Rat Burn Model to Study Full-Thickness Cutaneous Thermal Burn and Infection
Summary
A model mimicking the clinical scenario of burn injury and infection is necessary for furthering burn research. The present protocol demonstrates a simple and reproducible rat burn infection model comparable to that in humans. This facilitates the study of burn and infections following burn for developing new topical antibiotic treatments.
Abstract
Burn induction methodologies are inconsistently described in rat models. A uniform burn wound model, which represents the clinical scenario, is necessary to perform reproducible burn research. The present protocol describes a simple and reproducible method to create ~20% total body surface area (TBSA) full-thickness burns in rats. Here, a 22.89 cm2 (5.4 cm diameter) copper rod heated at 97 °C in a water bath was applied to the rat skin surface to induce the burn injury. A copper rod with a high thermal conductivity was able to dissipate the heat deeper in the skin tissue to create a full-thickness burn. Histology analysis shows attenuated epidermis with coagulative damage to the full-thickness extent of the dermis and the subcutaneous tissue. Additionally, this model is representative of the clinical situations observed in hospitalized burn patients following burn injury such as immune dysregulation and bacterial infections. The model can recapitulate the systemic bacterial infection by both Gram-positive and Gram-negative bacteria. In conclusion, this paper presents an easy-to-learn and robust rat burn model that mimics the clinical situations, including immune dysregulation and bacterial infections, which is of considerable utility for the development of new topical antibiotic drugs for burn wound and infections.
Introduction
Burn injuries are among the most devastating forms of trauma, with mortality rates reaching 12% even in specialized burn centers1,2,3. According to recently published reports, ~486,000 burn patients require medical care annually in the United States, with nearly 3,500 deaths1,2,3,4,5,6. Burn injury imposes a major challenge for patients' immune system and creates a significant open wound, which is slow to heal, leaving them susceptible to cutaneous, pulmonary, and systemic colonization with nosocomial, opportunistic bacteria. Immune dysregulation combined with the bacterial infection is associated with increased morbidity and mortality in burn patients7.
An animal burn and infection model is essential for studying the pathogenesis of bacterial infections following skin damage and immune suppression associated with burn trauma. Such models enable the design and evaluation of new methods for treating bacterial infections in burn patients. Rats and humans share similar skin physiological and pathological characteristics that have been previously documented8. Additionally, rats are smaller in size, making them easier to handle, more affordable, and easier to procure and maintain than larger animal models.
These characteristics make rats an ideal model animal to study burns and infections9. Unfortunately, the technique for burn induction is inconsistent and often minimally described10,11,12,13,14. The present protocol is designed to develop a simple, cost-effective, and reproducible procedure for creating a consistent full-thickness burn injury in a rat model that simulates the clinical scenario and can be used to evaluate immune suppression and bacterial infection.
Protocol
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of The University of North Carolina and were conducted in accordance with its established guidelines. Male and female Sprague Dawley rats (250-300 g) aged 7-9 weeks old were used for the experiments. All animals were housed in a 12 h:12 h light-dark cycle with free access to food and water ad libitum. Always work with your institutional veterinarian about an analgesic plan prior to study initiation.
1. Preparing rats for the burn injury
- Prepare the animals for burn injury 24 h prior to the burn.
- Anesthetize the rat with 5% isoflurane in 100% oxygen in an induction chamber for 5 min (flow rate: 2 L/min) until breathing has slowed.
- Once the rat is deeply anesthetized (unresponsive to toe pinch on all limbs), move the rat over to a heating pad in a prone position and reduce the isoflurane to 1.5% in oxygen for maintenance through a nose cone.
- To prevent corneal drying after anesthesia and during the procedure, apply eye lubricant on the corneas of both eyes using a cotton-tipped applicator.
- Shave the dorsal area of the rat using an electric clipper (see the Table of Materials) and remove as much hair as possible in a large rectangle from the shoulder blades down to the base of the tail (Figure 2A).
- Clean the shaved area with a tissue soaked in saline to wipe out the loose hairs. Apply hair removal lotion to the shaved area using a cotton-tipped applicator and leave it on for ~3 min.
NOTE: Application of the referenced hair removal lotion for more than 3 min will induce red rashes on the skin. - Wipe the area with a wet gauze sponge twice to remove the lotion and prevent skin irritation.
- Turn off the isoflurane, remove the nose cone, and place the rat in the recovery cage.
NOTE: Put a heating pad in the recovery cage. - Transfer the recovered animal to a clean housing cage for the next day's burn procedure (it may take ~10-15 min for the rat to recover from the anesthesia).
2. Inducing the burn injury in rats
- On the day of the burn, set the water bath temperature to 97 °C and place all four copper rods (420 g each; Figure 1) in the water bath 1 h prior to the burn experiment to let the rods warm up uniformly.
NOTE: The rods must be immersed in the water. Check the accuracy of the digital temperature display using a thermometer before the experiment. - Anesthetize the rat as mentioned in section 1.
- Once the rat is unresponsive to toe pinch on all limbs, place it on a heating pad in a prone position with 1.5% isoflurane in oxygen for maintenance (Figure 2A).
- Inject morphine (20 mg/kg body weight) via the intraperitoneal (i.p.) route for pain management6.
- Check the temperature of the water in the water bath. Set up the timer and put on the heat-resistant gloves.
- Take out one heated copper rod from the water bath and touch it on the dorsum area of the rat for 7 s to induce the burn.
NOTE: Keep a minimum distance (10-15 cm) between the water bath and the animal to minimize heat loss, and do not put pressure on the rods while inducing the burn (i.e., contact must be maintained by gravity). - Apply four burns, using one rod per burn site, one immediately after the other to produce an approximately 20% TBSA full-contact burn (Figure 2B).
- After the burn, resuscitate the animal by i.p. injection of lactated Ringer's solution (0.1 mL/g body weight).
NOTE: Use a body temperature-adjusted lactated ringer's solution to resuscitate the rats. - Turn off the isoflurane, remove the nose cone, and place the rat on the heat mat for recovery.
3. Preparation of bacterial inoculum and infection
- Streak the frozen sample of Pseudomonas aeruginosa PAO1 and Staphylococcus aureus ATCC25923 on Muller Hinton Agar (MHA) plates, 2 days prior to the burn experiment.
- On the next day, select a single colony of grown bacteria from the plate, and using an inoculation loop, slightly scrape it off the plate. Then, place it in the culture tube to inoculate 10 mL of Muller Hinton Broth (MHB) and culture overnight at 37 °C in an incubator shaker.
- On the day of the burn and infection, centrifuge the culture at 4,000 × g for 5 min. Wash the pellet with normal saline (0.9% NaCl solution).
- Resuspend the bacterial pellet in saline and dilute up to 0.1 OD600nm (optical density at 600 nm). Dilute the bacterial inoculum by taking 200 µL of this bacterial suspension and mixing it with 800 µL of saline to get the desired bacterial inoculum of 2 × 107 CFU/mL.
- Inject 50 µL of P. aeruginosa or S. aureus inoculum prepared in the previous step (infection dose 1 × 106 CFU) into the anesthetized rat 15 min after the burn, using a 29 G needle subcutaneously as close to the burn wound as possible.
- After infecting the burn wound, place the rat on the heating pad for recovery. Once the animal recovers (~15-20 min), house it in a clean cage.
NOTE: After the burn injury, house one rat per cage. Use water wet food pellets for easy chewing and place them on the cage floor for easy reach. - Fill the water bottles in the cage with morphine-spiked water (0.4 mg/mL) for pain management.
NOTE: Oral Morphine mirrors the clinical situation with human burn patients. This study utilized oral morphine to keep these experiments comparable to human burn patients after consulting with the veterinary staff on numerous occasions. Drinking and weight logs were maintained throughout the experiment. Use the same drinking system during all the procedures. Other analgesics, such as Buprenorphine, can be administered subcutaneously/intraperitoneally as per institutional animal care guidelines. - Fill out the monitoring checklist and monitor the animals closely for distress or illness for the entire duration of the experiment.
4. Evaluation of the burn injury
- Evaluate the skin burn injury morphologically in terms of color and margin immediately after the burn injury.
- Stain the burned skin with hematoxylin and eosin (H&E) to visualize the burn wound structure and epithelial gap15 (see step 5.6 for sample processing).
5. Postprocessing of rat samples and bacterial enumeration
- Euthanize the rat at 24, 48, and 72 h post burn with an overdose of anesthesia.
- Withdraw blood samples from the rats via cardiac puncture and collect them in a mini collect tube.
- Analyze complete blood counts from the blood samples to determine the effect of burn induction on the host immune system.
- Harvest skin, subcutaneous tissue, muscle, lung, and spleen at the time of euthanasia.
NOTE: Keep one part (~1 cm × 1 cm; weighing ~200-300 mg) of the skin for H&E staining and another part for bacterial enumeration. - Collect the tissues in a 10 mL collection tube and place them in normal saline on ice for bacterial enumeration.
- Normalize the tissue weight with normal saline and homogenize the samples using a tissue homogenizer (see the Table of Materials).
- Serially dilute the tissue homogenates in normal saline.
- Plate 100 µL of undiluted homogenate and all dilutions of each tissue sample on cetrimide agar plates for samples collected from rats infected with P. aeruginosa.
NOTE: Use mannitol agar plates for plating samples collected from rats infected with S. aureus. - Incubate the plates at 37 °C in an incubator for 16-18 h.
- The next day, count the bacterial colonies on the plates, multiply by the dilution ratio to get the CFU/mL count, and normalize with the tissue's weight to calculate the CFU/g tissue.
- Employ data analysis software to plot the bacterial counts in different organs at the different sampling time-points.
- Perform H&E staining of burned skin to visualize the wound structure and epithelial gap.
- Using scissors and toothed forceps, cut a skin patch of 1 cm x 1 cm from the burn area and immerse it in a fixative (10% neutral buffered formalin, NBF) for 48 h at room temperature.
NOTE: Swirl the container to ensure all tissues are completely immersed in the fixative, with the volume of the fixative 30x the tissue volume. - Dehydrate the skin tissue with 70% (v/v) ethanol for 72 h at room temperature.
- Process the dehydrated samples in paraffin blocks to cut the sections and stain with H&E15.
- Digitally image the stained slides in a slide scanner (see the Table of Materials) using a 40x objective.
- Analyze the scanned image using software (see Supplementary File 1 for processing of the image for analysis; see the Table of Materials).
- Examine all fields of the stained skin section to evaluate the condition of the epidermis, dermis, subcutaneous tissue, and skeletal muscle.
- Using scissors and toothed forceps, cut a skin patch of 1 cm x 1 cm from the burn area and immerse it in a fixative (10% neutral buffered formalin, NBF) for 48 h at room temperature.
Representative Results
The protocol presented here is highly reproducible and resulted in a third-degree, full-thickness burn injury in rats. The burn wound appears waxy white after burn induction (Figure 2B). The color of the burn injury changed from white to brown over the course of 72 h post burn (Figure 2B–E).
Histological analysis confirmed a full-thickness burn (depth >2.61 mm at 24 h post burn; Figure 3B). Compared to intact non-burn skin, skin samples from burn animals showed evidence of injury across all layers at 24, 48, and 72 h post burn injury (Figure 3). Additionally, histological analysis showed complete destruction of the epidermal layer and damage to the full thickness of the dermis with involvement of subcutaneous fat and skeletal muscle (Figure 3B).
To evaluate bacterial clearance, various tissues were harvested at 24, 48, and 72 h following infection with P. aeruginosa and S. aureus. Bacteria were recovered from the infection site for all burn injury rats (Figure 4A,B). Furthermore, the number of bacteria recovered from the skin of burn rats was less than the initial inoculum for P. aeruginosa at 24 h post infection, whereas tissue samples obtained at 48 and 72 h post burn and infection showed an increase in the bacterial burden (Figure 4A). In contrast, a 2 log10 increase was observed at all time-points for S. aureus in the skin compared to the initial inoculum (Figure 4B). This suggests that S. aureus was able to establish infection because of its active replication in the tissues and not solely because of immunosuppression induced by the burn injury.
Different layers of the skin (i.e., subcutaneous tissue, muscles, and distal organs) were also analyzed to examine bacterial dissemination. The subcutaneous tissue and muscles showed a higher bacterial load than the lung and spleen. Taken together, these data show that burn rats develop a systemic infection 24 h or 48 h following wound inoculation with P. aeruginosa (Figure 4A) or S. aureus, respectively (Figure 4B). Complete blood counts were also obtained using a hematology analyzer (see the Table of Materials) at baseline and 72 h post burn injury. Total white blood cell counts decreased over time, indicating immune suppression. Neutrophil counts declined after burn but increased following infection at 72 h compared to the baseline (Table 1). However, increases in red blood cell and platelet counts were observed after burn and infection, indicating systemic inflammation.

Figure 1: Copper rod employed to inflict burn induction. The weight of the custom-made rod is 420 g with a 5.4 cm diameter and 6.4 cm height. Please click here to view a larger version of this figure.
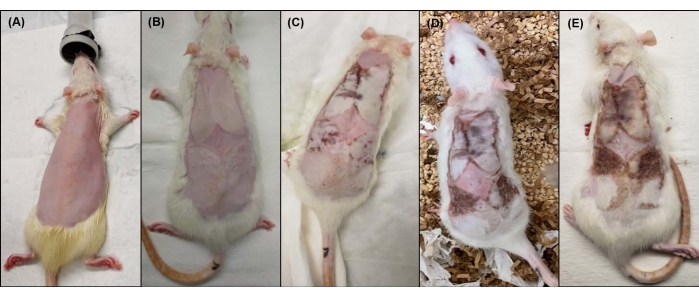
Figure 2: Macroscopic view of the rat dorsal side before and after burn induction. (A) Rat dorsum after shaving, (B) immediately after burn injury, (C) 24 h post burn, (D) 48 h post burn, and (E) 72 h post burn. Please click here to view a larger version of this figure.
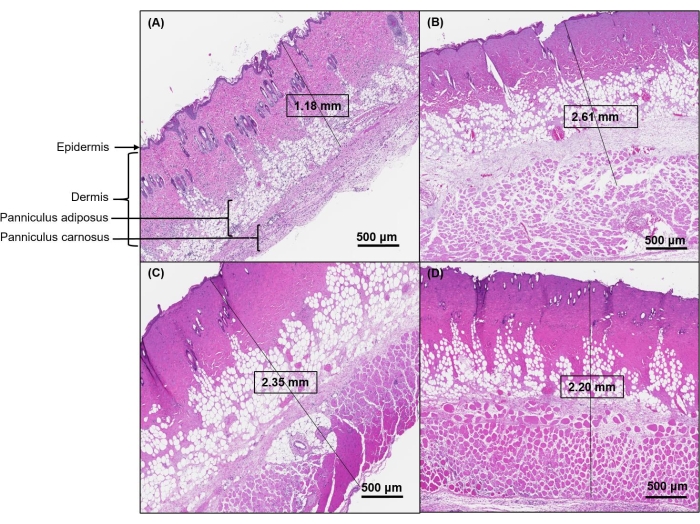
Figure 3: Representative images of H&E-stained cross-sections for each level of burn severity. (A) The histology of sham rat skin shows a clear distinction between epidermis, dermis, and subcutaneous tissue layers. (B) Skin histology 24 h post burn shows attenuated epidermis with coagulative damage to the full thickness of the dermis and the subcutaneous tissue with a maximum burn depth of >2.61 mm. (C) At 48 h post burn, the maximum burn depth was 2.35 mm, and (D) at 72 h post burn, the maximum burn depth was 2.20 mm. Images were scanned at 40x magnification. Scale bars = 500 μm (A–D). Abbreviation: H&E = hematoxylin and eosin. Please click here to view a larger version of this figure.
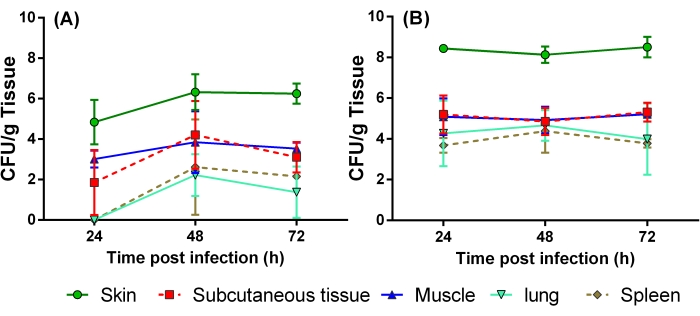
Figure 4: Quantification of bacterial load in different organs after infection of burn wound. Rats were infected with 6 log CFU of the bacteria via subcutaneous injections 15 min after the burn injury. Skin, subcutaneous tissue, muscle, lung, and spleen were collected at 24, 48, and 72 h post infection to determine the progression of systemic disease. Three rats were used at each time point.(A) Pseudomonas aeruginosa PA01, (B) Staphylococcus aureus ATCC25923. Abbreviation: CFU = colony-forming units. Please click here to view a larger version of this figure.
| Cell type | Baseline (average ± SD) | 72 h Un-infected (average ± SD) | 72 h-Infected (average ± SD) |
| White Blood Cells (109/L) | 16.9 ± 4.9 | 7.1 ± 2.0 | 6.50 ± 5.5 |
| Neutrophil (109/L); (%) | 4.0 ± 1.1; (24.3 ± 2.8) | 1.4 ± 0.4; (20.2 ± 5.7) | 1.88 ± 1.0; (35.0 ± 12.4) |
| Lymphocytes (109/L); (%) | 11.6 ± 4.1; (68.5 ± 1.7) | 4.8 ± 1.7; (66.5 ± 7.6) | 3.54 ± 3.9; (46.4 ± 17.0) |
| Monocytes (109/L); (%) | 0.9 ± 0.3; (5.4 ± 1.5) | 0.8 ± 0.2; (11.5 ± 1.6) | 1.0 ± 0.6; (17.3 ± 5.5) |
| Red blood cells (1012/L) | 7.5 ± 0.3 | 7.1 ± 0.8 | 10.0 ± 1.1 |
| Hemoglobin (g/dL) | 14.3 ± 0.7 | 13.4 ± 1.0 | 18.6 ± 2.0 |
| Platelets (109/L) | 723.3 ± 353.1 | 942.7 ± 43.1 | 1359.0 ± 228.5 |
| HCT (%) | 45.6 ± 3.0 | 39.9 ± 3.7 | 55.7 ± 8.2 |
Table 1: Hematology parameters before and after burn infliction and infection. Abbreviation: HCT = hematocrit.
Supplementary File 1: Steps to analyze H&E images in Aperio ImageScope. Abbreviation: H&E = hematoxylin and eosin. Please click here to download this File.
Discussion
Several burn models have been presented to study the pathophysiology of burn injury8,12,16,17. In the present study, we employed a rat model to develop a simple and reproducible protocol to induce a full-thickness burn followed by bacterial infection to simulate an infected burn trauma in patients. The choice of the rat as the animal model to mimic human conditions is based on a balance of cost, ease of use, reproducibility, and reliability of the data. The rat model used herein has many advantages over others: it is easy to handle and is the most commonly used burn model, which allows for comparisons across the literature. Despite the rat being widely used in the experimental setting, rat and human integuments are not histologically identical18,19. The integument of the rat is composed of the skin, a fatty layer known as panniculus adiposus, and beneath this layer is a sheath of loose connective tissue associated with white adipose tissue and smooth muscle forming a layer known as panniculus carnosus. The latter layer is absent in most of the human integument. This is of significance, since its smooth muscle cells promote fast and ample wound contraction20. Additionally, it must be noted that the wound healing mechanisms of rats are substantially different from those of humans8. Hence, researchers should bear this in mind when interpreting the results of the protocol described in this paper. Notwithstanding, the utility of the rat model for studying localized burn injuries and postburn sepsis is unquestionable and has produced copious data that are clinically reliable and transferable21. Additionally, rats have more surface area compared to other small animals, which permits the induction of relatively larger burn wounds, making it a good model for clinically relevant burn studies.
Different methods of burn induction have been published, including boiling water16, heated brass bar22, heated aluminum template17, a constant temperature hot plate placed over stainless steel rods23, and scalding over 45% of body surface24. An ideal experimental protocol would have the capacity to achieve burn wounds that are consistent in size and depth. In the present study, 420 g copper rods heated in water at 97 °C were used to transfer the heat through direct conductance to induce the burn. During burn induction, rods were directly touched to the skin surface without applying any external pressure, as the thermal energy conductance from a solid structure to a skin surface does not depend upon the pressure employed but rather on the temperature gradient25 and distance between the solid structure and the skin17,25. The factors that determined the choice of metal included thermal conductivity and the ability to resist rust and corrosion.
Copper has a high thermal conductivity (398 W/mK; where W is heat in Watts, m is area in meters, K is temperature in kelvin) compared to stainless steel, aluminum, or brass with 16 W/mK, 225 W/mK, and 109 W/mK, respectively9. High-thermal conductivity metal rods would dissipate heat energy faster to the skin tissues than low-thermal conductivity rods and induce a deeper level of burn within the same duration of exposure. Additionally, the size and weight of the rod was allometrically scaled from the burn model in mice7,26,27 and induces an approximately 20% TBSA burn. A 1.9 cm diameter rod (total burn area is 11.3 cm2 in a mouse after four applications) was scaled to 5.4 cm diameter (total burn area is 91.6 cm2 in a rat after four applications) to induce a similar ~20%-30% TBSA burn in rat (TBSA of a 220 g rat is 356.0 cm2)28, considering that the TBSA of rat is 6x bigger than the mouse (TBSA of a 20 g mouse is 61.2 cm2)29. The results clearly demonstrate that this method induced full-thickness burn, and histological analysis indicated excellent contrast between normal and burned skin tissues at different time points post burn (Figure 3). This model was also able to capture immune suppression, which is observed in patients after burn injury30,31 (Table 1).
Bacterial infections are an important threat that compromise the healing process of burn patients and often are the leading cause of morbidity and mortality after burn injuries. To simulate similar conditions, the rat was infected following a burn injury with either P. aeruginosa or S. aureus. Initially, we tried topical application of the bacteria, but the waxy appearance of the burn surface inhibited absorption of the bacterial inoculum. This model was also able to recapitulate systemic disease progression following bacterial infection of the burn site as seen with bacterial burden recovered from the lung and spleen (Figure 4). In conclusion, we have demonstrated a simple and reproducible method for creating full-thickness burns that exhibit many of the features observed in human burn injuries. This protocol can aid in studying a wide variety of novel topical therapeutics for the treatment of infected burn wounds. This model can also be used as a cost-effective model for evaluating different wound dressings.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors thank the Division of Comparative Medicine at the University of North Carolina for the provision and care of animals. We thank Lauren Ralph and Mia Evangelista in the Pathology Services Core for expert technical assistance with Histopathology/Digital Pathology, including tissue sectioning and imaging. This research was supported by a research grant from the Department of Defense (Award number W81XWH-20-1-0500, GR and TV).
Materials
| 1 mL syringe | BD, USA | 309597 | Used to inject the analgesic |
| 1.7 mL Microtube | Olympus, USA | 24-282 | Used to carry morphine |
| 10% NBF | VWR, USA | 16004-115 | Used to fix the skin piece for staining |
| 30 mL syringe | BD, USA | 302832 | Used to inject the lactate ringer solution |
| 70% ethyl alcohol | Fischer Scientific, USA | BP28184 | |
| Aperio AT2 Digital Pathology Slide Scanner with ImageScope software | Aperio, Technologies Inc., Vista, CA, USA | n/a | Scanning of H & E slides and analysis |
| Cetrimide agar plates | BD, USA | 285420 | Selective media plates for Pseudomonas aeruginosa growth |
| Copper rods | n/a | n/a | Used to induce the burn injury |
| Cotton tipped applicators | OMEGA Surgical supply, USA | 4225-IMC | Used to apply eye ointment |
| Electric shaver | Oster, USA | Golden A5 | Used to remove the dorsal side hairs |
| Eye lube | Dechra, UK | n/a | The eye wetting agent to provide long lasting comfort and avoid eye dryness |
| Fluff filled underpads | Medline, USA | MSC281225 | Used in the burn procedure |
| Forcep | F.S.T. | 11027-12 | Used to hold the skin piece |
| Gauze sponges | Oasis, USA | PK412 | Used to clean the applied nair cream from the dorsal side |
| Heat-resistant gloves | n/a | n/a | Used to hold the heated copper rods |
| Hematology Analyzer | IDEXX laboratories, USA | ProCyte Dx | |
| Induction chamber | Kent Scientific, USA | vetFlo-0730 | Used to anesthesize the animals |
| Insulin syringe | BD, USA | 329461 | |
| Isoflurane | Pivetal, USA | NDC46066-755-04 | Used to anesthesized rats to induce a loss of consciousness |
| Isoflurane vaporiser | n/a | n/a | |
| Lactated ringer's solution | icumedical, USA | NDC0990-7953-09 | Used to resuscitate the rats |
| L-shaped spreader | Fischer Scientific, USA | 14-665-230 | |
| Mannitol Agar | BD, USA | 211407 | Selective media plates for Staphylococcus aureus growth |
| Minicollect tubes (K2EDTA) | greiner bio-one, USA | 450480 | Used to collect the blood |
| Morphine | Mallinckrodt, UK | NDC0406-8003-30 | This analgesia was used to induce the inability to feel burn injury pain |
| Muller Hinton Broth | BD, USA | 275730 | |
| Muller Hinton II Agar | BD, USA | 211438 | |
| Nair hair removal lotion | Nair, USA | n/a | Used to remove the residual hairs on dorsal side |
| Needle 23 G | BD, USA | 305193 | Used to inject the lactate ringer solution |
| Normal saline | n/a | n/a | |
| Spectrophotometer | ThermoScientific, USA | Genesys 30 | |
| Sprague-Dawley rats, male and female | Charles River Labs | n/a | 7-9 weeks old for burn induction |
| Surgical Scissor | F.S.T. | 14501-14 | Used to cut the desired skin piece |
| Tissue collection tubes | Globe Scientific | 220101236 | |
| Tissue Homogenizer | Kinematica, Inc, USA | POLYTRON PT2100 | Used to homogenize the tissue samples |
| Water bath | Fischer Scientific, USA | n/a | Used to induce the burn injury |
| Weighted heating pad | Comfytemp, USA | n/a | Used during the procedure to keep rat's body warm |
References
- Peck, M., Molnar, J., Swart, D. A global plan for burn prevention and care. Bulletin of the World Health Organization. 87, 802-803 (2009).
- American Burn Association. Burn incidence and treatment in the United States: 2011 fact sheet. Chicago: American Burn Association. , (2011).
- Miller, S. F., et al. National burn repository 2007 report: a synopsis of the 2007 call for data. Journal of Burn Care & Research. 29 (6), 862-870 (2008).
- Kruger, E., Kowal, S., Bilir, S. P., Han, E., Foster, K. Relationship between patient characteristics and number of procedures as well as length of stay for patients surviving severe burn injuries: analysis of the American Burn Association National Burn Repository. Journal of Burn Care & Research. 41 (5), 1037-1044 (2020).
- American Burn Association. Burn incidence and treatment in the United States: 2016. Burn Incidence Fact Sheet. Chicago: American Burn Association. , (2016).
- Willis, M. L., et al. Plasma extracellular vesicles released after severe burn injury modulate macrophage phenotype and function. Journal of Leukocyte Biology. 111 (1), 33-49 (2022).
- Kartchner, L. B., et al. One-hit wonder: late after burn injury, granulocytes can clear one bacterial infection but cannot control a subsequent infection. Burns. 45 (3), 627-640 (2019).
- Abdullahi, A., Amini-Nik, S., Jeschke, M. Animal models in burn research. Cellular and Molecular Life Sciences. 71 (17), 3241-3255 (2014).
- Cai, E. Z., et al. Creation of consistent burn wounds: a rat model. Archives of Plastic Surgery. 41 (4), 317 (2014).
- Pessolato, A. G. T., dos Santos Martins, D., Ambrósio, C. E., Mançanares, C. A. F., de Carvalho, A. F. Propolis and amnion reepithelialise second-degree burns in rats. Burns. 37 (7), 1192-1201 (2011).
- Gurung, S., Škalko-Basnet, N. Wound healing properties of Carica papaya latex: in vivo evaluation in mice burn model. Journal of Ethnopharmacology. 121 (2), 338-341 (2009).
- Eloy, R., Cornillac, A. Wound healing of burns in rats treated with a new amino acid copolymer membrane. Burns. 18 (5), 405-411 (1992).
- Upadhyay, N., et al. Safety and healing efficacy of Sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats. Food and Chemical Toxicology. 47 (6), 1146-1153 (2009).
- El-Kased, R. F., Amer, R. I., Attia, D., Elmazar, M. M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Scientific Reports. 7 (1), 1-11 (2017).
- Fan, G. -. Y., et al. Severe burn injury in a swine model for clinical dressing assessment. Journal of Visualized Experiments. (141), e57942 (2018).
- Davenport, L., Dobson, G., Letson, H. A new model for standardising and treating thermal injury in the rat. MethodsX. 6, 2021-2027 (2019).
- Kaufman, T., Lusthaus, S., Sagher, U., Wexler, M. Deep partial skin thickness burns: a reproducible animal model to study burn wound healing. Burns. 16 (1), 13-16 (1990).
- Casal, D., et al. Blood supply to the integument of the abdomen of the rat: a surgical perspective. Plastic and Reconstructive Surgery Global Open. 5 (9), (2017).
- Casal, D., et al. A model of free tissue transfer: the rat epigastric free flap. Journal of Visualized Experiments. (119), e55281 (2017).
- Naldaiz-Gastesi, N., Bahri, O. A., Lopez de Munain, A., McCullagh, K. J., Izeta, A. The panniculus carnosus muscle: an evolutionary enigma at the intersection of distinct research fields. Journal of Anatomy. 233 (3), 275-288 (2018).
- Weber, B., et al. Modeling trauma in rats: similarities to humans and potential pitfalls to consider. Journal of Translational Medicine. 17 (1), 1-19 (2019).
- Nguyen, J. Q. M., et al. Spatial frequency domain imaging of burn wounds in a preclinical model of graded burn severity. Journal of Biomedical Optics. 18 (6), 066010 (2013).
- Sobral, C., Gragnani, A., Morgan, J., Ferreira, L. Inhibition of proliferation of Pseudomonas aeruginosa by KGF in an experimental burn model using human cultured keratinocytes. Burns. 33 (5), 613-620 (2007).
- Olivera, F., Bevilacqua, L., Anaruma, C., Boldrini Sde, C., Liberti, E. Morphological changes in distant muscle fibers following thermal injury i n Wistar rats. Acta Cirurgica Brasileira. 25, 525-528 (2010).
- Davies, J. W. . Physiological Responses to Burning Injury. , (1982).
- Neely, C. J., et al. Flagellin treatment prevents increased susceptibility to systemic bacterial infection after injury by inhibiting anti-inflammatory IL-10+ IL-12-neutrophil polarization. PloS One. 9 (1), e85623 (2014).
- Dunn, J. L., et al. Direct detection of blood nitric oxide reveals a burn-dependent decrease of nitric oxide in response to Pseudomonas aeruginosa infection. Burns. 42 (7), 1522-1527 (2016).
- Gouma, E., et al. A simple procedure for estimation of total body surface area and determination of a new value of Meeh’s constant in rats. Laboratory Animals. 46 (1), 40-45 (2012).
- Dawson, N. The surface-area/body-weight relationship in mice. Australian Journal of Biological Sciences. 20 (3), 687-690 (1967).
- Moins-Teisserenc, H., et al. Severe altered immune status after burn injury is associated with bacterial infection and septic shock. Frontiers in Immunology. 12, 529 (2021).
- Robins, E. V. Immunosuppression of the burned patient. Critical Care Nursing Clinics. 1 (4), 767-774 (1989).

