Assessment of Ex Vivo Murine Biventricular Function in a Langendorff Model
Summary
Presented here is a protocol to reliably quantify the right and left ventricular function of donor hearts after cold preservation using an ex vivo perfusion system.
Abstract
Primary graft dysfunction (PGD) remains the leading cause of early death following heart transplantation. Prolonged ischemic time during cold preservation is an important risk factor for PGD, and reliable evaluation of cardiac function is essential to study the functional responses of the donor heart after cold preservation. The accompanying video describes a technique to assess murine right and left ventricular function using ex vivo perfusion based in a Langendorff model after cold preservation for different durations. In brief, the heart is isolated and stored in a cold histidine-tryptophan-ketoglutarate (HTK) solution. Then, the heart is perfused with a Kreb buffer in a Langendorff model for 60 min. A silicone balloon is inserted into the left and right ventricle, and cardiac functional parameters are recorded (dP/dt, pressure-volume relationships). This protocol allows the reliable evaluation of cardiac function after different heart preservation protocols. Importantly, this technique allows the study of cardiac preservation responses specifically in native cardiac cells. The use of very small murine hearts allows access to an enormous array of transgenic mice to investigate the mechanisms of PGD.
Introduction
Heart transplantation improves survival and the quality of life in patients with end-stage heart failure1. Unfortunately, the shortage of heart donors limits the number of patients who could benefit from this therapy and limits the ability of clinicians to optimally match donors with recipients2,3,4. Furthermore, the new allocation system has contributed to longer ischemic times and significantly increased the use of marginal donors since 20185. Consequently, the mean age of heart donors and the ischemic time is increasing over time, leading to a higher rate of primary graft dysfunction (PGD) despite significant improvements in the strategies for heart preservation 6.
PGD can affect the left, the right, or both ventricles, and remains a life-threatening complication that represents the leading cause of early deaths after heart transplantation. Investigating the mechanisms of PDG and the development of strategies for better heart preservation are important considerations, given the potential life-saving impact on heart recipients. Therefore, experimental models that allow a robust and reliable assessment of donor heart function after a prolonged storage time are essential to increase our understanding of PGD and facilitate the development of novel therapies. The ability to accurately assess cardiac function in the mouse heart allows access to a vast repertoire of transgenic murine models that can accurately identify PGD mechanisms.
In physiologic and pharmacologic studies, the Langendorff retrograde perfusion model is widely used to assess heart function7. Specifically, cardiac performance is detected by a silicone balloon connected to a pressure transducer within the left ventricular (LV) cavity. A key feature of PGD is the inadequate contraction and relaxation of the ventricular muscle. Prior Langendorff studies have focused on using an LV balloon to produce reliable and reproducible results in LV functional assessment8,9,10. However, the use of an intracavitary balloon to assess right ventricular (RV) function using the balloon system is less well recognized.
Given a significant PGD rate involving the RV after transplantation11, experimental methods to study both LV and RV function would help determine the molecular and physiological mechanisms that contribute to RV PGD. This protocol shows that intracavitary silicone balloons can provide reliable assessments of LV and RV function in the same murine heart12. To evaluate the potential use of the Langendorff system in the PGD study, we examined the heart functions with different periods of storage and found decreased cardiac function in contraction and relaxation with the prolonged cold storage of murine hearts. Interestingly, the LV has a higher functional reduction than the RV. In summary, the protocol described here can be used for assessing the effect of a candidate drug and molecular pathways on both LV and RV function. The ability to use this method on murine hearts will facilitate the performance of detailed mechanistic studies.
Protocol
All animal experiments in this protocol were approved by the Institutional Animal Care and Use Committee at the University of Michigan, Ann Arbor. All mice were housed at a 12:12 light cycle in pathogen-free rooms. See the Table of Materials for details related to all materials, animals, and equipment used in this protocol.
1. Construction of the silicone balloon catheter
NOTE: The silicone balloon is made as described previously13.
- Add 9.5 mL of distilled water, 14.2 mL of light corn syrup, and 33.8 g of sucrose to a 100 mL beaker. Heat and stir the solution until the sugar is completely dissolved.
- Prepare the dough by mixing 10 g of wheat flour and 5 g of water until an even consistency is achieved and allow it to rest for 10 min.
- Shape a small piece of dough into an oval-the "head"-and attach it to the end of a dry spaghetti strand. Then, dip this head into the sugar solution and slowly remove from the solution, as the head is now completely coated.
NOTE: The dough should be smooth and of even texture. The sizes of dough can be varied to generate different sizes of balloons, from 5 mm (short diameter) to 7 mm (long diameter). Try to cover them with a thin film of sugar solution. - Suspend the spaghetti strand on a polystyrene foam block, or other holders, to form a glossy cover evenly over the head and dry overnight.
- Dip the mold into silicone dispersion (silicone elastomer dispersed in xylene). Place the spaghetti strand back into the polystyrene foam block at 37 °C for 2 h or until dry. Repeat this step once.
NOTE: It is essential to prevent the silicone dispersion gel from oxidizing due through air exposure, as this will generate an uneven balloon thickness. - Place the mold into the water to separate and collect the balloon. Store the balloon in 0.02% sodium azide.
- Cut a two-blunt-end tip from a 22 G needle; mount one blunt end to the silicone balloon and another blunt end to PE tubing. Use 4-0 silk to tie the balloon in place on the needle.
NOTE: Test the balloon integrity by injecting water into it. Once the balloon is filled, press the balloon softly to test if the balloon maintains tension inside. Use a new balloon if it is leaking. The mounted balloons can be stored for future use.
2. Preparation of the heart perfusion system
- Make 1 L of Krebs-Henseleit (KH) perfusion buffer and transfer it to the water reservoir Langendorff system.
- Connect the air tube to the water reservoir and turn on the airflow to balance the KH buffer with 5% CO2 and 95% O2 for at least 30 min.
- Set the water bath at 41.5 °C and circulate the water in the outer layer of the Langendorff system to warm up the system and KH buffer.
NOTE: The water bath temperature requires optimization for each system. For this system, the water bath temperature will maintain the KH at 37-37.5 °C when perfusing into the heart.
3. Isolation, mounting, and cannulating of the mouse heart
- For anticoagulation, adminster 200 units of heparin in Saline by intraperitoneal (i.p.) injection into the right-hand quadrant of C57/B6 mouse abdomen. Aspirate the syringe before injection to confirm the bevel of the needles is not in the bladder or lumen of the GI tract. Use at least four mice in each experimental condition (but also consider the size of the treatment effect).
- After 30 min, administer 80 mg/kg ketamine and 10 mg/kg of xylazine i.p. to anesthetize the mouse. Check if the anesthetized mouse is unconscious by performing a toe pinch and ensuring no response is observed. Acepromazine 2mg/kg can be added to the ket/xyl cocktail if the used strain of mice does not reach an adequate level of anesthesia with ket/xyl only.
- Make an incision right below the sternum. Use scissors to open the chest by cutting the diaphragm and the ribs. Fold the anterior chest wall to fully expose the chest. Cut at the descending aorta (closed to the aorta arch). Transfer the heart, lungs, and thymus of the mouse to cold histidine-tryptophan-ketoglutarate (HTK) buffer. Isolate the organs under ice-cold HTK buffer. Expose the aorta by removing any connective tissues.
NOTE: Maximize the aorta length by including both the ascending aorta and aortic arch region in the excision to allow enough space to connect to a needle.
- Connect the end of the aorta to a 22 G needle, and tie with a 6-0 silk suture. Ensure the cannula is above the aortic root so as not to interfere with the aortic valve. Perfuse the aorta with 10 mL of cold (4 °C) HTK buffer over approximately 10 min.
NOTE: It takes less than 15 min from heart removal to cannulation of the aorta; However, it is important to keep the perfusion speed at the appropriate level. Injections that are too fast and vigorous can generate high pressures and cause vascular/cardiac damage. - Store the heart in a 50 mL tube with ice-cold HTK for 8 h or immediately perform the perfusion (do not store the control) and avoid direct contact with ice.
NOTE: Direct contact of the heart tissue with ice may lead to cold injury. - Connect the needle-mounted heart to the cannula in the Langendorff apparatus and tie it with a silk suture.
NOTE: To standardize the procedure, wait for a total of 3 min for the cannulation process before perfusion. - Start the perfusion with a constant flow mode at 3 mL/min; then, change to constant pressure mode at 70-80 mmHg and adjust the heart to ~6 mL/min.
NOTE: Palpating the heart softly can help accelerate cardiac reanimation. If the perfusion flow rate is much higher than 6 mL/min at constant pressure mode, there could be a leak in the cannulation or the aorta valve may not be working properly. Adjust the connections to fix the leak. The constant flow mode overrides the vascular tone self-regulation of the heart. The constant pressure mode allows the heart to regulate its coronary perfusion flow. Therefore, the constant pressure mode will precisely measure the cardiac function and preservation quality of the heart. - Connect a deflated, water-filled balloon to a pressure transducer and a water-filled syringe with a three-way tap. After a 15-20 min equilibration period, cut the right atrium (RA) and insert the balloon to the RV through the RA. Use tape to hold the balloon inside the RV. Minimize the open area of the RA to help constrain the balloon in the ventricle (see Figure 1 for the setup).
NOTE: A period of equilibration is necessary, as the cardiac contraction and relaxation are not stable at the beginning, and the measure is less accurate and representative. If the AV node is damaged during the opening of the RA, the heart will display frequent arrhythmias. - After 20 min of RV functional data collection, cut the left atrium (LA) and insert a deflated water-filled balloon to the LV through the LA. Use tape to hold the balloon inside the LV.
NOTE: The heart should maintain stable hemodynamics for more than 1.5 h.
4. Functional data recording
- Calibration of the pressure transducer
- Fill a 10 mL syringe with warm saline and connect the syringe to the dome through a three-way tap. Open the tap and slowly fill the dome with saline, and then close all taps and remove the syringe. Attach the filled dome to the transducer; connect the pressure gauge to the third end of the three-way tap.
- In the recording software, select the Bridge Amp from the dropdown menu of the channel connecting to the transducer. Rename the channel as Perfused pressure. Click Zero to zero the transducer.
- Start the recording by clicking Start so that the transducer is now reading 0 mmHg. After several seconds of recording, slowly push the syringe and increase the pressure à 100. Click stop to stop recording.
- In the Units conversion dialog, select an area of recording for 0 mmHg and click the arrow à Point 1 and type 0 mmHg. Select area of recording for 100 mmHg, click the arrow à Point 2, and type 100 mmHg. Click OK to calibrate the transducer.
- Rename the channel corresponding to the pressure transducer with the balloon as Ventricle pressure. Start the recording when the heart is connected to the system. After inserting the balloon into the ventricle, adjust the water volume in the balloon using a micrometer syringe through the three-way tap to maintain the end-diastolic pressure at 5-10 mmHg.
NOTE: The end-diastolic measure could decrease during the measurement, preferably starting at close to 10 mmHg. - Rename an empty channel as dP/dt. From the dropdown menu, select Derivative | source channel as Ventricle Pressure. The channel will record the ratio of pressure change in the ventricular cavity during the contraction period.
- Select a stable period of measure, then click Setting on the Blood pressure module.
- Select the Ventricle pressure as the input channel and click selection for a period of calculation | OK.
- Click Classifier view to remove the outlier cardiac cycle (e.g., abnormal cycle time or pressure).
- Click table view to generate the tables of the average of max dP/dt (contraction) and min dP/dt (relaxation) for the selected period.
NOTE: Store the recording file for each sample and save the table of average heart function for statistical analysis.
Representative Results
Adult C57Bl/6 mouse hearts, 3 months of age, were harvested and mounted to the Langendorff system. The donor heart was stored in HTK for 0 and 8 h, and then perfused with oxygenated KH buffer. A silicone balloon connecting to a pressure transducer was used to measure the contraction and relaxation of LV and RV function.
The aortic pressures were maintained in the 70-80 mmHg range. The heart rate was comparable in mouse hearts with 0 and 8 h of storage. The LV and RV function were examined by measuring systolic and diastolic pressure. dP/dt, a derivative to calculate the ratio of pressure change, was calculated to determine the pressure dynamics. The absolute number of max dP/dt and min dP/dt could represent the level of muscular contraction and relaxation. At 0 h of storage, the LV had higher systolic pressure compared to the RV (Figure 2C and Figure 3A). The LV showed more muscular contraction and relaxation than the RV after perfusion of 0 h storage (Figure 2C and Figure 3B,C). However, after 8 h of cold storage, both the LV and RV showed a significant functional reduction compared to a 0 h baseline (Figure 2A–D and Figure 3B,C). The decreases in cardiac contraction were more severe in the LV. After 8 h of storage, the contraction and relaxation of the LV was 25.1% and 30.7% of the 0 h baseline, while the RV had 32.5% and 29.1% of function compared to the 0 h baseline (Figure 3B,C). These results showed that the PGD of the LV after prolonged storage had a more significant cardiac contraction reduction than the RV.
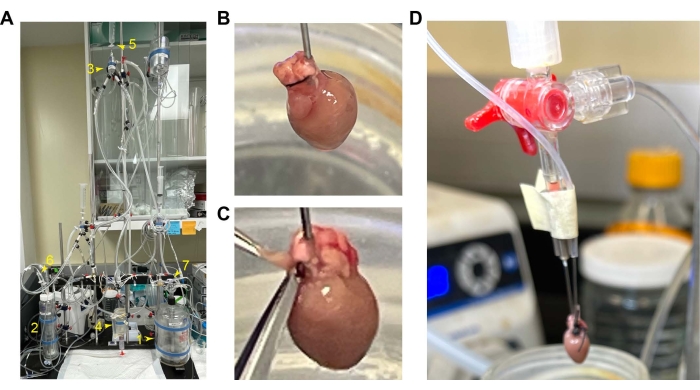
Figure 1: Mounting and cannulation of the mouse heart. (A) Overall setup of the perfusion setup. 1. Perfusion reservoir. 2. Oxygenation chamber. 3. Air trap chamber. 4. Heart chamber. 5. Value switch for constant flow and pressure. 6 and 7. Oxygen inflow. (B) Cannulated hearts with the RV in the front. (C) Position of the RV to cut for opening its cavity. (D) Tap the balloon tube with the cannula. Abbreviation: RV = right ventricle. Please click here to view a larger version of this figure.
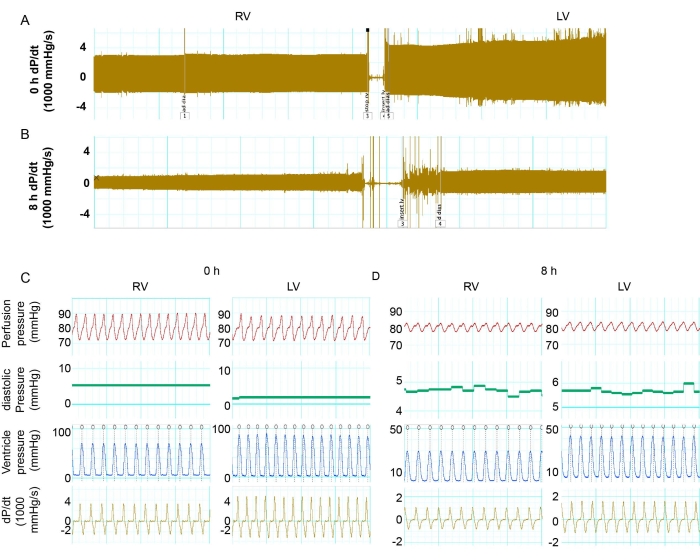
Figure 2: Comparing the function of the LV versus RV. (A) Tracing record of max and min dP/dt in the RV and LV in the donor heart with 0 h of storage. (B) The record of max and min dP/dt in the RV and LV in the donor heart with 8 h of storage. (C,D) Details of dP/dt, LV pressure, heart rate, and perfusion pressure in the LV and RV at 0 h and 8 h. Abbreviations: RV = right ventricle; LV = left ventricle; dP/dt = pressure-time relationship. Please click here to view a larger version of this figure.
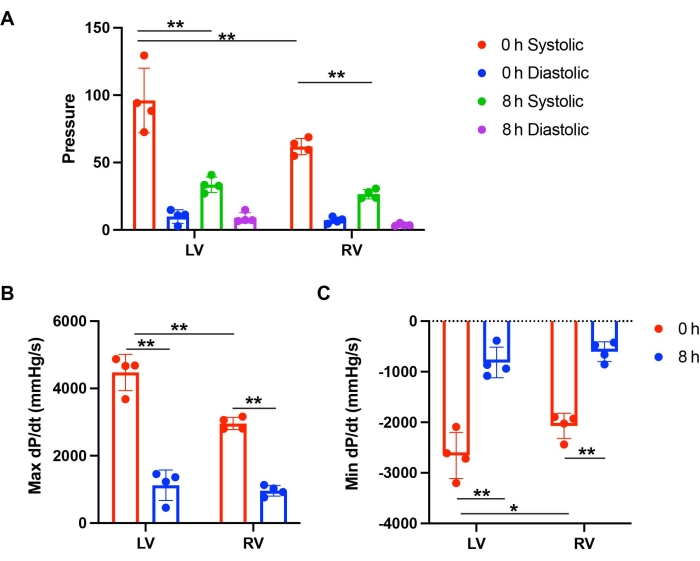
Figure 3: Comparing the function of the LV versus RV after storage and perfusion. (A) Systolic and diastolic pressure of the LV and RV after 0 h and 8 h of storage. (B) Max dP/dt and (C) Min dP/dt of the LV and RV after perfusion with 0 h and 8 h of storage. This figure is from Lei et al.12. Please click here to view a larger version of this figure.
Discussion
This protocol describes the retrograde perfusion Langendorff method via aortic cannulation. This technique can be used to evaluate the LV and RV function of murine hearts after cold storage. The results show that the prolonged cold storage of donor hearts leads to reduced cardiac function in both the LV and RV using this protocol.
The studies of acute and chronic rejection after heart transplantation widely focus on immunobiology14. The effects of native cells on PGD during cold storage are less well examined. PGD occurs in ~10%-20% of heart transplants and accounts for 66% of early death within 30 days following transplantation. In particular, the incidence of PGD affecting the LV versus the RV differ after transplantation11. Without the contribution of recipient cellular responses, this ex vivo method focuses on the contributions of native cardiac cells to PGD after cold preservation of donor hearts. Further studies may incorporate recipient responses in a murine heart transplant model.
In this protocol, the Langendorff perfusion of cold preserved donor hearts focused on the native cardiac responses to warm crystalloid perfusion without infiltrating cellular immunity. To achieve reproducible results, several critical steps were standardized. The mouse hearts were arrested using HTK solution and stored in ice-cold HTK, similar to clinical practice. The perfusion volume and infusion time of the HTK solution for every heart was closely monitored with a timer. The donor heart was kept in prechilled tubes on ice containing HTK in a 4 °C room. The cannulation time waas standardized to ~3 min prior to perfusion. All these steps ensured that cold preservation duration was the major variable in the study.
A period of irregular cardiac contractility for ~20 min was commonly seen at the beginning of perfusion. This equilibration and recovery period was facilitated by gradual warming and oxygenation of cardiac tissues. A relatively stable period was expected after the initial 20 min. The balloon was inserted into the ventricle cavity at ~18 min after the initial equilibration period. We started recording hemodynamics after the heart was stable for ~25 min, once the balloon was inserted. Perfusion with KH buffer maintained stable cardiac performance for ~1.5-2 h. We therefore elected to record hemodynamics for 20 min in each of the left and right ventricles.
There are several limitations of retrograde perfusion for studying the PGD of hearts after cold storage. First, due to the balloon size and a lack of space in each ventricular cavity (in particular, the RV), the simultaneous insertion of two balloons into both the LV and RV is very challenging. Thus, we measure the function of RV and LV sequentially. It is important to note that the interventricular septum contributes significantly to both left and right ventricular function. The septum contributes to ~50% of right ventricular function, so there is interventricular dependence15. It is also important to note that, while the procedures for reperfusion of the murine heart in the Langendorff device take ~3 min, surgical implantation of the human heart in the relatively warm surgical field takes ~45 min. In comparison, the murine heart in this Langendorff system incurs less ischemic time. This should be taken into account when considering clinical translation.
Since we used KH buffer to perfuse the heart without blood, this may also have less efficiency in oxygen delivery. However, the heart function is relatively stable through the initial 1.5-2 h of perfusion, thus allowing reliable hemodynamic measurements. Unfortunately, there are currently no viable working heart perfusion models for these smaller murine hearts, and the effect of ventricular loading cannot be evaluated in this system. Despite this, the perfusion system is highly reproducible and less labor-intensive and time-consuming than transplant models. It is also less costly than transplant studies, which may make it more suitable for screening different therapeutic options and various molecular pathways. With modifications to preservation solutions by adding candidate drugs, this platform can be used to evaluate the effects of pharmacological agents on reducing PGD in both the LV and RV.
Divulgations
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| 4-0 silk suture | Braintree Scientific | SUTS108 | |
| 6-0 Silk suture | Braintree Scientific | SUTS104 | |
| All purpose flour | Kroger | ||
| BD General Use and PrecisionGlide Hypodermic Needles 22 G | Fisher scientific | 14-826-5A | |
| BD Syringe with Luer-Lok Tips (Without Needle) | Fisher scientific | 14-823-16E | |
| Corn Syrup | Kroger | ||
| Custodiol HTK Solution | Essential Pharmaceuticals LLC | ||
| Dissecting Scissors | World Precision Instruments | 14393/14394 | |
| Falcon 50 mL conical tubes | Fisher scientific | 14-959-49A | |
| Heparin sodium salt from porcine intestinal mucosa | Sigma | H4784 | |
| Krebs Henseleit buffer | Sigma | K3753 | |
| Nusil silicone dispersions | Avantor | ||
| Perfusion system | Radnoti | 130101BEZ | |
| PowerLab | ADInstruments | PL3508 | |
| Sodium azide | Sigma | S2002 | |
| Sodium bicarbonate | Sigma | S5761 | |
| Sucrose | Sigma | S0389 | |
| Sucrose | Sigma | S0389 | |
| Xylazine | Sigma | X1126 |
References
- Kim, I. C., Youn, J. C., Kobashigawa, J. A. The past, present and future of heart transplantation. Korean Circulation Journal. 48 (7), 565-590 (2018).
- Gaffey, A. C., et al. Transplantation of "high-risk" donor hearts: Implications for infection. The Journal of Thoracic and Cardiovascular Surgery. 152 (1), 213-220 (2016).
- Hsich, E. M. Matching the market for heart transplantation. Circulation: Heart Failure. 9 (4), 002679 (2016).
- Piperata, A., et al. Heart transplantation in the new era of extended donor criteria. Journal of Cardiac Surgery. 36 (12), 4828-4829 (2021).
- Huckaby, L. V., Hickey, G., Sultan, I., Kilic, A. Trends in the utilization of marginal donors for orthotopic heart transplantation. Journal of Cardiac Surgery. 36 (4), 1270-1276 (2021).
- Singh, S. S. A., Dalzell, J. R., Berry, C., Al-Attar, N. Primary graft dysfunction after heart transplantation: a thorn amongst the roses. Heart Failure Reviews. 24 (5), 805-820 (2019).
- Bell, R. M., Mocanu, M. M., Yellon, D. M. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. Journal of Molecular and Cellular Cardiology. 50 (6), 940-950 (2011).
- Rossello, X., Hall, A. R., Bell, R. M., Yellon, D. M. Characterization of the Langendorff perfused isolated mouse heart model of global ischemia-reperfusion injury: impact of ischemia and reperfusion length on infarct size and LDH release. Journal of Cardiovascular Pharmacology and Therapeutics. 21 (3), 286-295 (2016).
- Matsuura, H., et al. Positive inotropic effects of ATP released via the maxi-anion channel in Langendorff-perfused mouse hearts subjected to ischemia-reperfusion. Frontiers in Cell Development Biology. 9, 597997 (2021).
- Tse, G., Hothi, S. S., Grace, A. A., Huang, C. L. Ventricular arrhythmogenesis following slowed conduction in heptanol-treated, Langendorff-perfused mouse hearts. The Journal of Physiological Sciences. 62 (2), 79-92 (2012).
- Kobashigawa, J., et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. The Journal of Heart and Lung Transplantation. 33 (4), 327-340 (2014).
- Lei, I., et al. Differential inflammatory responses of the native left and right ventricle associated with donor heart preservation. Physiological Reports. 9 (17), 15004 (2021).
- Miller, A., Wright, G. L. Fabrication of murine ventricular balloons for the Langendorff heart preparation. Journal of Biotechnology & Biomaterials. 1 (101), (2011).
- Madsen, J. C. Advances in the immunology of heart transplantation. The Journal of Heart and Lung Transplantation. 36 (12), 1299-1305 (2017).
- Voelkel, N. F., et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 114 (17), 1883-1891 (2006).

