamics3,4. The power of holography as the simplest interferometry method is based on its ability to detect the smallest displacement within the system. First, we exploited holography to observe and reveal the photophoretic effect5 (i.e., the displacement of deformation of a nanostructure due to a light-induced thermal gradient), in different biological structures. For a true presentation of the method, representative samples were selected from a number of tested biological specimens6. Wings of the Queen of Spain fritillary butterfly, Issoria lathonia (Linnaeus, 1758; I. lathonia), were used in the framework of this study.
After having successfully demonstrated the occurrence of photophoresis at the nanoscale in biological tissues, a similar protocol was applied to monitor the spontaneous symmetry breaking process7 caused by a phase transition in an oscillatory chemical reaction. In this part, the phase transition from a low concentration of iodide and iodine (called state I) to a high concentration of iodide and iodine with solid iodine formation (defined as state II) that occurs in a chemically nonlinear BR reaction was studied8,9. Here, we reported for the first time a holographic approach that allows studying such a phase transition and spontaneous symmetry breaking dynamics at the nanoscale occurring in condensed systems.
Protocol
1. Precharacterization
- Perform a full precharacterization of the sample.
- Perform all experiments on dry specimens purchased from a commercial source. Store the samples in the laboratory, in a dry and dark place, at room temperature.
- Prior to holographic measurements, perform a complete sample characterization by scanning electronic microscope (SEM), linear optical spectroscopy, and nonlinear optical microscopy (NOM)10 (Figure 1).
- In addition to the optical properties of samples measured by linear techniques, gather supplementary information with higher intensity laser beams that allow characterization of their nonlinear optical properties.
- Use the corresponding nonlinear optical susceptibilities to quantify the nonlinear optical response and form the basis of nonlinear optical techniques such as nondestructive multiphoton excitation fluorescence and second harmonic generation (SHG), which are used to characterize various biological samples.
- For the nonlinear chemical phenomena occurring in the oscillating BR reaction, carry out the study of interferometric monitoring of the in situ phase transition from state I to state II with the following concentrations of reactants: [CH2(COOH)2]0 = 0.0789 mol dm-3, [MnSO4]0 = 0.0075 mol dm-3, [HClO4]0 = 0.03 mol dm-3, [KIO3]0 = 0.0752 mol dm-3, and [H2O2]0 = 1.269 mol dm-3 (0 after the bracket stands for the initial concentration at the beginning of the process). Make the total volume used for the BR reaction equal to 2.5 mL.
NOTE: The concentration values used here are equal to the ones in the study by Pagnacco et al.8, but with reaction volume divided by 10.
- Prepare the sample for the experiment.
- Use wings of the Queen of Spain fritillary butterfly, I. lathonia, for this experiment. Place the wing on a hard surface and make a section with a 10 mm diameter cutter. Place the sample in the sample box, which can be any container with a lid.
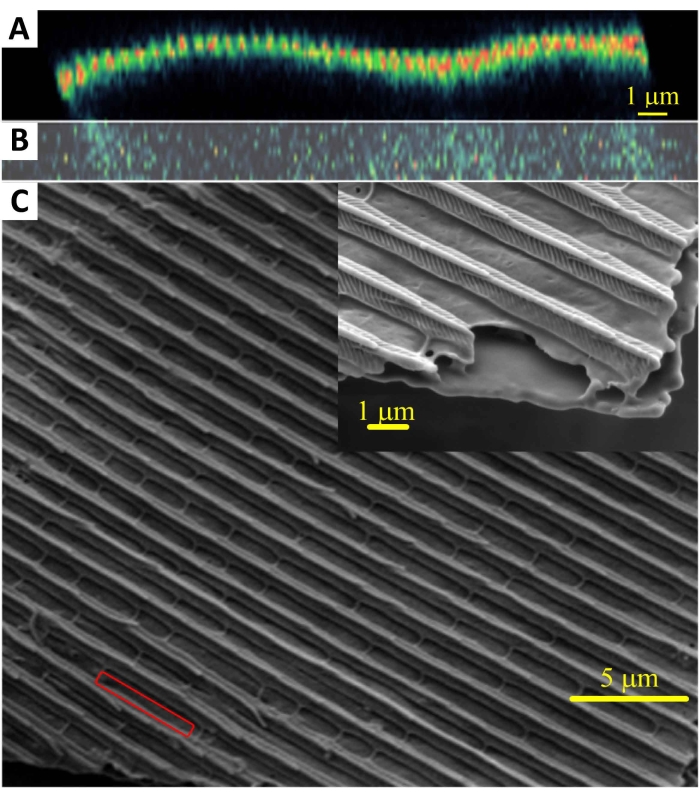
Figure 1: Wavy cross-section of butterfly wing scale. The cross-section was recorded on a nonlinear optical scanning microscope (A,B). A SEM observation (C) of a wing of the Queen of Spain fritillary butterfly, I. lathonia, was also done. This figure has been modified from14. Please click here to view a larger version of this figure.
2. Experimental setup
- Holographic setup
NOTE: The holographic interferometry measurements were performed with a tailor-made optical setup (Figure 2).- Adjust the laboratory temperature to be 23 °C ± 0.2 °C. Turn the laser on. Use a laser (details given in the Table of Materials) with an excitation wavelength of 532 nm for these holographic observations.
- Check the alignment of the optical elements (Figure 2). First, check that setup is made according to the scheme in Figure 2.
- Align the laser beam perfectly with the concave mirror M. Check and adjust the position of the optical beam expander (L).
- Determine the beam part that impinges on sample S and ensure that it forms a reflex beam O. Check if the rest of the beam is collected on a spherical mirror CM, to be used to generate the reference beam R. Check if the detector C is placed within the interference zone of the two specified beams.
NOTE: A complementary metal oxide semiconductor (CMOS) sensor is used as detector. - Set up the cameras according to the instructions for the camera used. Set up an optical/photographic camera for the holographic experiment as shown in Figure 2 (C is the camera; details given in the Table of Materials). Set up a second optical/photographic camera to view visible changes in BR reaction and a thermal camera with a thermal resolution of 50 mK and a focal length of 13 mm above the optical table.
NOTE: The camera used in the holographic experiment does not use an objective lens; the light directly impinges on the chip.
- Prepare the sample into holographic setup.
- Prepare the wing sample as in step 1.2.1. Place the prepared sample on a round metal support with a diameter of 15 mm. The support has three existing holes for the screws to which the metal ring holding the sample is attached.
- Attach the ring to the support. Place the attached sample in the part of the sample mount located on the optical table.
- Prepare the sample for chemical reaction monitoring. On the optical table, in the intended place, place a support with a flat adhesive surface on which the cuvette/vessel will be placed.
- Prepare the reagent used to initialize the reaction as in step 1.1.5. Fill the reactants into the cuvette, and mix in cuvette in the following order of volumes and concentrations: 0.7 mL of 0.2817 mol dm-3 CH2(COOH)2; 0.5 mL of 0.0375 mol dm-3 MnSO4; 0.5 mL of 0.15 mol dm-3 HClO4; 0.5 mL of 0.376 mol dm-3 KIO3 ; and 0.3 mL of 10.575 mol dm-3 H2O2.
- Ensure that the total volume in the cuvette is 2.5 mL, and place it on the support in the setup.
- Set up additional instruments if needed. For monitoring the photophoretic effect, use an additional laser (details given in Table of Materials) for local heating.
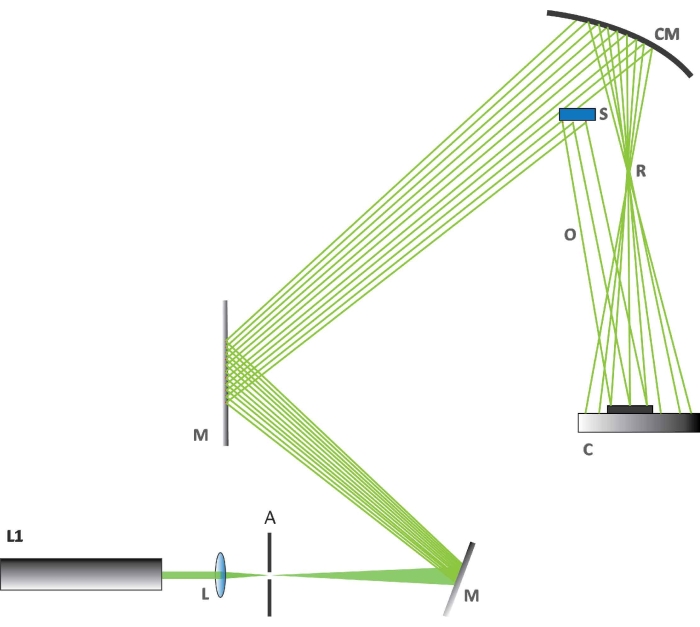
Figure 2: The holographic setup. The figure shows how the various components are arranged for the holographic experiment. Abbreviations: L1 = laser at 532 nm, L = biconvex lens, A = aperture, M = a flat mirror used to deflect the laser beam, CM = concave mirror, C = CMOS camera, S = butterfly wing section, R = reference beam, O = object beam. Please click here to view a larger version of this figure.
3. Setup of the software used
NOTE: Home-built C++ software based on Fresnel approximation11 is used to analyze data from holographic experiments. The software developed for the presented study can be found at .12 The details of software cannot be published at the moment; however, additional information will be provided on request. Fresnel approximation is extremely useful in digital holography since it focuses on different surfaces and zooms in on the area of the first diffraction order, which contains complete information about the recorded scene.
- Turn on the computer and run the software.
NOTE: The step for running the software depends on the software itself. There is no commercial software for this purpose.
4. Perform the experiment
- Switch off the external lights. Carry out the whole experiment in a dark room.
- Synchronize the cameras by using a chosen interval. For this experiment, start the holographic camera after 60 s, and the two other cameras immediately after it, using either a software or manually.
- Press the recording buttons and define in the software when the recording starts.
- Induce dynamical changes in the system of interest. The method of initiation depends on the type of sample; in the case of photophoretic effect, externally heat the sample by using the available lasers: 450 nm, 532 nm, 660 nm, 980 nm. In the case of the BR reaction, start the reaction by mixing the chemical reactants. Observe the holographic experiment.
- Set the photographic and thermal camera to follow the whole experiment and determine the moment of the end of the holographic recording from the optical and thermal measurements.
- Pronounce the end of the process. The end of the recording is preprogrammed, according to the estimated duration of the process. For the BR reaction, use solidification as the end of the reaction. In the case of the photophoretic effect, there is no such specific moment. In any case, this step emphasizes the importance of triple recording.
5. Acquisition of results12
- Save the results. Precisely sort the files as a function of time for reconstructing holograms and deeper data analysis.
NOTE: In this step, the data is transferred from the camera used for holography to the computer (hard disk) in folders named after the shooting dates. Use copy/paste and rename buttons. - Check the probe hologram for appropriate settings. In this way, the best settings are selected on the first hologram by looking at it, and then used for the reconstruction of all holograms.
- Choose one hologram by clicking on one of them from the folder you previously made (step 5.1) and make a reconstruction by clicking on the Reconstruct button.
- Change the settings to achieve the best image and make the reconstruction again. Options for adjusting parameters such as sampling, offset, and Fresnel distance will appear on the screen (software menu). Repeat these steps until the best settings are defined.
- Perform the reconstructions. Choose all the holograms by clicking the Open File button and choosing all files. Apply the desired parameters for numerical reconstruction of holograms; they remain unchanged after the step 5.2.1, so do not perform any action this time.
- Carry out the reconstructions using the Reconstruct button, and the interferograms by inserting the file names in the start with/end with field and then by clicking the button Batch. The interferograms appear in the previously made folder (in step 5.1).
NOTE: After recording a series of holograms in time, the first hologram represents an unperturbed state, while the action of an external force causes subsequent holograms. It is necessary to reconstruct the holograms using shifted Fresnel transform13. - Obtain the interferograms by subtraction (in terms of complex numbers) of a particular hologram in time with the first hologram obtained.
NOTE: This protocol allows observing the effect of the force on the object. The change in the interference pattern as a function of time is a consequence of deformation or displacement that occurs within the system during the measurement. These changes are used to monitor the system's dynamics at the nanoscale.
6. Analyses of the results
- Perform a visual analysis as the first quality control step of the process. In this step, look for visible changes in interference pattern and try to match the changes in the interference pattern with results obtained by optical and thermal measurements.
- Perform a cross-examination of all recordings. In this second phase of the analysis, thoroughly analyze the images visually from both the optical and thermal cameras with the holographic reconstructions in order to reveal dynamics at the nanoscale. In this way, the reaction moment is seen simultaneously in holographic, thermal, and photographic images.
- Make a graphical representation of results based on numerical/software analysis and present them in the form of graphs (1D, 2D, or 3D), charts, histograms etc. After a complete analysis of results, draw conclusions and anticipate further research based on this.
Representative Results
A photophoretic effect was induced and monitored in a first experiment on the wing of a Morpho menelaus butterfly5. The effect was initiated by the action of LED lasers of different wavelengths (450 nm, 532 nm, 660 nm, and 980 nm). Here, the wings from an I. lathonia butterfly14 were used. After the recording procedure, the hologram image was reconstructed.
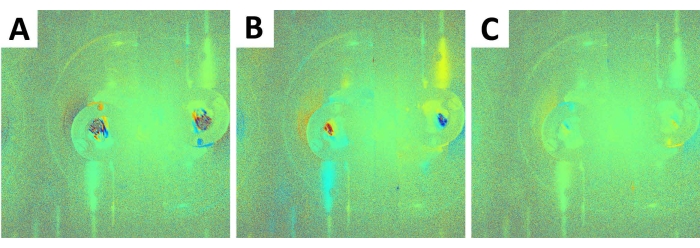
Figure 3: I. lathonia wings' holographic reconstructions. The reconstruction was done at 450 nm initiation (A), 532 nm initiation (B), and 980 nm initiation (C). The images show an obvious difference in the visual sense, where depending on the wavelength, the colored area appears in different sizes. Please click here to view a larger version of this figure.
The fringes observed in Figure 3A–C are the consequence of the interference. This figure clearly shows that changes occur only during the irradiation of the sample with a second laser (placed to hit the sample with a beam that does not interfere with the beam from the primary laser; put into operation at any time during the recording), and confirms that holographic interferometry can be used to monitor the deformation or displacement of the biological tissues.
Figure 3A–C shows how different wavelengths between 450 nm (Figure 3A), 532 nm (Figure 3B), and 980 nm (Figure 3C) affect the interferometric pattern by causing different morphological displacements within the tissues.
In the second experiment regarding the oscillatory BR reaction, this reaction started immediately after the addition of hydrogen peroxide, producing a large amount of oxygen (Figure 4A). As the transition from state I to state II (Figure 4) is essentially irreproducible for an individual kinetic run8, the moment of transition is very difficult to monitor. Therefore, the presented results are the consequence of a large number of attempts. In the analysis of interferograms, a change in the fringe pattern was noticed at the exact moment when the reaction occurred (i.e., when transition from state I to state II occurred). Figure 4E shows a moment before the reaction occurred (left) and the exact moment (right). The wavelength used here is 573 nm. When calculating the displacement data from the amplitude image, the method of direct fringe counting was used. One fringe corresponds to a displacement of half the wavelength (i.e., 286.5 nm). If the displacement data is calculated from the phase, the following relation applies: Δl/λ = ΔΦ/2π.
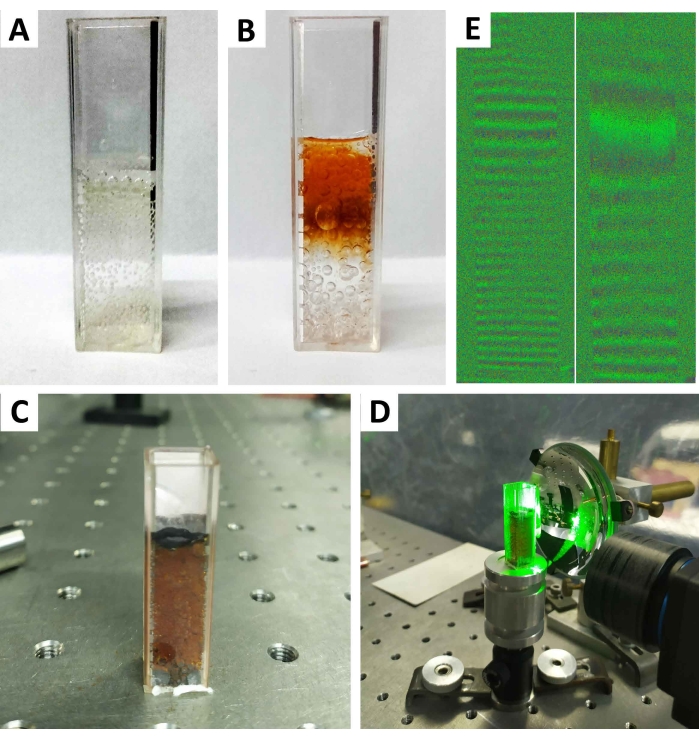
Figure 4: The transition from state I to state II in Briggs-Rauscher (BR) reaction. The different recordings for the transition from state I to state II in Briggs-Rauscher (BR) reaction. (A) The beginning of the BR reaction with bubbles corresponds to oxygen and carbon dioxide formation. (B) The state I to state II reaction course. (C) The end of state I to state II transition. (D) Cuvette in setup. (E) Interferogram of the moment before reaction (left) and the moment of reaction (right). Please click here to view a larger version of this figure.
Nonlinear chemical phenomena have been known for more than 100 years15, but despite this, there are still doubts about their full mechanism and dynamics16,17. The results obtained open new possibilities for the investigation and monitoring of such complex chemical phenomena in situ by a holographic technique.
Discussion
In the presented biophotonic study, it is shown that a novel holographic method can be used to detect minimal morphological displacement or deformation caused by low-level thermal radiation.
The most critical step in holographic measurement with biological samples is the preparation step. The preparation of the sample (cutting/gluing to match the size of the holder) depends on the sample's mechanical properties, and it is not possible to have a standard protocol for this step.
Regarding the BR study, it is vital to have a transparent reaction vessel and relatively clear optical path, since every obstacle during a chemical reaction, or physical transformation (like the release of oxygen, impurity) will affect interference pattern and therefore recorded results.
In general, the most significant limitation of the described method is the sample size that can be studied. The sample must have an appropriate dimension to be inserted within the optical setup.
Here we show that holographic interferometry (HI) should be considered as an essential complementary tool for the characterization of samples. For example, a classic optical/IR image captures information only regarding the intensity, while the information about the phase is totally lost18. Holographic interferometry provides all information regarding the intensity and phase, and additionally can be used to monitor their changes in real time.
The importance of exploiting this method in condensed matter science is to reveal in situ the slightest changes in system dynamics. For example, the BR reaction can reveal the first cause of the symmetry-breaking process. Is the symmetry-breaking process predetermined by physical constraints connected with nonlinear dynamics, or is the process truly random? On the other hand, in another way, can the minor differences in BR oscillatory period duration cause a significant deviation in transition appearance?
The presented results are the first step that will lead to a deeper understanding of dynamics at the nanoscale. Since the potential of holography in condensed science research has still not been fully recognized, the purpose of this article is to highlight the power of holography for future material science research and applications; for example, particle trapping and levitation such as movement of unburnt hydrocarbons in the atmosphere or separation of various aerosols19, breaking down of microplastics in water and fractionation of particles in general20, and characterisation of temperature and thermal conductivity properties of micron-size fuel particles21.
Divulgations
The authors have nothing to disclose.
Acknowledgements
M. S. P., D. G., D. V., and B. K. acknowledge support of the Biological and bioinspired structures for multispectral surveillance, funded by NATO SPS (NATO Science for Peace and Security) 2019-2022. B. K., D. V., B. B., D. G., and M. S. P. acknowledge funding provided bythe Institute of Physics Belgrade, through the institutional funding bythe Ministry of Education, Science, and Technological Development of theRepublic of Serbia. Additionally, B. K. acknowledges support from F R S – FNRS. M. P. acknowledges support from the Ministry of Education, Science and Technological Development of the Republic of Serbia, Contract number 451-03-9/2021-14/200026. S. R. M. was supported by a BEWARE Fellowship of the Walloon Region (Convention n°2110034), as a postdoctoral researcher. T. V. acknowledges financial support from the Hercules Foundation. D.V., M.S.P., D.G., M.P., B.B., and B.K. acknowledge the support of the Office of Naval Research Global through the Research Grant N62902-22-1-2024. This study was conducted in partial fulfillment of the requirements for the PhD degree of Marina Simović Pavlović at the University of Belgrade, Faculty of Mechanical Engineering.
Materials
| Active Vibration Isolation, Four Optical Table Supports | Thorlabs | PTR502 | High Load Capacity: 2,500 kg, Height 600 mm |
| Cuvette | Standard glass cuvette | ||
| Holographic camera (optical camera for holography) | Cannon | EOS 50D | Sensor Size 22.3 x 14.9 mm; Pixel pitch 4.69 µm; Max. resolution 4752 x 3168; JPEG file format |
| Hydrogen peroxide, H2O2 | Merck (Darmstadt, Germany) | ||
| Laser | Laser Quantum | Torus 532 laser | Wavelength 532 nm; Power 390 mW; Coherence length 10 m |
| LED lasers | |||
| Malonic acid, C3H4O4 | Acr s Organics (Geel, Belgium) s Organics (Geel, Belgium) |
||
| Manganese sulphate, MnSO4 | Fluka (Buchs, Switzerlend) | ||
| Nonlinear optical microscope | IPB | ||
| Optical accessories | Thorlab | ||
| Optical spectroscope | |||
| Optical table | Thorlabs | TOP450II PTR52509 | dimensions 2000*1250*310 mm |
| Perchloric acid, HClO4 | Merck (Darmstadt, Germany) | ||
| Potassium iodate, KIO3 | Merck (Darmstadt, Germany) | ||
| Software | Home-build software made by one of the authors: Dusan Grujic. This software was conducted in partial fulfillment of the requirements for the PhD deegree of D.G. | ||
| Thermal camera | Flir | A65 | 640×512 pixel; Thermal resolution 50 mK |
| Video camera | Nikon | 1v3 | 18.4 Mpixel; 60 fps |
References
- Pietrzyk, D. J., Frank, C. W. Development of an analytical method. Analytical Chemistry. , 10-19 (1979).
- Ostrovsky, Y. I., Shchepinov, V. P., Yakovlev, V. V. . Holographic Interferometry in Experimental Mechanics. 60, (2013).
- Pedrini, G., Osten, W., Gusev, M. E. High-speed digital holographic interferometry for vibration measurement. Applied Optics. 45 (15), 3456-3462 (2006).
- Pantelić, D. V., Grujić, D. &. #. 3. 8. 1. ;., Vasiljević, D. M. S. i. n. g. l. e. -. b. e. a. m. dual-view digital holographic interferometry for biomechanical strain measurements of biological objects. Journal of Biomedical Optics. 19 (12), 127005 (2014).
- Grujić, D., et al. Infrared camera on butterfly’s wing. Optics Express. 26 (11), 14143-14158 (2018).
- Mouchet, S. R., Deparis, O. . Natural Photonics and Bioinspiration. , (2021).
- Pagnacco, M. C., et al. Spontaneous symmetry breaking: the case of crazy clock and beyond. Symmetry. 14, 413 (2022).
- Pagnacco, M. C., Maksimovic, J. P., Potkonjak, N. I., Božić, B. &. #. 2. 7. 2. ;., Horvath, A. K. Transition from low to high iodide and iodine concentration states in the Briggs-Rauscher reaction: evidence on crazy clock behavior. The Journal of Physical Chemistry A. 122 (2), 482-491 (2018).
- Pagnacco, M. C., Maksimović, J. P., Janković, B. &. #. 3. 8. 1. ;. Analysis of transition from low to high iodide and iodine state in the Briggs-Rauscher oscillatory reaction containing malonic acid using Kolmogorov-Johnson-Mehl-Avrami (KJMA) theory. Reaction Kinetics, Mechanisms and Catalysts. 123 (1), 61-80 (2018).
- Mouchet, S. R., et al. Unveiling the non-linear optical response of Trichtenotoma childreni longhorn bestle. Journal of Biophotonics. 12 (9), 201800470 (2019).
- Shimobaba, T., et al. Computational wave optics library for C++: CWO++ library. Computer Physics Communications. 183 (5), 1124-1138 (2012).
- Grujic, D. . Application of digital holography for detection of infrared radiation on biophotonic structures. , (2022).
- Muffoletto, R. P., Tyler, J. M., Tohline, J. E. Shifted Fresnel diffraction for computational holography. Optical Express. 15 (9), 5631-5640 (2007).
- Pavlović, D., et al. Naturally safe: Cellular noise for document security. Journal of Biophotonics. 12 (12), 201900218 (2019).
- Bray, W. C. A periodic reaction inhomogeneous solution and its relation to catalysis. Journal of the American Chemical Society. 43 (6), 1262-1267 (1921).
- Nicolis, G. Self-organization in nonequilibrium systems. Dissipative Structures to Order through Fluctuations. , 339-426 (1977).
- Prigogine, I., Hiebert, E. N. From being to becoming: Time and complexity in the physical sciences. Physics Today. 35 (1), 69 (1982).
- Nikolova, L., Ramanujam, P. S. . Polarization Holography. , (2009).
- Haisch, C., Kykal, C., Niessner, R. Photophoretic velocimetry for the characterization of aerosols. Analytical Chemistry. 80 (5), 1546-1551 (2008).
- Kononenko, V. L., et al. Feasibility studies on photophoretic effects in field-flow fractionation of particles. Journal of Liquid Chromatography & Related Technologies. 20 (16-17), 2907-2929 (1997).
- Zhang, X., Bar-Ziv, E. A novel approach to determine thermal conductivity of micron-sized fuel particles. Combustion Science and Technology. 130 (1-6), 79-95 (1997).

