Single-Molecule Analysis of Sf9 Purified Superprocessive Kinesin-3 Family Motors
Summary
This study details purification of KIF1A(1-393LZ), a member of kinesin-3 family, using Sf9-baculovirus expression system. In vitro single-molecule and multi-motor gliding analysis of these purified motors exhibited robust motility properties comparable to motors from mammalian cell lysate. Thus, Sf9-baculovirus system is amenable to express and purify motor protein of interest.
Abstract
A complex cellular environment poses challenges for single-molecule motility analysis. However, advancement in imaging techniques have improved single-molecule studies and has gained immense popularity in detecting and understanding the dynamic behavior of fluorescent-tagged molecules. Here, we describe a detailed method for in vitro single-molecule studies of kinesin-3 family motors using Total Internal Reflection Fluorescence (TIRF) microscopy. Kinesin-3 is a large family that plays critical roles in cellular and physiological functions ranging from intracellular cargo transport to cell division to development. We have shown previously that constitutively active dimeric kinesin-3 motors exhibit fast and superprocessive motility with high microtubule affinity at the single-molecule level using cell lysates prepared by expressing motor in mammalian cells. Our lab studies kinesin-3 motors and their regulatory mechanisms using cellular, biochemical and biophysical approaches, and such studies demand purified proteins at a large scale. Expression and purification of these motors using mammalian cells would be expensive and time-consuming, whereas expression in a prokaryotic expression system resulted in significantly aggregated and inactive protein. To overcome the limitations posed by bacterial purification systems and mammalian cell lysate, we have established a robust Sf9-baculovirus expression system to express and purify these motors. The kinesin-3 motors are C-terminally tagged with 3-tandem fluorescent proteins (3xmCitirine or 3xmCit) that provide enhanced signals and decreased photobleaching. In vitro single-molecule and multi-motor gliding analysis of Sf9 purified proteins demonstrate that kinesin-3 motors are fast and superprocessive akin to our previous studies using mammalian cell lysates. Other applications using these assays include detailed knowledge of oligomer conditions of motors, specific binding partners paralleling biochemical studies, and their kinetic state.
Introduction
An immensely crowded cell environment poses many challenges in sorting destined proteins and molecules. This intense workload of organization and spatiotemporal distribution of molecules within the cytoplasm is facilitated by molecular motors and cytoskeletal tracks. Molecular motors are the enzymes that hydrolyze the energy currencies such as ATP and utilize that energy during motion and force generation1. Based on the amino acid sequence similarity, kinesins are grouped into 14 families and despite this similarity, each motor contributes uniquely to the functioning of a cell. Kinesin-3 family motors constitute one of the largest, comprising five subfamilies (KIF1, KIF13, KIF14, KIF16, and KIF28)2, associated with diverse cellular and physiological functions, including vesicle transport, signaling, mitosis, nuclear migration, and development 3,4,5. Impairment in kinesin-3 transport function implicates in many neurodegenerative disorders, developmental defects, and cancer diseases6,7,8,9.
Recent work has demonstrated that kinesin-3 motors are monomers but undergo cargo-induced dimerization and result in fast and superprocessive motility compared to conventional kinesin10,11,12,13. Their biochemical and biophysical characterization needs a large quantity of purified, active proteins. However, their production in the prokaryotic expression system resulted in inactive or aggregated motors, presumably due to incompatible protein synthesis, folding and modification machinery14,15,16,17,18. To circumvent such limitations and increase the yield, here we have established a robust Sf9-baculovirus expression system to express and purify these motors.
The baculovirus expression system uses Sf9 insect cell lines as a host system for high-throughput eukaryotic recombinant protein expression19,20. Baculovirus possesses a strong polyhedrin promoter that assists in heterologous gene expression and the production of soluble recombinant proteins17. Due to its cost-effectiveness, safe to handle and high amount of active protein expression, it has become a powerful tool21. To express a protein of interest, a key step is to generate a recombinant bacmid. Since the commercially available bacmid generating kits are expensive and we will be working with more samples, we developed an in-house protocol for both large and small inserts of kinesin-3 motors into bacmids. Sf9-purified kinesin-3 motors were used to characterize in vitro single-molecule and multi-motor microtubule gliding properties using total internal reflection fluorescence (TIRF) microscopy. Motors are C-terminally tagged with 3-tandem fluorescent molecules (3xmCit) to provide enhanced signal and decreased photobleaching. Due to its increased signal-to-noise ratio, less phototoxicity, and selective imaging of a very small area close to the coverslip, TIRF imaging has been widely used to visualize protein dynamics at the single-molecule level in vivo and in vitro.
This study discusses the purification of kinesin-3 motors by employing Sf9-baculovirus expression system and in vitro single-molecule imaging and multi-motor gliding analysis of motors using TIRF microscopy. Altogether, this study shows that the motility properties of Sf9 purified motors are identical to that of motors prepared from mammalian cell lysates. Hence, we believe that the Sf9-baculovirus system can be adapted to express and purify any motor protein of interest.
Protocol
1. Sf9 culture, transfection, and virus generation
NOTE: Maintain Sf9 cells in 30 mL of Sf-900/SFM medium in 100 mL sterile, disposable conical flask without any antibiotic/antimycotic at 28 °C. Keep the suspension culture in an orbital shaker at 90 rpm. Supply of CO2 and humidity maintenance is not required. Cells are usually subcultured every fourth day by inoculating 0.5 x 106 cells/mL to reach 2.0 x 106 cells/mL density on the fourth day.
- P0 virus stock generation
- For transfection, seed cells into a 35 mm dish with 4.5 x 105 cells/mL confluency and maintain at 28 °C without shaking.
- After 24 h, once cells are attached and look healthy, proceed for transfection as described below.
- Tube A: Mix 1 µg of bacmid DNA encoding for constitutively active KIF1A(1-393LZ)-3xmCit-FLAG specific kinesin-3 motor with 100 µL of unsupplemented Grace's media.
- Tube B: Mix 6 µL of transfection reagent with 100 µL of unsupplemented Grace's media.
- Carefully transfer the content of Tube A into Tube B and mix thoroughly by pipetting up and down (approximately 20 times).
- Incubate the mixture for ~45 min at room temperature.
- After completing the incubation, add 0.8 mL of unsupplemented Grace's media to the above mixture and mix slowly by pipetting.
- Gently aspirate the Sf-900/SFM media from cells (to remove any traces of serum that may affect transfection efficiency).
- Add the transfection mixture from step 1.1.3 dropwise on the top of the cells and incubate the plate for 6 h at 28 °C.
- After the incubation, carefully remove the transfection mixture, add 2 mL of Sf-900/SFM media and incubate further for 48 h at 28 °C.
- Check for the motor protein expression under an inverted fluorescence microscope. The motor protein is tagged with a fluorescent protein, mCitrine, a variant of yellow fluorescent protein.
NOTE: Transfected cells were visualized under an inverted microscope equipped with differential interference contrast (DIC) and epifluorescence illumination with a 20x objective (200 times magnification), mercury lamp and an EM-CCD camera. Check the efficiency of virus generation and infection by monitoring the expression of mCitrine-tagged motors in cells through mCitrine excitation and emission filter cube. In addition, check for morphological changes of infected cells, such as enlarged cells/nuclei (Figure 1A-C). - Again, check the cells 72 h post-infection. Usually, cells start detaching from the surface (Figure 2).
- If >5% of cells detached from the surface, harvest the media with infected cells in 1.5 mL sterile microcentrifuge tubes and spin for 5 min at 500 x g.
- Collect the supernatant and snap freeze aliquots of 1 mL in liquid nitrogen and store as P0 stock at -80 °C or use it to generate P1 virus stock.
- P1 virus stock generation
- To further amplify the P0 baculovirus stock and confirm protein expression, grow Sf9 cells in liquid suspension culture.
- In a sterile 100 mL conical flask, add 10 mL of Sf-900/SFM media with a cell density of 2 x 106 cells/mL and 1 mL of P0 virus stock. Incubate at 28 °C with constant shaking at 90 rpm.
- After 72 h of infection, check for the protein expression as described earlier (step 1.1.7). If the protein expression is good (>90% of cells show a bright mCitrine fluorescence signal) and shows significant cell death (approximately 10%-15%), spin down the cells in a 15 mL sterile conical tube at 500 x g for 5 min.
- Collect the supernatant, snap freeze aliquots of 1 mL (P1 stock) in liquid nitrogen and store at -80°C or proceed for large-scale infection and protein purification.
- Large-scale infection
- For large-scale protein expression, infect 30 mL of the suspension culture at 2 x 106 cells/mL density with 1 mL of P1 virus stock and incubate at 28 °C with constant shaking at 90 rpm.
- After ~72 h post-infection, check for the protein expression (in general, maximum protein expression is achieved between 65-75h post-infection).
- If >90% of cells show a bright fluorescent signal with minimal cell death (<5%), collect the cells in a sterile 50 mL conical tube and spin down at 500 x g at 4 °C for 15 min.
NOTE: There should be minimal cell death (<5%), because dead cells release the cellular contents into the media, which leads to loss of expressed protein. - Discard the supernatant, collect the cell pellet, and proceed with protein purification.
2. Sf9 purification of kinesin-3 motors
- To the above cell pellet, add 3 mL of ice-cold lysis buffer (Supplemental Table 1) freshly supplemented with 5 mM DTT, 5 µg/mL of aprotinin, 5 µg/mL of leupeptin, and 5 µg/mL of PMSF and lyse the cells by pipetting 20-25 times without generating any air bubbles. For all the buffer compositions and reagents, please refer to Supplemental Table 1.
- Spin the cell lysate at 150,000 x g for 30 min at 4 °C.
- Collect the supernatant into a fresh, sterile tube and mix with ~40 µL of 50% anti-FLAG M2 affinity resin. Incubate the mixture for 3 h at 4 °C with end-to-end tumbling.
- After incubation, pellet the FLAG resin by spinning at 500 x g for 1 min at 4 °C. Gently aspirate the supernatant without disturbing the pellet with a 26 G needle and discard.
- Wash the FLAG resin pellet three times with ice-cold wash buffer (Supplemental Table 1) freshly supplemented with 2 mM DTT, 5 µg/mL of aprotinin, 5 µg/mL of leupeptin, and 5 µg/mL of PMSF. Pellet the beads by spinning at 500 x g for 1 min at 4 °C in each wash.
- After the third wash, carefully drain the wash buffer as much as possible without disturbing the pellet. For protein elution, add ~70 µL of wash buffer containing 100 µg/mL FLAG peptide to the resin pellet and incubate overnight at 4 °C with end-to-end tumbling.
- On the subsequent day, spin down the resin at 500 x g for 1 min at 4 °C. Collect the supernatant containing purified protein into a fresh tube and supplement with 10% glycerol. Snap freeze aliquots of 5 µL in liquid nitrogen and store at -80 °C until further use.
- Run the SDS-PAGE gel to determine the protein concentration and yield. Along with purified protein of interest, a standard protein control, BSA of known concentrations ranging from 0.2 µg, 0.4 µg, 0.6 µg, 0.8 µg, and 1 µg are loaded to generate a standard curve. Stain the gel with Coomassie Brilliant blue (Figure 3).
- Analyze the gel using a built-in gel quantification tool in ImageJ software. First, measure the band intensity of known concentrations of BSA and generate the standard curve. Then, measure the intensity of the purified protein band and determine the protein concentration. To do so open the Image J software, click on the Analyze option in the menu bar and select Gels in the drop-down menu.
3. In vitro single-molecule motility assay using Sf9-purified kinesin-3 motors
NOTE: The Sf9-purified kinesin-3 motors can be used to study biochemical and biophysical properties such as ATP turnover rate, microtubule affinity, velocity, run length, step size, and force generation. Here, a detailed protocol for in vitro single-molecule motility analysis of KIF1A(1-393LZ) using Total Internal Reflection Fluorescence (TIRF) microscopy is described. For all the buffers composition and reagents, please refer to Supplemental Table 1.
- Microtubule polymerization
- In a pre-chilled 0.5 mL microcentrifuge tube, prepare a polymerization mix by pipetting in the following order: 12.0 µL of BRB80 buffer, pH 6.9; 0.45 µL of 100 mM MgCl2, 1 µL of 25 mM GTP.
- Take out a 10 µL aliquot of 10 mg/mL tubulin stored in liquid nitrogen, thaw immediately, and add into the above polymerization mixture. Mix gently by pipetting 2-3 times without creating any air bubbles.
NOTE: Perform the above steps quickly and strictly on ice. - Let the above mixture sit on ice for 5 min.
NOTE: This is a critical step to prevent denaturing of tubulin or to avoid the formation of short microtubule seeds. - Transfer the tube into a pre-warmed 37 °C heat block/water bath and incubate for 30 min for polymerization of microtubules.
- While microtubules are polymerizing, thaw an aliquot of P12 buffer and bring it to room temperature.
- Before completing 30 min of incubation, start preparing MT stabilization buffer by pipetting 100 µL of P12 buffer into a fresh microcentrifuge tube. To this, add 1 µL of 1 mM taxol and immediately vortex the mixture.
NOTE: Start preparing the MT stabilization buffer approximately 5 min before completing the incubation in step 3.1.4. Taxol stabilizes the polymerized microtubules by binding to β-tubulin. - Warm the microtubule stabilization buffer for 2-3 min at 37 °C and gently add it to the polymerized microtubules without disturbing the polymerization mixture at the bottom.
NOTE: Warming the stabilization buffer will bring it to the same temperature as the polymerization mixture. - Do not tap or pipette the mixture and incubate further at 37 °C for 5 min.
- Gently tap the mixture and mix it with a beveled cut tip (200 µL capacity) slowly using the pipette.
NOTE: From this point onward, always handle microtubules with a beveled cut tip to avoid microtubule shearing. - Take 10-15 µL of polymerized microtubules in a flow cell to check proper microtubule polymerization.
NOTE: One can visualize the unlabeled microtubules using a DIC microscopy setup. - If microtubules are concentrated, dilute them in P12 buffer supplemented with 10 µM taxol.
- Preparation of motility flow cell chamber
- Prepare a motility chamber using a glass slide, double-sided tape and glass coverslip (22 mm x 30 mm).
- Take a glass slide, place a drop (~70 µL) of deionized distilled water in the middle and wipe it with a lint-free tissue paper.
- Cut two strips of double-sided tape (~35 x 3 mm) and firmly stick them to the glass slide parallelly, leaving an ~4-5 mm gap between two strips to create a narrow passage.
- Next, take a coverslip and add a drop (~20 µL) of deionized distilled water in the middle. Place a strip of lint-free lens cleaning tissue paper on the water drop until it absorbs the water. Then, slide it slowly toward one end of the coverslip.
NOTE: The coverslip should be completely dry. No water should be visible on the coverslip. - Place the coverslip on the double-sided strips stuck on the slide and press the coverslip evenly along the strips to stick firmly.
NOTE: Please make sure that the cleaned side of the coverslip faces the glass slide. - Ensure that together this creates a narrow chamber of 10-15 µL capacity for performing motility assay (Figure 4A).
- In vitro single-molecule motility assay
NOTE: To study the microtubule-based single-molecule motility properties of motors, microtubules need to be adsorbed onto the coverslip surface in the motility chamber.- Dilute the polymerized taxol-stabilized microtubules in P12 buffer supplemented with 10 µM taxol at 1:5 ratios and mix by pipetting slowly with a beveled tip.
- Keep the flow chamber in a slant position (~15-20°). Flow 30 µL of diluted microtubule solution through the flow chamber from the upper end while keeping a lint-free tissue paper at the lower end to absorb the liquid. This creates a shear force to align the microtubule in the flow direction and helps to adsorb the microtubules straight and align parallel.
- Leave a small drop of liquid on both ends of the chamber and keep the flow cell in an inverted position (coverslip facing the bottom) in a closed, moist chamber to prevent drying of the motility chamber.
- Let it sit for ~30 min so that microtubules adsorb to the surface of the coverslip inside the motility chamber.
- In the meantime, prepare blocking buffer by mixing 500 µL P12-BSA buffer with 5 µL of 1 mM taxol.
- Flow 40-50 µL of blocking buffer and incubate the slide in an inverted position for 10 min in a moist chamber.
- Prepare the motility mixture by pipetting the following components into a 500 µL capacity sterile microcentrifuge tube in the following order: 25 µL of P12 buffer with taxol, 0.5 µL of 100 mM MgCl2, 0.5 µL of 100 mM DTT, 0.5 µL of 20 mg/mL Glucose oxidase, 0.5 µL of 8 mg/mL Catalase, 0.5 µL of 2.25 M Glucose, and 1.0 µL of 100 mM ATP.
NOTE: Fluorescence imaging has been widely used for biological applications22,23,24,25,26. Photoexcitation of fluorescent proteins generates reactive oxygen species (ROS), which can cause photobleaching of the fluorescent proteins and damage to the biological samples27,28. Oxygen scavengers such as glucose, glucose oxidase and catalase are routinely used in motility assays to limit photodamage and prolong the bleaching time of fluorescent proteins. - Finally, add 1 µL of the purified motor to the above motility mix and mix well before flowing into the motility chamber.
- Seal both the ends of the motility chamber with liquid paraffin wax and immediately image under TIRF illumination using 100X TIRF objective of 1.49 NA with 1.5x magnification.
- In order to focus the coverslip surface, first, focus on one of the inner edges of double-sided tape in the motility chamber under differential interference contrast (DIC) illumination.
NOTE: The bright and uneven surface will be visible. - Then, move the focus into the motility chamber. Using fine adjustment, focus the coverslip surface and look for the microtubules adsorbed on the coverslip surface.
- Once the microtubules are focused, switch to TIRF illumination with a 488 nm excitation laser and adjust the illumination depth by changing the excitation beam angle to get the best and uniform TIRF illumination (Figure 4B).
- Focus the individual mCitrine-tagged motors moving processively along the microtubule surface with 100 ms exposure and record the motion using an EM-CCD camera.
NOTE: Although the motility assays were performed on unlabeled microtubules, the maximum intensity z-projection function can be used to reveal the outline of the microtubule track. Preferentially, events on long microtubule tracks were considered for tracking analysis. - Manually track the position of the fluorescently tagged individual motors walking on long microtubule tracks frame-by-frame using a custom-written plugin in ImageJ (nih.gov) software as described previously29.
- Generate the histograms of velocity and run length for the motor population by plotting the number of events in each bin. Fit these histograms to a single Gaussian peak function to obtain average velocity and run length12 (Figure 4C-E).
4. In vitro microtubule gliding assay
NOTE: To understand the collective behavior of kinesin-3 motors, in vitro microtubule gliding assay was performed18,30,31. Where motors are immobilized onto the coverslip in an inverted position and upon adding microtubules into the chamber, microtubules land on motors and glide along as the motors try to walk on them (Figure 5A,B).
- Fluorescent microtubule polymerization: Polymerize the microtubules following the protocol described previously except for mixing rhodamine labeled tubulin (3 mg/mL) with unlabeled tubulin (10 mg/mL) in a ratio 1:10.
- After 30 min of polymerization, gently add 30 µL of pre-warmed microtubule stabilization buffer and incubate further for 5 min at 37 °C.
- Next, shear the microtubules by pipetting with the capillary-loading tip (~25-30 times).
- Prepare the motility flow chamber as described previously, flow 50 µL of Sf9-purified GFP nanobodies (2.5 µL of 100 nM diluted in 50 µL of P12 buffer) and incubate for 30 min at room temperature with the coverslip facing downward in a moist chamber.
NOTE: The motors are C-terminally tagged with mCitrine. GFP nanobodies are used to immobilize the motors due to their low dissociation constant32. - Block the coverslip surface by flowing 50 µL of block buffer into the flow chamber to prevent nonspecific protein adsorption and incubate further for 5 min.
- Prepare the motor mix by pipetting 50 µL of block buffer, 1 µL of 100mM ATP, and 5 µL of 100 nM Sf9-purified kinesin-3 motors. Mix gently before flowing into the chamber and incubate for 30 min at room temperature in a moist chamber.
- Wash the chamber twice with 50 µL of P12 casein.
- In a sterile microcentrifuge tube, prepare the gliding assay mixture in the following order, 45 µL of P12-casein with 10 µM taxol, 1 µL of 100 mM ATP, 0.5 µL of 8 mg/mL catalase, 0.5 µL of 20 mg/mL glucose oxidase, 0.5 µL of 2.25 M glucose and 1 µL of sheared fluorescent microtubules. Gently mix the content before flowing into the motility chamber and seal the ends of the chamber with liquid paraffin wax.
- Image microtubule gliding under TIRF illumination at 100 ms exposure and capture the images with attached EM-CCD camera.
NOTE: The average microtubule gliding velocity was determined by manually tracking approximately 100 individual microtubules frame-by-frame using a custom-written plugin in ImageJ29. Determine the average microtubule gliding velocity by generating a histogram and fitting to a Gaussian function (Figure 5C,D).
Representative Results
To express and purify active and functional recombinant motor proteins at a large scale using the Sf9-baculovirus expression, the system needs generation of viral particles stably carrying a coding sequence to infect Sf9 cells. To achieve this, Sf9 cells were transfected with recombinant bacmid encoding KIF1A(1-393LZ)-3xmCit-FLAG. After 72 h, a significant population of cells showed expression of green fluorescent protein (mCitrine) with enlarged cells and nuclei (Figure 1 and Figure 2), suggesting successful generation of viral particles (P0). These viral particles were further expanded by infecting fresh Sf9 population at a higher volume to generate P1 viral stocks for storage and large-scale infection. For purification of kinesin-3 motor, a 30 mL culture of Sf9 cells were infected with 1 mL of P1 viral particles. After 72 h of infection, cells brightly expressing green fluorescent protein were harvested, lysed, and purified using C-terminal FLAG tag affinity purification with anti-FLAG antibody-coated resin. Interestingly, the addition of FLAG peptide to the beads attached with kinesin-3 motors eluted most of the protein in a single fraction and the purified protein was of high purity, shown as a band of ~120 kDa in Lane 7 of Figure 3.
The successful polymerization of long and healthy microtubules to study the motility properties of S9-purified kinesin-3 motors at a single-molecule level requires pure tubulin. In the present study, the microtubules were polymerized using 2X cycled tubulin purified from the goat brain at a critical concentration between ~2.5-3.0 mg/mL for 30 min in the absence of taxol, as taxol decreases the critical concentration of microtubule nucleation and generates a large number of short microtubules33,34,35. Taxol was added post-polymerization to stabilize the microtubules and prevent depolymerization. Microtubules polymerized using this method resulted in long and uniform structures (Video 1) with an average length of 60-70 µm.
In vitro single-molecule motility analysis of Sf9-purified kinesin-3 motor, KIF1A(1-393LZ) under TIRF illumination (Figure 4B) exhibited unidirectional, fast, and superprocessive motion along the microtubule (Video 2), as shown in the kymograph as long diagonal white lines (Figure 4C). Tracking analysis of these long superprocessive motility events showed an average velocity and run length of 2.44 ± 0.02 µms-1 and 10.79 ± 0.28 µm (Figure 4D–E), respectively. These results are in agreement with our previously published data12,13 using motors prepared from mammalian cell lysate by overexpression. Furthermore, the multi-motor microtubule-gliding assay using Sf9-purified motors attached on the coverslip under TIRF illumination exhibited long, uniform, and unidirectional motion (Figure 5C and Video 3). Tracking analysis of these long microtubule gliding events showed an average velocity of 1.37 ± 0.01 µms-1 (Figure 5D).
Together, these results suggest that the Sf9-baculovirus system provides an excellent system for the expression and purification of active motor proteins that are generally difficult using the prokaryotic system. FLAG tag offers an advantage of the single-step purification of protein in its pure form. Furthermore, microtubule polymerization using fresh and pure tubulin and their stabilization after the polymerization yields long and uniform microtubules. Additionally, in vitro single-molecule motility analysis suggests that Sf9-purified motors were robust and exhibited motility properties similar to motors expressed in mammalian systems.
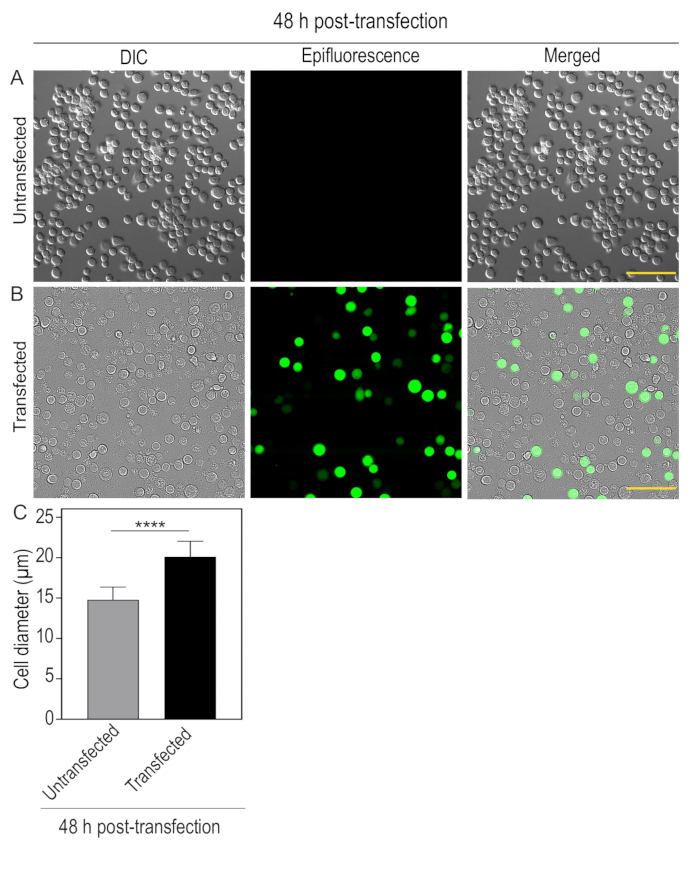
Figure 1: 48 h post-transfection of Sf9 cells with fluorescently-tagged kinesin-3 motor: Sf9 cells were plated on a 35 mm dish at a density of 4.5 x 105 cells/mL. After 24 h, cells were transfected with recombinant bacmid DNA encoding specific kinesin-3 motor, KIF1A(1-393LZ)-3xmCit-FLAG using transfection reagent. After 48 h of transfection, images were captured under differential interference and contrast (DIC) and Epifluorescence illumination. (A) Untransfected cells, small, round, and firmly attached. (B) Transfected cells expressing KIF1A(1-393LZ)-3xmCit-FLAG tagged protein showing enlarged cell diameter and loosely attached to the surface. Scale bar, 100 µm. (C) Bar graph indicating average cell diameter of untransfected and transfected cells. Error bars represent mean ± SD. N = 50 cells. Student's t-test is used to find the significance value (****p < 0.0001). Please click here to view a larger version of this figure.
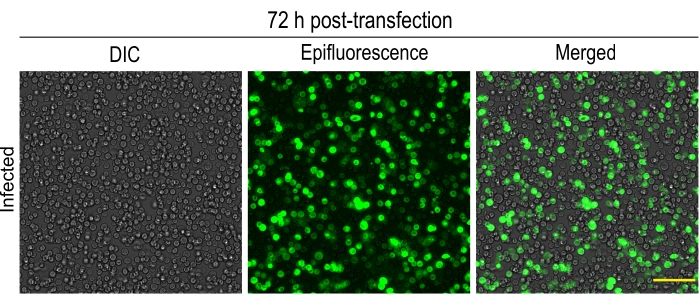
Figure 2: 72 h post-transfection of Sf9 cells with fluorescently-tagged kinesin-3 motor: Images showing more than 90% of Sf9 cells expressing fluorescently-tagged kinesin-3 motor, KIF1A(1-393LZ). Cells are loosely bound to the surface. Please click here to view a larger version of this figure.
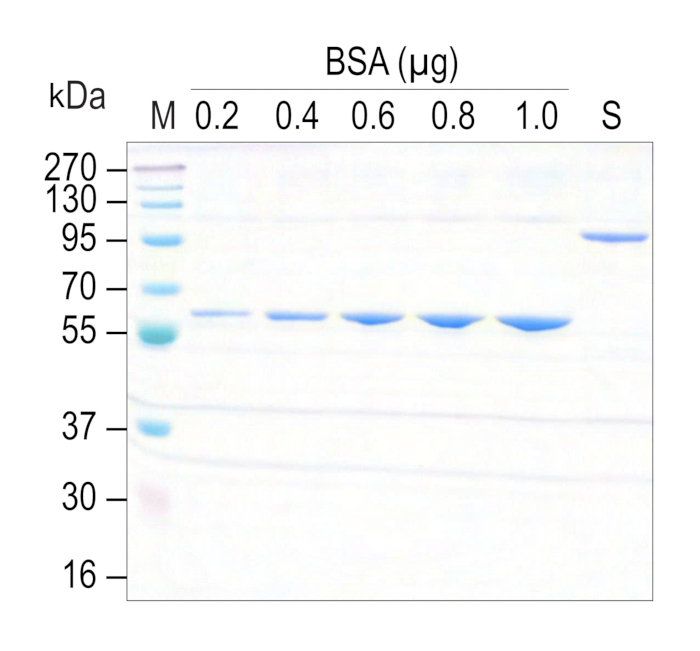
Figure 3: Sf9-purified kinesin-3 motor: Coomassie-stained SDS-PAGE gel representing Sf9-purified kinesin-3 motor, KIF1A(1-393LZ) C-terminally tagged with 3xmCit-FLAG. From left to right, Protein ladder (M), BSA standard protein control, 0.2 µg (lane 2), 0.4 µg (lane 3), 0.6 µg (lane 4), 0.8 µg (lane 5), and 1.0 µg (lane 6), purified kinesin-3 motor (S) (lane 7). BSA was used as a standard to estimate purified protein concentration. Please click here to view a larger version of this figure.
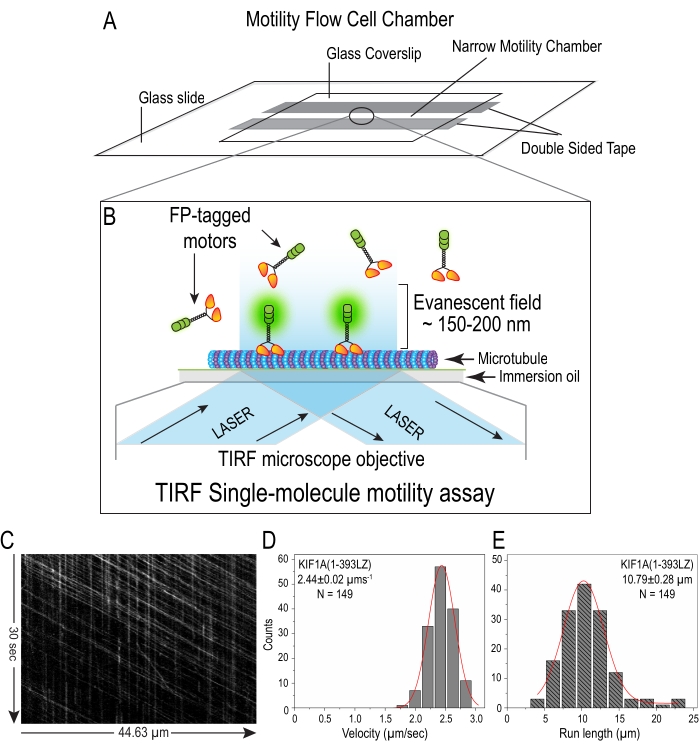
Figure 4: In vitro single-molecule motility assay using Sf9 purified kinesin-3 motors:(A) Schematic flow cell motility chamber prepared using a glass slide, double-sided tape, and glass coverslip. (B) Cartoon diagram illustrating in vitro single-molecule imaging of fluorescently tagged motors moving along the microtubule surface adsorbed onto the coverslip using Total Internal Reflection Fluorescence (TIRF) microscopy. Only the molecules very close to the coverslip get excited due to the evanescent field (~150-200 nm) formed by the complete reflection of incident light when illuminated at an angle beyond the critical angle. (C) Kymograph depicts kinesin-3 motor walking processively (diagonal white lines) along the microtubule surface. Time on the y-axis (vertical arrow) and distance on the x-axis (horizontal arrow). (D–E) The histograms of velocity (D) and run length (E) were determined by tracking individual motors walking on the microtubule surface frame-by-frame and histograms were plotted for each population of motors and fit to a single Gaussian. The peak represents the average velocity and run length and values are shown on the graph as mean ± SEM. N = Number of events counted. Please click here to view a larger version of this figure.
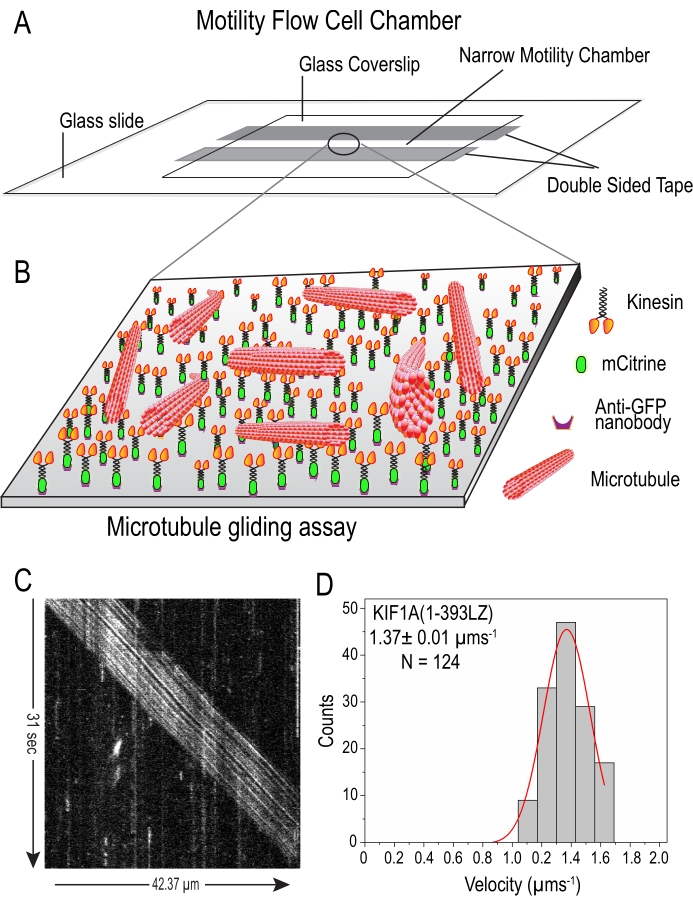
Figure 5: In vitro microtubule gliding assay using Sf9 purified kinesin-3 motors: (A) Schematic of flow cell chamber prepared using a glass slide, double-sided tape, and glass coverslip. (B) Cartoon diagram illustrating rhodamine-labeled microtubules gliding on the surface of constitutively active kinesin-3 motors. As kinesin-3 motors are C-terminally tagged with mCitrine, GFP-nanobodies were used to attach them to the glass surface. Subsequently, sheared rhodamine-labeled microtubules were introduced into the flow chamber and images of microtubule gliding movement were captured under TIRF illumination. (C) The kymograph represents smooth microtubule gliding (diagonal white stripe) by the kinesin-3 motors. Time on the y-axis (vertical arrow) and distance on the x-axis (horizontal arrow). D. Histogram of the velocity were plotted for a population of microtubules and fit to a single Gaussian peak. Average gliding velocity is indicated on the top-left corner as mean ± SEM. N = Number of molecules counted. Please click here to view a larger version of this figure.
Video 1: Polymerized microtubules. TIRF imaging of polymerized microtubules before diluting for motility assay. The movie was acquired at 100 ms exposure and the video is played at 60 frames/s. Scale bar, 10 µm. Please click here to download this Video.
Video 2: In vitro single-molecule assay of the constitutively active kinesin-3 motor. Fluorescently-tagged KIF1A(1-393LZ) motors purified from Sf9 cells progressively moving along the microtubule surface (unlabeled) adsorbed on the coverslip in a motility assay chamber. Imaging was performed in TIRF illumination and images were acquired at 100 ms exposure. Video is played at 30 frames/s. Scale bar, 10 µm. Please click here to download this Video.
Video 3: Microtubule gliding assay of the constitutively active kinesin-3 motor. Rhodamine-labeled microtubules smoothly gliding on the surface of the glass coverslip coated with Sf9 purified KIF1A(1-393LZ) motor. Imaging was performed in TIRF illumination and images were acquired at 100 ms exposure. Video is played at 60 frames/s. Scale bar, 10 µm. Please click here to download this Video.
Supplemental Table 1: Buffers and reagents composition. Please click here to download this File.
Discussion
The Sf9-baculovirus expression system is one of the most versatile and successful methods for high-throughput protein production19,36,37. The posttranslational modification ability of Sf9 cells is highly comparable to the mammalian system15. A considerable disadvantage of using this system is that it is slow and sensitive to contamination. One of the most critical steps is efficient infection and successful expression in Sf9 cells, which requires proper handling and maintenance of Sf9 cells without any contamination. The system requires dedicated dust-free space and instruments such as a temperature-controlled incubator and shaker. Irregular cell culture practice and use of overgrown culture will result in poor infection, slow growth, and significant cell death.
The FLAG tag purification of protein expressed in Sf9 cells has several advantages. First, Sf9 cells can be lysed efficiently to get most of the protein in its active and soluble form. Secondly, FLAG resin has less nonspecific protein adsorption than HIS and GST resin38. As a result, a significant amount of the protein gets released in a single-step elution in its pure form. The Sf9-purified kinesin-3 motors showed robust microtubule-stimulated ATP turnover rates in the biochemical ATPase assays14. Similarly, in in vitro single-molecule motility assays, these motors showed fast and superprocessive motility on the surface of the microtubule. Interestingly, these motility properties showed remarkable similarity with the motility properties measured using motors prepared from mammalian cell lysates11,12,13. Additionally, the measured stepping rate of kinesin-3 motors in the in vitro single-molecule motility assay closely matches the rate of ATP hydrolysis measured in biochemical ATPase assay, suggesting motors hydrolyze one ATP molecule per step14. Together, these results suggest that kinesin3 motors purified from the Sf9-baculovirus expression system yielded a significantly large population of active and soluble proteins. Therefore, we believe that the Sf9-baculovirus system can be employed to purify motor proteins of interest with necessary modifications.
In vitro single-molecule motility assay of kinesin-3 motors that exhibit superprocessive motility needs polymerization of long polymerized microtubules. A critical step in in vitro polymerization of long microtubules is to prepare a polymerization mixture with critical tubulin concentration, incubation time, and appropriate buffer pH. The tubulin used for microtubule polymerization should be highly competent, pure, and stored in liquid nitrogen in small aliquots to avoid freezing and thawing. Tubulin, once thawed, should not be frozen back for future polymerization because tubulin loses its competency significantly in every freeze-thaw cycle. Incompetent tubulin will not contribute to the microtubule polymerization but will hinder the polymerization process and result in short microtubules with unknown defects. The optimal tubulin concentration in the polymerization mixture should be between 2.5-3 mg/mL and the mixture should be kept on ice for 5 min before incubating it at 37 °C for polymerization. The duration of microtubules polymerization should be between 20-30 min and should not exceed 30 min, as prolonged polymerization often results in the formation of short and fragmented microtubules.
Next, stabilization of polymerized microtubules is a second critical step. Taxol is poorly soluble in water and precipitates in an aqueous solution39. Therefore, prepare the stabilization buffer freshly with 10 µM taxol, add gently over the polymerized microtubules mixture, and incubate further without disturbing. Always use frozen taxol aliquot from the -20 °C freezer to prepare the stabilization buffer, as thawed aliquot results in the precipitation/aggregation of polymerized microtubules. Always handle the polymerized microtubules using beveled tips to avoid breakage. Microtubules polymerized using this method yield long microtubules with 60-70 µm long suitable for superprocessive motility analysis. The next critical step is the stable adsorption of microtubules to the coverslip surface in the motility chamber. To achieve this, coverslips should be stored in a dry place because exposure of coverslips to the humid environment significantly decreases the microtubule adsorption efficiency and their stability.
Preparing a motility assay mixture with proper ingredients at particular concentrations and optimal motor protein concentration is important to achieve robust motility events along the microtubule surface. The most crucial component in generating smooth and processive motility is using a stable ATP solution in the assay mixture. (Thus, care should be taken while preparing the ATP stock. Dissolving the sodium salt of ATP in 10 mM PIPES buffer pH 6.9, yields a clear solution with a very low pH. ATP is extremely unstable at low pH and rapidly hydrolyzes to ADP and phosphate, thus keeping the solution cold and immediately adjusting the pH to 7.0 using concentrated KOH prevents hydrolysis.) Flow the motility mixture into the flow cell and seal both the edges with liquid paraffin to prevent liquid flow and drying of the chamber while imaging under TIRF illumination.
As kinesin-3 motors are fast and superprocessive along the microtubule tracks, their imaging, data acquisition and analysis pose several challenges. Single-molecule imaging and data acquisition should be performed under low laser intensity to prevent photobleaching of fluorescent proteins before the motor completes its processive run. The acquired data would be of low intensity with poor signal-to-noise ratio. Consequently, tracking of these individual motility events using existing automated tracking software to characterize their motility events would be difficult. Therefore, data analysis has to be performed manually by accurately tracking fluorescently tagged motor spots moving along the microtubule tracks frame-by-frame with minimal errors. For data analysis, preferentially, choose the motility events on the long microtubule tracks to avoid motors reaching the microtubule end before completing their run. For consistent and reproducible results, consider only those motility events for tracking, at least, the last 10 frames (500 ms) with a clear starting and ending of the processive run.
Overall, the method offers several advantages over the existing method, such as simple and one-step purification of active motor proteins, preparation of long microtubules, and detailed step-by-step protocol with instructions to achieve good motility. In principle, the method can be applied to express and purify any class and type of proteins, including but not limited to myosin, kinesin, and dynein. Indeed, the baculovirus expression system has been successfully established to purify several viral vaccines, gene therapy vectors and other pharmaceutical proteins20.
Divulgations
The authors have nothing to disclose.
Acknowledgements
V.S. and P.S. thank Prof. Kristen J. Verhey (University of Michigan, Ann Arbor, MI, USA) and Prof. Roop Mallik (Indian Institute of Technology Bombay (IITB), Mumbai, India) for their unconditional support throughout the study. P.S. thanks Dr. Sivapriya Kirubakaran for her support throughout the project. V.S. acknowledges funding through DBT (Grant No.: BT/PR15214/BRB/10/1449/2015 and BT/RLF/Re-entry/45/2015) and DST-SERB (Grant No.: ECR/2016/000913). P.K.N acknowledges ICMR for funding (Grant No. 5/13/13/2019/NCD-III). P.S. acknowledges funding from DST (Grant No.: SR/WOS-A/LS-73/2017). D.J.S acknowledges fellowship from IIT Gandhinagar.
Materials
| Sf9 culture and transfection materials | |||
| anti-FLAG M2 affinity | Biolegend | 651502 | For motility purification |
| Aprotinin | Sigma | A6279 | For motility assay and purification |
| Cellfectin | Invitrogen | 10362100 | For Sf9 transfection |
| DTT | Sigma | D5545 | For motility assay mixture |
| FLAG peptide | Sigma | F3290 | For motility purification |
| Glycerol | Sigma | G5516 | For motility purification |
| HEPES | Sigma | H3375 | Preparing lysing Sf9 cells |
| IGEPAL CA 630 | Sigma | I8896 | Preparing lysing buffer for Sf9 cells |
| KCl | Sigma | P9541 | For motility purification |
| Leupeptin | Sigma | L2884 | For motility assay and purification |
| MgCl2 | Sigma | M2670 | For preparing lysis buffer |
| NaCl | Sigma | S7653 | For preparing lysis buffer |
| PMSF | Sigma | P7626 | For motility purification |
| Sf9 cells | Kind gift from Dr. Thomas Pucadyil (Indian Institute of Science Education and Research, Pune, India). | For baculovirs expression and purification | |
| Sf9 culture bottles | Thermo Scientific | 4115-0125 | For suspension culture |
| Sf-900/SFM medium (1X) | Thermo Scientific | 10902-096 -500ml | For culturing Sf9 cells |
| Sucrose | Sigma | S1888 | Preparing lysing buffer for Sf9 cells |
| Unsupplemented Grace’s media | Thermo Scientific | 11595030 -500ml | For Sf9 transfection |
| Mirotubule Polymerization and Single molecule assay materails | |||
| ATP | Sigma | A2647 | For motility and gliding assay |
| BSA | Sigma | A2153 | For blocking motility chamber |
| Catalase | Sigma | C9322 | For motility and gliding assay |
| DMSO | Sigma | D5879 | For dissolving Rhodamine |
| EGTA | Sigma | 3777 | For preparing buffers |
| Glucose | Sigma | G7021 | For motility and gliding assay |
| Glucose oxidase | Sigma | G2133 | For motility and gliding assay |
| GTP | Sigma | G8877 | For microtubule polymerization |
| KOH | Sigma | P1767 | Preparing PIPES buffer pH 6.9 |
| PIPES | Sigma | P6757 | For motility and gliding assay |
| Microtubule gliding assay materials | |||
| 26G needle | Dispovan | For shearing microtubules | |
| Casein | Sigma | C3400 | For microtubule glidning assay |
| GFP nanobodies | Gift from Dr. Sivaraj Sivaramakrishnan (University of Minnesota, USA) | For attaching motors to the coverslip | |
| Rhodamine | Thermo Scientific | 46406 | For preparing labelling tubulin |
| Microscope and other instruments | |||
| 0.5ml, 1.5 and 2-ml microcentrifuge tubes | Eppendorf | For Sf9 culture and purification | |
| 10ml disposable sterile pipettes | Eppendorf | For Sf9 culture and purification | |
| 10ul, 200ul, 1ml micropipette tips | Eppendorf | For Sf9 culture and purification | |
| 15ml concal tubes | Eppendorf | For Sf9 culture and purification | |
| 35mm cell culture dish | Cole Palmer | 15179-39 | For Sf9 culture |
| Balance | Sartorious | 0.01g-300g | |
| Benchtop orbial shaking incubator | REMI | For Sf9 suspenculture at 28oC | |
| Camera | EMCCD Andor iXon Ultra 897 | For TIRF imaging and acquesition | |
| Double sided tape | Scotch | For making motility chamber | |
| Glass coverslip | Fisherfinest | 12-548-5A | size; 22X30 |
| Glass slide | Blue Star | For making motility chamber | |
| Heating block | Neuation | Dissolving paraffin wax | |
| Inverted microscope | Nikon Eclipse Ti- U | To check protein expression | |
| Lasers | 488nm (100mW) | For TIRF imaging | |
| Liquid nitrogen | For sample freezing and storage | ||
| Microcapillary loading tip | Eppendorf | EP022491920 | For shearing microtubules |
| Microscope | Nikon Eclipse Ti2-E with DIC set up | For TIRF imaging | |
| Mini spin | Genetix, BiotechAsia Pvt.Ltd | For quick spin | |
| Objective | 100X TIRF objective with 1.49NA oil immersion | For TIRF imaging | |
| Optima UltraCentrifuge XE | Beckman Coulter | For protein purification | |
| Parafilm | Eppendorf | ||
| pH-meter | Corning | Coring 430 | To adjust pH |
| Pipette-boy | VWR | For Sf9 culture and purification | |
| Sorvall Legend Micro 21 | Thermo Scientific | For protein purification | |
| Sorvall ST8R centrifuge | Thermo Scientific | Protein purification | |
| ThermoMixer | Eppendorf | For microtubule polymerization | |
| Ultracentrifuge rotor | Beckman coulter | SW60Ti rotor | |
| Ultracentrifuge tubes | Beckman | 5 mL, Open-Top Thinwall Ultra-Clear Tube, 13 x 51mm | |
| Vortex mixer | Neuation | Sample mixing | |
| Wax | Sigma | V001228 | To seal motility chamber |
References
- Vale, R. D. The molecular motor toolbox for intracellular transport. Cell. 112, 467-480 (2003).
- Miki, H., Okada, Y., Hirokawa, N. Analysis of the kinesin superfamily: insights into structure and function. Trends in Cell Biology. 15 (9), 467-476 (2005).
- Hirokawa, N., Niwa, S., Tanaka, Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 68 (4), 610-638 (2010).
- Hirokawa, N., Takemura, R. Molecular motors and mechanisms of directional transport in neurons. Nature Reviews Neuroscience. 6 (3), 201-214 (2005).
- Patel, N. M., et al. KIF13A motors are regulated by Rab22A to function as weak dimers inside the cell. Scientific Advances. 7 (6), (2021).
- Franker, M. A., Hoogenraad, C. C. Microtubule-based transport – basic mechanisms, traffic rules and role in neurological pathogenesis. Journal of Cellular Science. 126, 2319-2329 (2013).
- Gunawardena, S., Anderson, E. N., White, J. Axonal transport and neurodegenerative disease: vesicle-motor complex formation and their regulation. Degernative Neurological and Neuromuscular Disease. 4, 29-47 (2014).
- Rath, O., Kozielski, F. Kinesins and cancer. Nature Reviews Cancer. 12 (8), 527-539 (2012).
- Wang, Z. Z., et al. KIF14 promotes cell proliferation via activation of Akt and is directly targeted by miR-200c in colorectal cancer. International Journal of Oncology. 53 (5), 1939-1952 (2018).
- Guo, S. K., Shi, X. X., Wang, P. Y., Xie, P. Run length distribution of dimerized kinesin-3 molecular motors: comparison with dimeric kinesin-1. Scientific Reports. 9 (1), 16973 (2019).
- Scarabelli, G., et al. Mapping the processivity determinants of the Kinesin-3 motor domain. Biophysical Journal. 109 (8), 1537-1540 (2015).
- Soppina, V., et al. Dimerization of mammalian kinesin-3 motors results in superprocessive motion. Proceedings of the National Academy of Sciences of the United States of America. 111 (15), 5562-5567 (2014).
- Soppina, V., Verhey, K. J. The family-specific K-loop influences the microtubule on-rate but not the superprocessivity of kinesin-3 motors. Molecular Biology Cell. 25 (14), 2161-2170 (2014).
- Soppina, V., et al. Kinesin-3 motors are fine-tuned at the molecular level to endow distinct mechanical outputs. BMC Biology. , (2022).
- Korten, T., Chaudhuri, S., Tavkin, E., Braun, M., Diez, S. Kinesin-1 Expressed in insect cells improves microtubule in vitro gliding performance, long-term stability and guiding efficiency in nanostructures. IEEE Transactions on Nanobioscience. 15 (1), 62-69 (2016).
- Schmidt, F. R. Recombinant expression systems in the pharmaceutical industry. Applied Microbiology and Biotechnology. 65 (4), 363-372 (2004).
- Kurland, C., Gallant, J. Errors of heterologous protein expression. Current Opinions in Biotechnology. 7 (5), 489-493 (1996).
- Tao, L., Scholey, J. M. Purification and assay of mitotic motors. Methods. 51 (2), 233-241 (2010).
- Kost, T. A., Condreay, J. P., Jarvis, D. L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nature Biotechnology. 23 (5), 567-575 (2005).
- Felberbaum, R. S. The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnology Journal. 10 (5), 702-714 (2015).
- Kumar, N., Pandey, D., Halder, A., Kumar, D., Gong, C. . Trends in Insect Molecular Biology and Biotechnology. , 163-191 (2018).
- Nagano, T. Development of fluorescent probes for bioimaging applications. Proceedings of the Japan Academy. Series B. Physical and Biological Sciences. 86 (8), 837-847 (2010).
- Hassan, M., Klaunberg, B. A. Biomedical applications of fluorescence imaging in vivo. Comparative Medicine. 54 (6), 635-644 (2004).
- Yang, Z., Samanta, S., Yan, W., Yu, B., Qu, J. Super-resolution microscopy for biological imaging. Advances in Experimental Medicine and Biology. 3233, 23-43 (2021).
- Ettinger, A., Wittmann, T. Fluorescence live cell imaging. Methods in Cell Biology. 123, 77-94 (2014).
- Waters, J. C. Live-cell fluorescence imaging. Methods in Cell Biology. 114, 125-150 (2013).
- Zheng, Q., Jockusch, S., Zhou, Z., Blanchard, S. C. The contribution of reactive oxygen species to the photobleaching of organic fluorophores. Photochemistry and Photobiology. 90 (2), 448-454 (2014).
- Wojtovich, A. P., Foster, T. H. Optogenetic control of ROS production. Redox Biology. 2, 368-376 (2014).
- Cai, D., Verhey, K. J., Meyhofer, E. Tracking single Kinesin molecules in the cytoplasm of mammalian cells. Biophysical Journal. 92 (12), 4137-4144 (2007).
- Bohm, K. J., Stracke, R., Baum, M., Zieren, M., Unger, E. Effect of temperature on kinesin-driven microtubule gliding and kinesin ATPase activity. FEBS Letters. 466, 59-62 (2000).
- Porter, M. E., et al. Characterization of the microtubule movement produced by sea urchin egg kinesin. The Journal of Biological Chemistry. 262 (6), 2794-2802 (1987).
- Muyldermans, S. Nanobodies: natural single-domain antibodies. Annual Review of Biochemistry. 82, 775-797 (2013).
- Verma, S., Kumar, N., Verma, V. Role of paclitaxel on critical nucleation concentration of tubulin and its effects thereof. Biochemical and Biophysical Research Communications. 478 (3), 1350-1354 (2016).
- Parness, J., Horwitz, S. B. Taxol binds to polymerized tubulin in vitro. Journal of Cell Biology. 91 (2), 479-487 (1981).
- Schiff, P. B., Fant, J., Horwitz, S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 277 (5698), 665-667 (1979).
- Kato, T., Kageshima, A., Suzuki, F., Park, E. Y. Expression and purification of human (pro)renin receptor in insect cells using baculovirus expression system. Protein Expression and Purification. 58 (2), 242-248 (2008).
- Liu, F., Wu, X., Li, L., Liu, Z., Wang, Z. Use of baculovirus expression system for generation of virus-like particles: successes and challenges. Protein Expression and Purification. 90 (2), 104-116 (2013).
- Terpe, K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Applied Microbiology and Biotechnology. 60 (5), 523-533 (2003).
- Huang, S. T., et al. Liposomal paclitaxel induces fewer hematopoietic and cardiovascular complications than bioequivalent doses of Taxol. International Journal of Oncology. 53 (3), 1105-1117 (2018).

