Zebrafish Animal Model for the Study of Allergic Reactions in Response to Tick Saliva Biomolecules
Summary
Here, zebrafish (Danio rerio) is used as a model to study allergic reactions and immune responses related to alpha-Gal syndrome (AGS) by evaluating allergic reactions to tick saliva and mammalian meat consumption.
Abstract
Ticks are arthropod vectors that cause disease by pathogen transmission and whose bites could be related to allergic reactions impacting human health worldwide. In some individuals, high levels of immunoglobulin E antibodies against the glycan Galα1-3Galβ1-(3)4GlcNAc-R (α-Gal) have been induced by tick bites. Anaphylactic reactions mediated by glycoproteins and glycolipids containing the glycan α-Gal, present in tick saliva, are related to alpha-Gal syndrome (AGS) or mammalian meat allergy. Zebrafish (Danio rerio) has become a widely used vertebrate model for the study of different pathologies. In this study, zebrafish was used as a model for the study of allergic reactions in response to α-Gal and mammalian meat consumption because, like humans, they do not synthesize this glycan. For this purpose, behavioral patterns and hemorrhagic anaphylactic-type allergic reactions in response to Ixodes ricinus tick saliva and mammalian meat consumption was evaluated. This experimental approach allows the obtention of valid data that support the zebrafish animal model for the study of tick-borne allergies including AGS.
Introduction
Ticks are vectors of pathogens that cause diseases and are also the cause of allergic reactions, affecting the health of humans and animals worldwide1,2. During tick feeding, biomolecules in tick saliva, especially proteins and lipids, facilitate the feeding of these ectoparasites, avoiding host defenses3. Some saliva biomolecules with glycan Galα1-3Galβ1-(3)4GlcNAc-R (α-Gal) modifications lead to the production of high anti-α-Gal IgE antibody levels after the tick bite, only in some individuals, which is known as α-Gal Syndrome (AGS)4. This is a disease associated with IgE-mediated allergy that may result in anaphylaxis to tick bites, non-primate mammalian meat consumption, and some drugs such as cetuximab5. Reactions to α-Gal are often severe and sometimes could be fatal6,7,8,9,10,11,12,13,14,15.
The α-Gal is found in all mammals except for Old World monkeys, apes, and humans that do not have the ability to synthesize α-Gal13. However, pathogens such as bacteria and protozoa express this glycan on their surface, which can induce the production of high amounts of anti-α-Gal IgM/IgG antibodies and may be a protective mechanism against these pathogens16,17. However, the production of anti-α-Gal antibodies increases the risk of developing IgE-mediated anti-α-Gal allergies7,13. Natural anti-α-Gal antibodies produced in humans, mainly of the IgM/IgG subtypes, could be associated with this modification present in bacteria from the gut microbiota16. AGS can be a challenging clinical diagnosis, as the main diagnostic method at the moment is based on a clinical history of delayed allergic reactions, especially associated with food allergies (i.e., pruritus, localized hives, or recurrent angioedema to anaphylaxis, urticaria, and gastrointestinal symptoms) and the measurement of IgE anti-α-Gal antibody levels9. Current findings suggest tick bites constitute one of the principal risks in the appearance of AGS18,19, a 20-fold or greater increase in IgE levels to α-Gal following a tick bite19, a history of tick bites in patients with AGS20,21,22, the existence of antibodies reactive to tick antigens in AGS patients19, and that anti-α-Gal IgE are strongly related to anti-tick IgE levels19,23 but further studies are needed to assess which biomolecules are actually involved.
In addition, another possible scenario is patients who present strong allergic reactions to tick bites and high levels of anti-α-Gal IgE antibodies but are tolerant to mammalian meat consumption12. Therefore, mammalian meat allergy could be a particular type of tick bite-related allergy. The principal tick species associated with AGS include Amblyomma americanum (USA), Amblyomma sculptum (Brazil), Amblyomma testudinarium and Haemaphysalis longicornis (Japan), Ixodes holocyclus (Australia), and Ixodes ricinus (the main vector of Lyme borreliosis in Europe)11,24.
The only model that has been used to evaluate IgE production related with tick bites is the mouse model genetically modified with the gene for α−1,3-galactosyltransferase knocked out (α-Gal KO) mice25,26 because like other mammals, mice also express α-Gal on proteins and lipids and do not produce IgE to α-Gal. However, zebrafish (Danio rerio) is a useful model for biomedical research applied to mammals because it shares many anatomical similarities with mammals and, like humans, is also unable to synthesize α-Gal. Since α-Gal is not produced naturally in zebrafish, it is an affordable model, easy to manipulate, and allows a high sample size for the study of α-Gal-related allergic reactions.
In this study, zebrafish is used as a model organism to characterize and describe local allergic reactions, behavioral patterns, and the molecular mechanisms associated with response to percutaneous sensitization to tick saliva26,27 and subsequent mammalian meat consumption. For this purpose, fish are exposed to tick saliva by intradermal injection and then are fed with dog feed, that contains mammalian meat-derived products suitable for animal use which contains α-Gal27, then possible related allergic reactions are evaluated. This method may be applied to the study of other biomolecules related to allergic processes, especially those related to AGS.
Protocol
All methods described here have been approved by the Ethics Committee on Animal Experimentation of the University of Castilla La Mancha under the study "Evaluation of the immune response to inactivated M. bovis vaccine and challenge with M. marinum in the zebrafish model number PR-2017-05-12."
Ticks were obtained from the laboratory colony, where representative samples of ticks in the colony were tested by PCR for common tick pathogensto confirm the absence of pathogens, and maintained at the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences (IP BC CAS), Czech Republic.All animal experiments were performed in accordance with the Animal Protection Law of the Czech Republic No. 246/1992 Sb (ethics approval No. 34/2018).
1. Zebrafish treatment
NOTE: The trial is designed to evaluate allergic reactions and the immune response in zebrafish treated with tick saliva in response to mammalian meat consumption.
- Treat the fish (as explained in section 4) with tick saliva, commercial Gala1-3Gal-BSA 3 (α-Gal) (see Table of Materials), used as a positive control, with phosphate-buffered saline (PBS) as a negative control. Adult zebrafish is randomly distributed into three gender-balanced groups (Figure 1).
NOTE: Any other desired compound related with AGS can be evaluated using this model.
2. Ixodes ricinus tick saliva extraction
- Use semi-engorged pathogen-free female ticks fed for 6-7 days on guinea pigs.
- Treat the tick with 5 µL of a 2% (wt/vol) solution of pilocarpine hydrochloride in PBS (see Table of Materials) at pH 7.4 into the hemocoel using a 50 µL syringe with a 0.33 mm needle as previously was described28 to induce tick saliva production.
NOTE: Ticks are handled using forceps; be cautious to not apply too much strength when gripping them. - Collect saliva using a 10 µL tip mounted on a micropipette.
- Introduce the tip inside the tick hypostome carefully.
- Keep the saliva in a 1.5 mL tube on ice, pool it, and store it at -80 °C as previously described27.
- Determine the saliva protein concentration, to establish the amount of protein to be injected into the fish as in previous studies27 using a BCA Protein Assay Kit (see Table of Materials) following the manufacturer's recommendations.
3. Maintenance of zebrafish
- Maintain zebrafish in a flow-through water system at 27 °C with a light/dark cycle of 14 h/10 h (Figure 2).
- Feed the fish twice daily at 9:30 a.m. and 1:30 p.m. with dry fish feed (50-70 μg/fish) until day 2.
- Feed the fish twice daily at 9:30 a.m. and 1:30 p.m. with dry dog feed (50-70 μg/fish) from day 2 after the treatment injection until the end of the experiment
4. Zebrafish injection
- Select 10 fish per group with a similar ratio of females/males and similar weight.
NOTE: Group 1 contains fish injected with PBS, group 2 contains fish injected with tick saliva, and group 3 contains fish injected with α-Gal. - Anesthetize the fish briefly by immersion in 0.02% tricaine methanesulphonate (MS-222) (Movie 1).
NOTE: Properly anesthetized fish show normal breathing and no swimming, while they could be placed at the bottom of the water tank or floating. Each fish must be individually anesthetized to avoid possible physiological damage. - Capture the anesthetized fish using a fishing net.
- Place the fish on its half side using forceps or hands carefully, on a wet sponge, with the caudal fin on the right side to inject the compounds in the same direction in order to control the lesions.
- Inject groups of fish intradermally, as in previous studies26, in the muscle at 5 mm to the caudal fin and at a 45° angle in relation to the body of the fish (Movie 2). Use the appropriate treatment at days 0, 3, and 8 as previously described27 with a 100 µL syringe fitted with a 1 cm, 29 G needle with 1 µL (with 9 µg/µL of protein) of I. ricinus saliva in 10 µL of PBS (tick saliva), 5 µg of α-Gal in 10 µL PBS (α-Gal)27, and 10 µL of PBS (Figure 3).
NOTE: Handling must be done quickly and carefully to avoid any physical damage to the animal.
Other biomolecules in tick saliva can be evaluated following this protocol. - Place the treated fish back in a freshwater tank without anesthesia for recovery.
NOTE: All the fish of the same group can be placed in the same water tank for recovery.
5. Zebrafish feeding
- Mash the dog food with a mortar and pestle.
- Feed 50-70 μg/fish twice daily at 9:30 a.m. and 1:30 p.m. with dry fish feed until day 2.
- Feed 50-70 μg/fish twice daily at 9:30 a.m. and 1:30 p.m. with mashed dog feed from day 2 after the treatment injection until the end of the experiment on day 8.
NOTE: If immunity markers or antibody titers to α-Gal or IgE antibody in response to the treatments or the feed throughout the different inoculations are to be evaluated, feeding would be necessary until the end of the experiment.
6. Evaluation of allergic reactions, lesions, and behavior in zebrafish
- Examine the hemorrhagic type of allergic reactions (skin redness, discoloration, and hemorrhage) using a magnifier or stereomicroscope for accuracy and indicate the location of their appearance on the fish following the categorization included in Table 1 (Figure 4A).
NOTE: The allergic reactions presented in Figure 4 appeared after the injection of tick saliva and the consumption of feed containing red meat. Therefore, the reactions described are the type of reactions associated with AGS, as similar reactions appear in the clinical context.- Observe if any reaction appears after treatments and while administering food twice a day while the fish are in the water tank.
- Examine the fish behavior by evaluating the changes27 in swimming patterns (mobility, speed, standing motionless at the bottom of the water tank, and zigzag swimming) following the categorization included in Table 1.
- Evaluate accumulated mortality, reporting the number of dead fish including the time/day of death (Figure 4B).
NOTE: All parameters are evaluated right after treatment or after the change of feed and followed daily until the end of the experiment on day 8 categorizing qualitative variables (Table 1). As a recommendation, this evaluation should be conducted by a professional with knowledge on zebrafish to consider behavioral changes based on their background and experience working with this animal model. - Calculate the number of zebrafish per day with reported allergic reactions, abnormal behavior, and feeding changes in each group and compare between groups by a one-way ANOVA test.
7. Sample collection
- Euthanize the fish by immersion in 0.04% MS-222 on day 8.
NOTE: Also collect the samples from the fish that die from allergic reactions during the trial. - Fix the fish on a paraffin plate with pins.
- Collect serum from the gill-blood vessels29 of the fish immediately after euthanasia, when the gills are still irrigated with blood, using a 0.5 mL syringe fitted with a 1 cm, 29 G needle. Store it in a 1.5 mL tube at -20 °C until use (Movie 3).
- Cut the fish sagittally with a scalpel blade and evaluate the internal lesions (hemorrhagic lesions or granulomas)27,30 if they appear.
NOTE: Lesions do not necessarily appear but must be registered if they do. - Collect the intestine (Movie 4) and kidney (Movie 5) from each fish in separate empty 1.5 mL tubes, as previously described31, and store them at -80 C (Figure 4C).
- Extract total RNA from the zebrafish intestine and kidney samples using an RNA purification kit (see Table of Materials).
- Analyze the expression of genes related to immune response as previously described30,32 (see Table 2 for primer sequences) in zebrafish, performing a quantitative reverse transcription-polymerase chain reaction (RT-qPCR) using a reverse transcription mix for RT-qPCR (see Table of Materials), according to the manufacturer's instructions. Normalize the mRNA cT values against D. rerio GAPDH, and compare between groups (fish treated with saliva, α-Gal, and the PBS-treated groups)using a Student t-test with unequal variance.
- Determine IgM antibody titers that recognize α-Gal in zebrafish in serum samples by ELISA as described previously27,30. Record the antibody titers as O.D.450 nm values, using a plate reader, and compare between groups (fish treated with saliva, α-Gal, and the PBS-treated groups)using a Student t-test with unequal variance.
NOTE: Determination of IgM antibody titers and expression gene analysis is optional and conducted only if immunological information is required. RT-qPCR mix is a first-strand cDNA synthesis kit for gene expression analysis using real-time qPCR.
Representative Results
The protocol presented here is based on several aspects of previously published experiments27,30 and results performed in our laboratory where the zebrafish model is established and validated for the study of AGS and the immune response to α-Gal because both humans and zebrafish do not synthesize this molecule13. This model allows the characterization and evaluation of a variety of allergic reactions as a result of the host response to tick saliva (Figure 4) and their implication in AGS. In addition, alterations in behavior such as slow swimming (Movie 6), lying on the bottom of the tank (Movie 7), and not eating, vibrating, or zigzagging motion (Movie 8) are observed in the fish in response to tick saliva treatment that is not observed in the control fish; these findings are particularly significant following the administration of dog food on day 2. At this point, the fish were already sensitized with alpha-gal and tick saliva, and the administration of red meat through the feed began. Finally, a significant incidence of allergic reactions is observed in fish treated with tick saliva (Figure 4A,B and Table 3), only zebrafish who had been exposed to tick saliva developed allergic reactions, showing rapid desensitization and tolerance. On the other hand, in previous studies, zebrafish fed with fish food did not develop any visible lesion or reaction27. The behavioral change was more pronounced in the fish treated with tick saliva than with just α-Gal (Figure 5). Additional analysis of the expression of the most representative immune response makers (IFN, TLR 2, IL1 β, and AKR2) was performed by RT-PCR (Table 3), in order to study different immune responses to the treatments. The results showed differences between zebrafish groups in the kidney, where the most immune response markers appeared to be downregulated in fish treated with saliva and α-Gal as compared to the control group (Figure 6), but no significant differences were found in gene expression in the intestine. Previous studies about allergic reactions to different tick saliva components in zebrafish showed similar results27. Additionally, as representative results, zebrafish treated with tick saliva and α-Gal using this protocol developed IgM antibodies against α-Gal that showed higher levels than in fish treated with PBS (Figure 7), as was found in previous studies27,30.
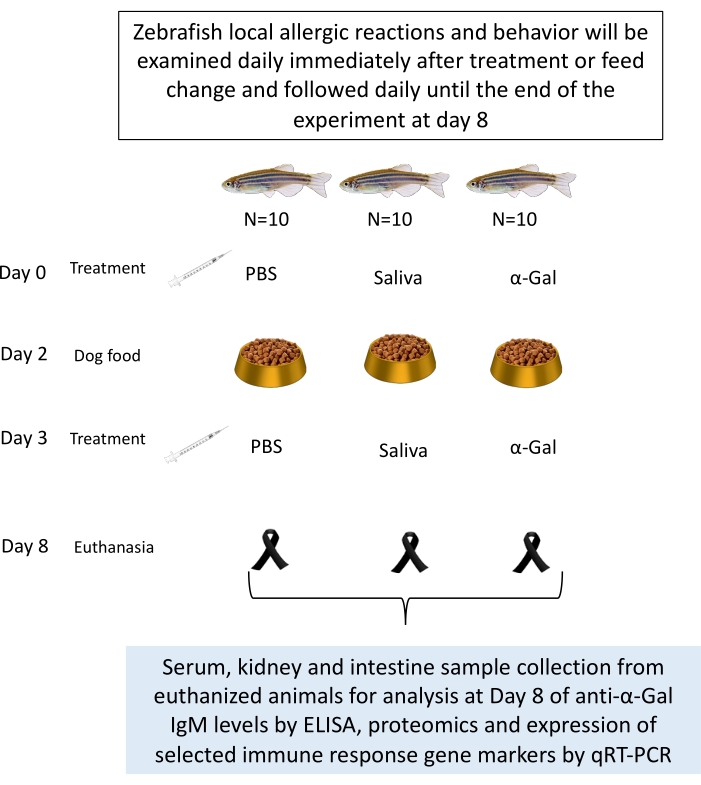
Figure 1: Experimental design for zebrafish trial. Fish are intradermally injected with α-Gal, tick saliva, and PBS as a negative control. Samples are collected after a fish dies or at the end of the experiment. Samples could be used for analysis of anti-α-Gal IgM levels and the expression of selected immune response gene markers by qRT-PCR27. Behavioral changes or allergic reactions are recorded throughout the experiment. Please click here to view a larger version of this figure.

Figure 2: Zebrafish experimental facility. The zebrafish are maintained in a flow-through water system at 27 °C with a light/dark cycle of 14 h/10 h. Please click here to view a larger version of this figure.
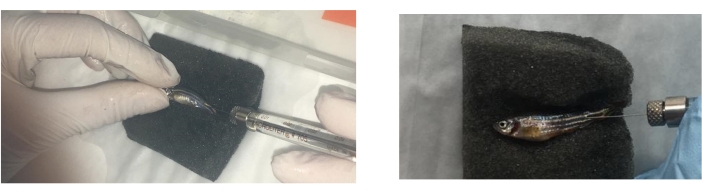
Figure 3: Zebrafish treatment injection. Zebrafish treatment injection with a 100 μL syringe fitted with a 1 cm, 29 G needle is performed intradermally at a distance of 5 mm from the caudal fin. The fish are anaesthetized and treated one by one over a sponge dipped in warm water. Please click here to view a larger version of this figure.
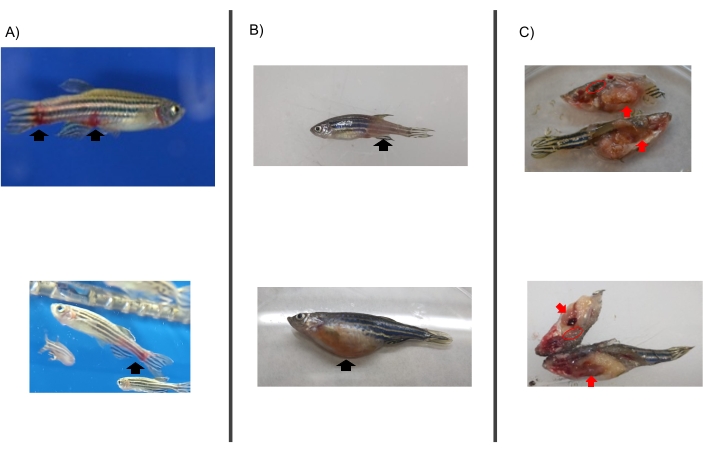
Figure 4: Evidence of anaphylactic-type hemorrhagic reactions in zebrafish injected with tick saliva and that died on day 2 prior to feeding change. (A) Fish with allergic reactions in the tank after treatment. (B) Fish dead from hemorrhagic anaphylactic reactions (allergic reaction type: discoloration and skin redness. (C) Sample collection.Red arrows indicate the intestine and red circles indicate the kidney. Please click here to view a larger version of this figure.
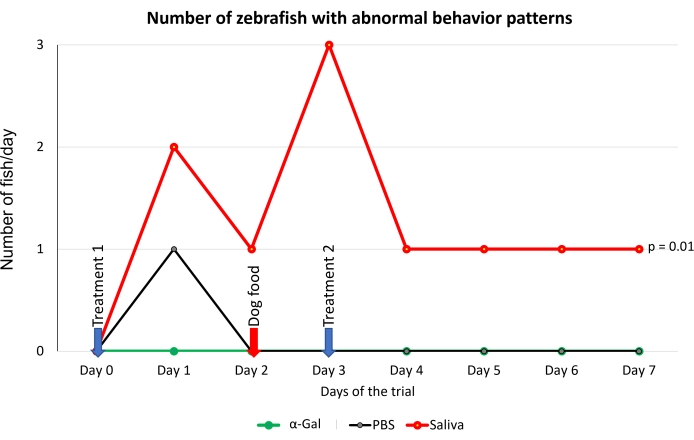
Figure 5: Behavioral pattern observed in fish. Abnormal behavior patterns consisted of slow swimming, standing stationary at the bottom of the water tank, and zigzag swimming. Blue arrows indicate the time of treatment, and the red arrow indicates the time to change from fish feed to dog feed. Fish fed with dog food were compared between saliva-treated and PBS-treated control fish by a one-way ANOVA test (p = 0.05; N = 5 fish/group). Please click here to view a larger version of this figure.
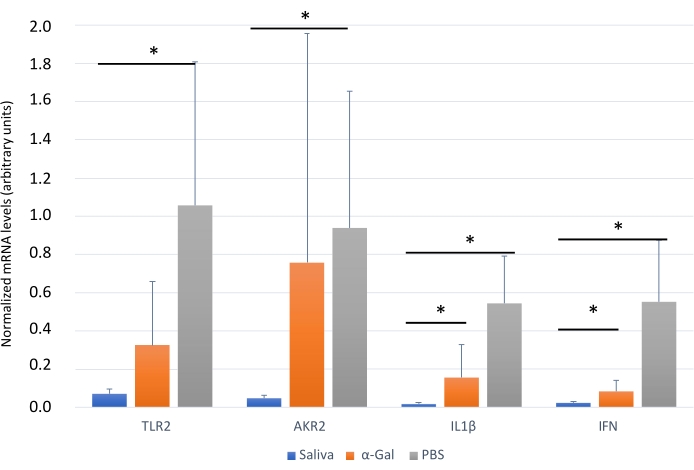
Figure 6: Expression of selected immune response markersin zebrafish kidney. Gene expression analysis by qRT-PCR in the kidney of zebrafish at the end of the experiment. The mRNA cT values are normalized against D. rerio GAPDH, presented as average ± SD, and compared between fish treated with saliva, α-Gal, and the PBS-treated control group by a Student t-test with unequal variance (*p < 0.05; N = 3-7). This figure has been adopted from27 and reproduced with permission. Please click here to view a larger version of this figure.
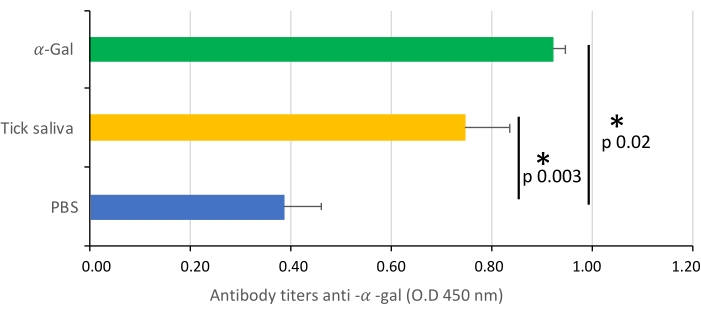
Figure 7: IgM antibody titers. The IgM antibody titers of zebrafish against α-Gal are determined by ELISA, represented as the average ± SD O.D. at 450 nm and compared between fish treated with saliva, α -Gal, and the PBS-treated control group by a Student t-test with unequal variance (*p < 0.005; N = 3-7). This figure has been adopted from27 and reproduced with permission. Please click here to view a larger version of this figure.
Table 1: Lesions and behavior patterns evaluated. Categorization of qualitative variables. The parameters that are evaluated qualitatively are injuries (on fins and scales), swimming, feeding and whether the death of the fish is caused by the test or by handling. As a subjective consideration, each variable is categorized from very mild to severe Please click here to download this Table.
Table 2: Oligonucleotide primers and annealing temperatures for qRT-PCR. This table has been adopted from30 and reproduced with permission. Please click here to download this Table.
Table 3: Representative results. Records of zebrafish allergies and deaths and expression of selected immune response markers are analyzed by qRT-PCR in the kidney and intestine of zebrafish. The mRNA cT values are normalized against D. rerio GAPDH and compared between fish treated with saliva, α -Gal, and the PBS-treated control group by a Student t-test with unequal variance (*p < 0.05; N = 3-7). This table has been adopted from27,30 and reproduced with permission. Please click here to download this Table.
Movie 1: Anesthetized fish. The anesthetized fish does not show movement or swim but continues breathing. Please click here to download this Movie.
Movie 2: Injection of the treatment in the fish. The fish are placed anesthetized on a wet sponge and injected at a 45° angle to their body with the indicated treatment. Please click here to download this Movie.
Movie 3: Serum collection from the gill-blood vessels. The fish is fixed on a paraffin plate with pins and serum is collected from the gills using a 0.5 mL syringe fitted with a 1 cm, 29 G needle. Please click here to download this Movie.
Movie 4: Intestine collection from a euthanized fish. The fish is cut sagittally using a scalpel blade and the intestine is collected with tweezers. Please click here to download this Movie.
Movie 5: Kidney collection from a euthanized fish. The swim bladder is removed, and the kidney is collected. Please click here to download this Movie.
Movie 6: Representative behavior aspects observed in treated zebrafish. One fish showed slow swimming. All fish from the same group are in the same tank, The video is an example to illustrate this behaviour, and several fish may have this behaviour at different times of the day. Please click here to download this Movie.
Movie 7: Representative behavior aspects observed in treated zebrafish. One fish stayed at the bottom of the tank. All fish from the same group are in the same tank, The videos is an example to illustrate this behaviour, and several fish may have this behaviour at different times of the day. Please click here to download this Movie.
Movie 8: Representative behavior aspects observed in treated zebrafish. One fish showed vibrating swimming. All fish from the same group are in the same tank, The videos is an example to illustrate this behaviour, and several fish may have this behaviour at different times of the day. Please click here to download this Movie.
Discussion
Zebrafish is a cost-effective and easy-to-handle model that also has been a very feasible tool for the study of molecular mechanisms of the immune response, pathogen diseases, novel drug testing, and vaccination and protection against infections33,34,35. The study on the behavior of zebrafish is useful since previous studies have found that some fish species remain motionless at the bottom of the tank when they are stressed, which affects their food consumption, eating less; in addition, zigzagging when they move could be also associated with fish stress and anxiety36,37. The information generated from studies by evaluating these parameters in zebrafish will provide a fundamental understanding of the tick-host molecular interactions and mechanisms involved in host immune response to α-Gal that may lead to the development of AGS, including allergy to mammalian meat consumption.
To avoid false positive reactions to the injected molecule, it is important to perform an intradermal injection not very deeply, parallel to the zebrafish body, and to evaluate if the fish is damaged at the time of injection. A fish with injury resulting from handling or needle penetration should not be included in the analysis. Additionally, it is highly recommended that a professional with knowledge of zebrafish evaluates changes in behavior such as swimming and feeding in order to consider behavioral changes based on their background and experience working with this model38. Another important consideration is anesthesia; an adequate dose is important for the optimal condition of the collected samples. In addition, during the injection treatment, a more pronounced stress response is avoided, which can compensate for possible difficulties related to stress diagnosis29.
The results showed that the zebrafish model could also advance the possibilities for evaluating the risks of developing AGS after a tick bite and other allergic reactions. Further, targets for the diagnosis, treatments, and prevention of these allergies can be applied to humans, since this method and the parameters that are evaluated allow more accurate characterization of allergic reactions in zebrafish.
This method could allow other salivary biogenic molecules, responsible for allergic reactions and present in tick saliva, to be evaluated. The α-Gal content in tick saliva has previously been quantified27, but it is not known what other compounds may be involved in the development of AGS. Allergic reactions were observed in groups treated with tick saliva and α-Gal but not in PBS groups (Table 3), however behavior is more affected in the tick saliva-treated group than in the α-Gal group (Figure 5). From this data, our hypothesis would be that other biomolecules in combination with alpha-Gal are involved in AGS, so further experiments should study which other molecules present in saliva have an influence on these findings. Additionally, anti-alpha-gal antibody titers were significantly higher in zebrafish treated with tick saliva and alpha-gal, which, as in previous studies26,29, showed an immune response to the alpha-gal present in tick saliva (Figure 7).
Finally, immune response markers appeared downregulated in zebrafish groups treated with tick saliva and alpha gal when compared with PBS treated group (Table 3 and Figure 6). These results are consistent with those obtained in other studies where other AGS-related biomolecules were tested27, but opposed to previous studies25 where α-Gal KO mice in response to tick bites and red meat consumption showed a IgE response and an upregulated expression of inflammatory Toll-like receptor (TLR) and IL-1 signaling pathways, which resulted in the activation of the Akr2. Therefore, further studies are needed to understand the activation pathways of these responses to tick saliva and other biomolecules in zebrafish that could be achieved by the application of this methodology.
Then, this methodology may allow screening for biomolecules that alone or in combination trigger allergic reactions and which could affect the host immune response leading to allergic diseases such as AGS and other tick-borne allergies27.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We would like to thank members of the SaBio group for their collaboration in the experimental design and technical assistance with the fish experimental facility and Juan Galcerán Sáez (IN-CSIC-UMH, Spain) for providing zebrafish. This work was supported by Ministerio de Ciencia e Innovación/Agencia Estatal de Investigación MCIN/AEI/10.13039/501100011033, Spain and EU-FEDER (Grant BIOGAL PID2020-116761GB-I00). Marinela Contreras is funded by the Ministerio de Ciencia, Innovación y Universidades, Spain, grant IJC2020-042710-I.
Materials
| 1.5 mL tube | VWR | 525-0990 | |
| All Prep DNA/RNA | Qiagen | 80284 | |
| Aquatics facilities | |||
| BCA Protein Assay Kit | Thermo Fisher Scientific | 23225 | |
| Disection set | VWR | 631-1279 | |
| Dog Food – Red Classic | Acana | ||
| ELISA plates-96 well | Thermo Fisher Scientific | 10547781 | |
| Gala1-3Gal-BSA 3 (α-Gal) | Dextra | NGP0203 | |
| iScript Reverse Transcription Supermix | Supermix | 1708840 | |
| Microliter syringes | Hamilton | 7638-01 | |
| Plate reader | any | ||
| Phosphate buffered saline | Sigma | P4417-50TAB | |
| pilocarpine hydrochloride | Sigma | P6503 | |
| Pipette tip P10 | VWR | 613-0364 | |
| Pipette tip P1000 | VWR | 613-0359 | |
| Premium food tropical fish | DAPC | ||
| Sponge Animal Holder | Made from scrap foam | ||
| Stereomicroscope | any | ||
| Thermal Cycler Real-Time PCR | any | ||
| Tricaine methanesulphonate (MS-222) | Sigma | E10521 |
References
- de la Fuente, J., Estrada-Pena, A., Venzal, J. M., Kocan, K. M., Sonenshine, D. E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Frontiers in Bioscience: A Journal and Virtual Library. 13 (18), 6938-6946 (2008).
- de la Fuente, J., et al. Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Frontiers in Cellular and Infection Microbiology. 7, 114 (2017).
- Villar, M., et al. Characterization of tick salivary gland and saliva alphagalactome reveals candidate alpha-gal syndrome disease biomarkers. Expert Review of Proteomics. 18 (12), 1099-1116 (2021).
- Chmelař, J., Kotál, J., Kovaříková, A., Kotsyfakis, M. The use of tick salivary proteins as novel therapeutics. Frontiers in Physiology. 10, 812 (2019).
- Chung, C. H., et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. The New England Journal of Medicine. 358 (11), 1109-1117 (2008).
- Van Nunen, S. A., O’Connor, K. S., Clarke, L. R., Boyle, R. X., Fernando, S. L. An association between tick bite reactions and red meat allergy in humans. The Medical Journal of Australia. 190 (9), 510-511 (2009).
- Cabezas-Cruz, A., et al. Environmental and molecular drivers of the α-Gal syndrome. Frontiers in Immunology. 10, 1210 (2019).
- de la Fuente, J., Pacheco, I., Villar, M., Cabezas-Cruz, A. The alpha-Gal syndrome: new insights into the tick-host conflict and cooperation. Parasites & Vectors. 12 (1), 154 (2019).
- Platts-Mills, T. A. E., et al. On the cause and consequences of IgE to galactose-α-1,3-galactose: A report from the National Institute of Allergy and Infectious Diseases workshop on understanding IgE-mediated mammalian meat allergy. The Journal of Allergy and Clinical Immunology. 145 (4), 1061-1071 (2020).
- Commins, S. P., et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. The Journal of Allergy and Clinical Immunology. 123 (2), 426-433 (2009).
- Platts-Mills, T. A. E., Schuyler, A. J., Tripathi, A., Commins, S. P. Anaphylaxis to the carbohydrate side chain alpha-gal. Immunology and Allergy Clinics of North America. 35 (2), 247-260 (2015).
- Mateos-Hernández, L., et al. Tick-host conflict: immunoglobulin E antibodies to tick proteins in patients with anaphylaxis to tick bite. Oncotarget. 8 (13), 20630-20644 (2017).
- Galili, U. Evolution in primates by "Catastrophic-selection" interplay between enveloped virus epidemics, mutated genes of enzymes synthesizing carbohydrate antigens, and natural anti-carbohydrate antibodies. American Journal of Physical Anthropology. 168 (2), 352-363 (2019).
- Hilger, C., Fischer, J., Wölbing, F., Biedermann, T. Role and mechanism of galactose-alpha-1,3-galactose in the elicitation of delayed anaphylactic reactions to red meat. Current Allergy and Asthma Reports. 19 (1), 3 (2019).
- Cabezas-Cruz, A., Valdés, J., de la Fuente, J. Cancer research meets tick vectors for infectious diseases. The Lancet. Infectious Diseases. 14 (10), 916-917 (2014).
- Yilmaz, B., et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 159 (6), 1277-1289 (2014).
- Cabezas-Cruz, A., et al. Regulation of the immune response to α-Gal and vector-borne diseases. Trends in Parasitology. 31 (10), 470-476 (2015).
- Weins, A. B., Eberlein, B., Biedermann, T. Diagnostics of alpha-gal syndrome: Current standards, pitfalls and perspectives. Der Hautarzt; Zeitschrift Fur Dermatologie, Venerologie, Und Verwandte Gebiete. 70 (1), 36-43 (2019).
- Commins, S. P., et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. The Journal of Allergy and Clinical Immunology. 127 (5), 1286-1293 (2011).
- Fischer, J., Yazdi, A. S., Biedermann, T. Clinical spectrum of α-Gal syndrome: from immediate-type to delayed immediate-type reactions to mammalian innards and meat. Allergo Journal International. 25 (2), 55-62 (2016).
- Hodžić, A., et al. Infection with Toxocara canis inhibits the production of IgE antibodies to α-Gal in humans: towards a conceptual framework of the hygiene hypothesis. Vaccines. 8 (2), 167 (2020).
- Kiewiet, M. B. G., et al. Clinical and serological characterization of the α-Gal syndrome-importance of atopy for symptom severity in a European cohort. The Journal of Allergy and Clinical Immunology. In Practice. 8 (6), 2027-2034 (2020).
- Steinke, J. W., Platts-Mills, T. A. E., Commins, S. P. The alpha-gal story: lessons learned from connecting the dots. The Journal of Allergy and Clinical Immunology. 135 (3), 589-596 (2015).
- Hashizume, H., et al. Repeated Amblyomma testudinarium tick bites are associated with increased galactose-α-1,3-galactose carbohydrate IgE antibody levels: A retrospective cohort study in a single institution. Journal of the American Academy of Dermatology. 78 (6), 1135-1141 (2018).
- Chandrasekhar, J. L., et al. Cutaneous exposure to clinically relevant lone star ticks promotes IgE production and hypersensitivity through CD4+ T cell- and MyD88-dependent pathways in mice. Journal of Immunology. 203 (4), 813-824 (2019).
- Araujo, R. N., et al. Amblyomma sculptum tick saliva: α-Gal identification, antibody response and possible association with red meat allergy in Brazil. International Journal for Parasitology. 46 (3), 213-220 (2016).
- Contreras, M., et al. Allergic reactions and immunity in response to tick salivary biogenic substances and red meat consumption in the zebrafish model. Frontiers in Cellular and Infection Microbiology. 10, 78 (2020).
- Poole, N. M., Mamidanna, G., Smith, R. A., Coons, L. B., Cole, J. A. Prostaglandin E(2) in tick saliva regulates macrophage cell migration and cytokine profile. Parasites & Vectors. 6 (2), 261 (2013).
- Seibel, H., Baßmann, B., Rebl, A. Blood will tell: what hematological analyses can reveal about fish welfare. Frontiers in Veterinary Science. 8, 616955 (2021).
- Pacheco, I., et al. Vaccination with alpha-gal protects against mycobacterial infection in the zebrafish model of tuberculosis. Vaccines. 8 (2), 195 (2020).
- Gupta, T., Mullins, M. C. Dissection of organs from the adult zebrafish. Journal of Visualized Experiments. (37), e1717 (2010).
- Lu, M. -. W., et al. The interferon response is involved in nervous necrosis virus acute and persistent infection in zebrafish infection model. Molecular Immunology. 45 (4), 1146-1152 (2008).
- Saralahti, A., et al. Adult zebrafish model for pneumococcal pathogenesis. Developmental and Comparative Immunology. 42 (2), 345-353 (2014).
- Gore, A. V., Pillay, L. M., Venero Galanternik, M., Weinstein, B. M. The zebrafish: A fintastic model for hematopoietic development and disease. Wiley Interdisciplinary Reviews. Developmental Biology. 7 (3), 312 (2018).
- Katoch, S., Patial, V. Zebrafish: An emerging model system to study liver diseases and related drug discovery. Journal of Applied Toxicology. 41 (1), 33-51 (2021).
- Kalueff, A. V., et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish. 10 (1), 70-86 (2013).
- Xin, N., Jiang, Y., Liu, S., Zhou, Y., Cheng, Y. Effects of prednisolone on behavior and hypothalamic-pituitary-interrenal axis activity in zebrafish. Environmental Toxicology and Pharmacology. 75, 103325 (2020).
- Aleström, P., et al. Zebrafish: Housing and husbandry recommendations. Laboratory Animals. 54 (3), 213-224 (2020).

