A Chronic High-Intensity Interval Training and Diet-Induced Obesity Model to Maximize Exercise Effort and Induce Physiologic Changes in Rats
Summary
This paper presents the morphometric responses and training performance outcomes of a high-intensity interval training (HIIT) protocol in a Sprague-Dawley rat model of diet-induced obesity. The purpose of this protocol was to maximize exercise intensity and determine the physiologic responses to HIIT in lean and obese rats.
Abstract
Compared to continuous-moderate or low-intensity training, high-intensity interval training (HIIT) is a more time-efficient alternative method that results in similar physiologic benefits. This paper presents a HIIT protocol that can be used to assess various health markers in a Sprague-Dawley rat model of diet-induced obesity. Female Sprague Dawley rats aged 21 days old were randomly assigned to the following groups: control (CON, n = 10), exercise-trained (TRN, n = 10), high-fat diet (HFD, n = 10), and high-fat diet/exercise training (HFD/TRN, n = 10). The control diets consisted of commercial laboratory chow with 10% kilocalories (kcal) from fat (3.82 kcal/g), and the high-fat diets (HFD) consisted of 45% kcal from fat (4.7 kcal/g). The animals had ad libitum access to their assigned diet throughout the study. After an 8 week diet induction period, the exercise cohorts completed four HIIT sessions per week for 8 weeks. Each HIIT session consisted of 10 intervals of 1 min sprints/2 min rest using a rodent treadmill with a motor-driven belt. After the 8 weeks of training, the animals were sacrificed for tissue collection. The results revealed no differences in the distance run between the TRN and HFD/TRN groups, and the training speed steadily increased over the duration of the study, with a final running speed of 115 cm/s and 111 cm/s for the TRN and HFD/TRN groups, respectively. The weekly caloric intake was decreased (p < 0.05) in the TRN group relative to the CON group but increased (p < 0.05) in the HFD/TRN group relative to the HFD group. Lastly, the animals on the HFD had greater (p < 0.05) adiposity, and the trained animals had reduced (p < 0.05) adiposity relative to controls. This protocol demonstrates an efficient method to evaluate the effects of HIIT on various physiologic outcomes in a diet-induced obesity model.
Introduction
Obesity and comorbid conditions, such as cardiovascular diseases, metabolic diseases, and cancer, continue to be some of the most serious, costly, and preventable of all health outcomes. Currently, over one-third of adults in the United States and more than 1.6 billion adults worldwide are classified as obese according to their body mass index (BMI; defined as weight in kilograms divided by the square of height in meters)1. Obesity as a disease results from a genetic predisposition, environmental exposures, and a breakdown in the normal mechanisms regulating energy intake and energy expenditure2. As the human and financial costs of the obesity epidemic continue to rise, there has been an intensified focus on trying to understand the mechanisms involved in energy balance and the effects of diet and exercise in combating metabolic disease.
Previous studies have demonstrated that exposure to highly palatable, energy-dense diets stimulates overeating in rat models3. Ad libitum access to highly palatable diets drives excessive weight gain as a result of increased caloric intake4. Studies have also shown that exercise can modulate appetite and improve the sensitivity of satiety signaling in obese individuals5. It is theorized that this recovery of the sensitivity of satiety signaling with exercise is partially mediated through the impact of exercise training on the reactivity of the central and peripheral tissues to leptin, a key adipocyte-derived regulatory hormone that suppresses appetite and stimulates energy expenditure5. While these studies have investigated a variety of exercise protocols, there is no clear consensus on which intervention is superior6,7. There is some evidence to suggest that high-intensity interval training (HIIT), which involves repeated bursts of strenuous exercise interwoven with intervals of recovery, may improve appetite regulation more than other forms of exercise, such as moderate-intensity continuous exercise training (MICT), vigorous-intensity continuous training, or voluntary physical activity8. However, there are gaps in knowledge surrounding the intersectionality of high-intensity interval training, diet, and appetite regulation.
Previous studies have also demonstrated that exercise is a powerful mediator of inactivity-related comorbidities, particularly from the perspective of alterations in muscle and adipose tissue9,10,11. It is hypothesized that these compositional changes lead to the promotion of an anti-inflammatory state that may be responsible for the amelioration of disease risk seen with exercise12. Myokines, which are cytokines, other small proteins, and proteoglycan peptides released from skeletal muscle during muscular contractions have been posited as moderating the anti-inflammatory outcomes associated with physical activity. In contrast, adipokines, cell-signaling molecules produced by adipose tissue, have been shown to primarily play a more deleterious role and contribute to the promotion of an inflammatory state13,14,15,16. While there is significant evidence demonstrating that the compositional alterations seen with MICT promote positive health outcomes, less work has been done to evaluate the potential benefits of HIIT17,18.
Finally, cardiovascular disease is well-established as the leading cause of morbidity in humans and is highly correlated with obesity, diet, and physical activity1. This protocol provides an efficient way in which to train rodents for the evaluation of the effects of cardiovascular training on numerous systems. In particular, cardiac hypertrophy is a marked adaptation that occurs with cardiovascular exercise. This hypertrophy allows for more robust cardiac contractions and delivery of blood and oxygen to the exercising tissues. Previous research suggests that high-intensity exercise is more likely to induce cardiac hypertrophy than moderate-intensity exercise19.
This protocol helps fill the gaps in the literature by providing an approach for examining the effects of HIIT on appetite regulation, compositional changes (thus, myokine and adipokine changes), and cardiovascular adaptations in a murine model of diet-induced obesity. Further, the performance-based increases in intensity maximize the training outcomes and ensure that animals are not adapting to the exercise training and approaching a moderate intensity later in the training protocol.
The overall goal of this method is to maximize the exercise effort and identify phenotypic changes in Sprague-Dawley rats in response to HIIT, diet-induced obesity, and the interaction of these stimuli. This protocol is unique compared to other techniques due to its ability to maximize the effort throughout the training period, even with increases in the skill and fitness levels of the rats. It also allows for the simultaneous analysis of exercise and obesity, rather than solely focusing on one or the other. Specifically, this study intended to test the following hypotheses. (1) Exercise speeds may increase throughout the training, and the distance covered by rats in the TRN group may be larger than in the HFD/TRN group20. (2) The average weekly caloric intake of the trained rats may be greater than controls, and this may be evident within each diet cohort21. (3) The average daily gain in mass may be greater in control rats than exercised rats, and control rats may have higher fat mass at sacrifice21. (4) The mass of the heart and liver may be larger in the HFD/TRN rats versus the TRN rats19.
Protocol
All procedures described in the present study followed the Guide for the Care and Use of Laboratory Animals, 8th Edition. The experimental design was approved by the Office of Research and Sponsored Programs (ORSP) under the Institutional Animal Care and Use Committee (IACUC) 2019-5 at the West Virginia School of Osteopathic Medicine. Refer to the Table of Materials and Table 1 for additional details about all the materials used in this protocol. A general outline of the protocol timeline is displayed in Figure 1.
1. Experimental design
- Use 40 female, 21 day old Sprague-Dawley rats from a commercial source (see Table of Materials).
- Use proper protective equipment when handling the animals in accordance with the IACUC guidelines. These safety measures include, but are not limited to, wearing single-use sterile gloves, a lab coat, shoe covers, etc.
- Weigh each animal, and calculate the mean and standard error of the mean to ensure that the groups do not differ in weight. If the groups differ, match the groups for body weight by redistributing the heavier individuals into the lighter groups and the lighter individuals into the heavier groups.
- Randomly divide the animals into four groups: control (CON, n = 10), control diet/exercise-trained (TRN, n = 10), high-fat diet/control (HFD, n = 10), and high-fat diet/exercise-trained (HFD/TRN, n = 10).
- House the rats in individual cages (one animal per cage) in a controlled environment (12 h light/dark cycles, 21 °C ± 2 °C, 60% ± 10% humidity), and wean all the rats to a control diet of commercially bought laboratory chow (see Table of Materials) for a 1 week acclimatization period. Supply each cage with enrichment devices (shelter, gnawables, and nesting material).
NOTE: The CON diet consists of commercially bought laboratory chow (see Table of Materials and Table 1 for additional details) with 10% kcal from fat (3.82 kcal/g). - Allow for ad libitum access to food and water throughout the experiment.
- Following the 1 week acclimatization period, begin the 8 week diet period by supplying the HFD and HFD/TRN groups with HFD chow. The HFD chow (see Table of Materials and Table 1 for additional details) consists of 45% kcal from fat (4.7 kcal/g), representing the macronutrient breakdown found in a typical Western diet. Ensure all animals continue to have ad libitum access to food and water.
- At the beginning of each week, weigh out and record the mass of chow given to each animal. Use 140 g of chow to feed each animal for a full week.
- To weigh the chow, place a weigh boat on a precision electronic digital scale (see Table of Materials), and tare the scale by pressing the "tare" button. Place 140 g of chow in the weigh boat, and record the weight (g) from the scale. This is the "before" weight.
- Place the chow into the feed trough in each individual animal's housing cage.
- If an animal starts to run low on chow, weigh an additional allotment (20 g for each remaining day), and add that chow to the food tray. Record how much additional chow is given to each animal. Weight may need to be added on top of the food in the hopper to allow greater ease of consumption if the animals are struggling to consume the pellets (as evidenced by rounded pellets in the hopper).
- At the end of each week, weigh the remaining chow for each animal. Every animal should have leftover chow to ensure they were able to eat ad libitum. Using the same scale, record the food remaining. This is the "after" weight.
- Subtract the "after" weight from the "before weight" for each individual animal to record the food intake (g) per week.
- Following the 8 weeks diet induction period, begin the HIIT training protocol for rats in TRN and HFD/TRN. This consists of an 8 week HIIT regimen with training sessions every week on Monday, Tuesday, Thursday, and Friday (see "HIIT Training Protocol" below) between 08:00 A.M. and 10:00 A.M. Ensure all animals have ad libitum access to their assigned experimental diets throughout the protocol.
NOTE: There is no standardization of the protocol between the groups, as this protocol is designed to maximize the performance of each cohort, and each cohort may differ (due to phenotypes induced by diet). - Euthanize the rats 48 h after their last exercise session via vital tissue harvest following anesthesia induction using inhaled isoflurane (5%).
- Begin by making sure there is adequate oxygen and isoflurane in the system to induce anesthesia. Open the oxygen tank by turning the main valve (typically on top of the tank) counterclockwise. There may or may not be a regulator valve that needs to be opened on the oxygen tank as well, depending on the size of the oxygen tank. Additionally, check that the exhaust tubing is properly secured and that the collection canister is not overweight.
- Weigh the canister before use, and note the date and weight on the side of the canister. Check to make sure the stopcock is open to the induction chamber and the stopcock to the nose cone is closed.
- To induce anesthesia, place the animal in the induction chamber, and seal the chamber by securing the lock devices. Set the isoflurane to 5% by depressing the safety lock and turning the dial counterclockwise.
- Next, turn the dial at the base of the oxygen flowmeter counterclockwise until the meter reads between 1.5-2 L/min.
- After 1-2 min, when the animal is no longer conscious, turn off the isoflurane by rotating the dial clockwise while depressing the safety lock. Flush the induction chamber with oxygen by pressing the oxygen release valve for 3-5 s. Unlock the induction chamber, and remove the unconscious animal.
- Place the unconscious animal on its back, and secure a nose cone to deliver further anesthesia. Open the stopcock for facemask delivery, and close the stopcock for the induction chamber. Deliver 5% isoflurane with 100% oxygen for anesthesia via the facemask until pedal reflexes are absent.
- Check the pedal reflexes by applying a pinching pressure to the toes of the anesthetized animal and looking for a reflex response.
- Sacrifice the animal according to IACUC-approved methods (which may vary by study), and carefully dissect out the target tissues for measurement and further analysis (subcutaneous adipose tissue, perirenal adipose tissue, skeletal muscle, liver, gonads, and heart). Depending on the IACUC protocols, euthanasia can be completed by decapitation with a guillotine or by vital tissue (heart) harvest.
- To collect the heart, make an incision below the ribs and through the diaphragm.
- Locate the heart, and cut the vasculature (aorta, vena cava, pulmonary artery, pulmonary vein) with surgical scissors. Grab the heart with forceps, and cut any connective tissue to release the heart. Working quickly, rinse the heart with saline solution, dab off the excess fluid with gauze, and record the weight. If needed, separate the left ventricle, right ventricle, and septum with surgical scissors, and weigh them individually.
- Place the heart tissue samples in a cryovial, and flash-freeze in liquid nitrogen.
- Next, make a longitudinal incision down the abdomen with a scalpel and two lateral incisions from the umbilical region to the lateral side of the animal to allow for access to the abdominal organs.
- Using forceps and surgical scissors, remove any organs of interest.
NOTE: For this study, the liver, visceral (abdominal) adipose tissue, pancreas, and gastrocnemius were collected. The abdominal adipose tissue was removed in one or two large sections by gently trimming the connective tissue around the organs and body cavity wall. The subcutaneous fat was not collected, similar to previous methods22. - For the organs, after removal, place them in a clean weigh boat on a tared scale. Record the weight (g), and place the samples in cryovials for flash-freezing.
- Using forceps and surgical scissors, remove any organs of interest.
- For the gastrocnemius, make two incisions down the lateral sides of the lower leg and one horizontally across the Achilles tendon.
- Cut or tear the connective tissue connecting the skin to the musculature to expose the gastrocnemius. Cut the Achilles tendon with surgical scissors as close to the muscle as possible, and grab the gastrocnemius with forceps.
- Follow the gastrocnemius to the upper connection point, and make a similar cut to free the muscle.
- Weigh the sample on a clean, tared weigh boat, place in a cryovial, and flash-freeze in liquid nitrogen.
- To collect the heart, make an incision below the ribs and through the diaphragm.
- Immediately place any other collected tissue samples in cryovials, flash-freeze in liquid nitrogen, and store at −80 °C. These tissues can be saved for future laboratory analyses such as PCR, western blot, or other methods according to the research goals.
2. HIIT training protocol
- To begin a training session, turn on the treadmill (see Table of Materials) by flipping the power switch on the back of the control unit.
- Adjust the treadmill shock to 0.00 mA by turning the dial on the control unit counterclockwise until the monitor reads 0.00 mA.
- Set the inclination of the treadmill to 5.0% by loosening the locking nut on the bottom of the treadmill and setting the incline to the first notch. Retighten the locking nut to secure the treadmill incline in this position.
- Supporting the animal’s body with one hand, gently grasp the base of the tail with the other hand and place the animal in an individual lane on the treadmill.
- Repeat the process until all five individual lanes on the treadmill are occupied by a rat from the same cohort.
- Adjust the treadmill speed to 45 cm/s by rotating the speed dial clockwise until the monitor reads 45 cm/s. Press the Stop/Run button to start the treadmill, and allow it to run for 5 min. Press the Stop/Run button again to stop the treadmill after 5 min. No electric shock is used during this time.
NOTE: The animals may need encouragement with stiff bristle brushes to stay off the shock grid during the earlier stages of the protocol to facilitate their learning of how to use the treadmill. - At the end of the 5 min, allow a 2 min rest before beginning the training period. Turn the dial on the control unit clockwise until the monitor reads the corresponding starting velocity of the training bout. Use an initial running speed for the first session of 55 cm/s. For the first sprint of each new training day, use a starting speed that is 4 cm/s slower than the highest speed achieved the prior day.
- Start the treadmill by pressing the Start button, have the animals run until the monitor reads 1:00 (1 min), and then stop the treadmill by pressing the Stop/Run button again.
- Agitate the animals with brushes to encourage forward motion if the animal reaches the shock grid (located at the rear of the treadmill). If any animal per training group fails to respond to the brushes more than twice per training bout, turn on the shock grid to 2.0 mA for the remainder of the session.
- After the sprint, let the animals rest for 2 min. At the end of the 2 min rest, begin the next sprint by starting the treadmill by pressing the Stop/Run button on the control unit. The details regarding the treadmill speed are defined below.
- Increase the speed by 4 cm/s for the following sprint interval from the previous speed used if all the five animals within a cohort complete the sprint interval without needing motivation (encouragement with a stiff bristle brush or touching the shock grid more than five times) for a full 1 min sprint interval. The speed is increased by turning the speed knob on the control unit clockwise.
- Use the same interval speed as the previous sprint interval if the brushes are used to encourage running or if any animal touches the shock grid more than five times in a single 1 min sprint.
- Reduce the speed for the following interval by 4 cm/s if an animal struggles excessively during a sprint interval (more than 20 s of accumulated time on the shock grid).
NOTE: In our experience, 100% of the animals were able to complete the required running. Nevertheless, animals may need to be removed from the study at the investigator’s discretion if they demonstrate an unwillingness to run or experience excessive shocks. - Record the speed and distance run for each bout.
- Repeat the process for a total of 10 HIIT training bouts each training day. Each training bout consists of 1 min of high-intensity running followed by 2 min of rest.
- At the end of the training session, remove each animal from the treadmill, and place it in its individual cage.
- For every new day of training, the initial running speed for the first bout begins at 4 cm/s slower than the fastest speed obtained in the prior day's workout, with a minimum speed of 55 cm/s.
3. Statistical analysis
- Report the morphometrics and other outcome measures as means and standard errors.
- Determine the differences between the groups in an analysis software (see Table of Materials) using a mixed-effects model allowing for multiple comparisons.
NOTE: Šidák correction was implemented to account for multiple comparisons. A repeated-measures model was implemented when appropriate. Significant differences were determined by p < 0.05.
Representative Results
Figure 2 demonstrates that the training performance increased over the duration of the protocol. The final running speeds of the TRN and HFD/TRN groups were 115 cm/s and 111 cm/s respectively. The total running distance did not differ between differ between the TRN and HFD/TRN groups (Figure 3).
The average weekly feed intake for the animals on the control diet was higher (p < 0.0001) than for those on the high-fat diet (103 g/week ± 1.0 g/week vs. 91 g/week ± 1.0 g/week, respectively). The average weekly feed intake was also greater (p < 0.001) in trained groups than the non-trained groups (98 g/week ± 1.3 g/week vs. 92.2 g/week ± 1.0 g/week, respectively). When looking at the interactions, the CON versus TRN groups did not differ from each other but had greater (p < 0.05) weekly intake than the HFD/TRN group, which ate more (p < 0.05) than the HFD group (Figure 4). When translating feed intake to kcal intake, the animals on the high-fat diet had a higher (p < 0.0001) caloric intake than those on the control diet (430 kcal/week ± 4.6 kcal/week vs. 396 kcal/week ± 3.7 kcal/week, respectively). This resulted in differences (p < 0.05) in the weekly caloric intake among all four groups, with the HFD/TRN group showing the greatest weekly caloric intake, followed by the HFD, CON, and TRN groups sequentially (Figure 5).
Body weight did not differ between groups until week 8 of the feeding period, when the HFD and HFD/TRN groups reached a greater (p < 0.05) mass than the CON and TRN groups (293 g ± 10.1 g and 298 g ± 13.1 g vs. 270 g ± 8.6 g and 264 g ± 6.8 g, respectively). The HFD and HFD/TRN groups remained heavier (p < 0.05) than the CON and TRN groups for the remainder of the study (reaching 332 g ± 14.4 g, 347 g ± 16.3 g, 304 g ± 10.3 g, and 304 g ± 10.1 g for the HFD, HFD/TRN, CON, and TRN groups, respectively). The average daily gain (ADG) was greater (p < 0.05) in the trained versus non-trained animals over the exercise portion of the study (0.8 g/day ± 0.11 g/day vs. 0.5 g/day ± 0.09 g/day, respectively), and there were no differences in ADG between the CON versus HFD groups over this period. Together, this resulted in greater (p < 0.05) ADG in the HFD/TRN group than in the HFD group and no differences between the CON and TRN groups (Figure 6) over the training period. However, the 8 week training period did not induce a difference in weight between the HFD/TRN and HFD groups (347 g ± 16.3 g vs. 331.5 g ± 14.4 g, respectively).
After the completion of the training protocol, the tissue retrieval revealed that animals on the HFD had greater (p < 0.05) visceral adiposity than the CON group (25 g ± 2.1 g vs. 19 g ± 1.5 g, respectively), and the exercise-trained animals had reduced (p < 0.05) visceral adiposity relative to the control animals (21 g ± 2.4 g vs. 25 g ± 2.1 g, respectively). The HFD group had a greater (p < 0.05) visceral adiposity than the TRN and HFD/TRN groups (Figure 7). The heart mass was greater in the HFD/TRN group than in the CON, TRN, and HFD groups (p < 0.05; 1.3 g ± 0.2 g vs. 1.1 g ± 0.1 g, 1.1 g ± 0.1 g, and 1.0 g ± 0.1 g, respectively). There were no differences observed in the liver mass among the groups. No differences were identified in the mass of any other organs or tissues.
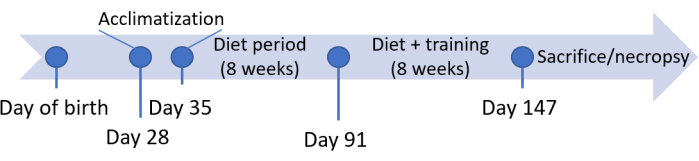
Figure 1: Study protocol timeline by animal age in days. Please click here to view a larger version of this figure.
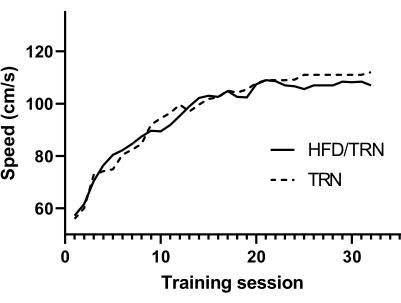
Figure 2: HIIT speed throughout the training protocol for the TRN and HFD/TRN animals by session. HIIT was performed on four different days every week for 8 weeks, resulting in 32 training sessions. The mean data per workout are presented. Please click here to view a larger version of this figure.
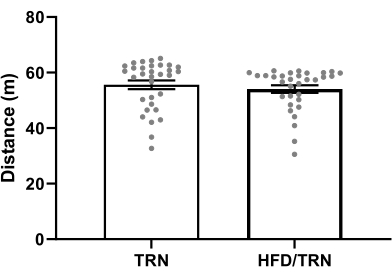
Figure 3: Average distance covered per sprint in the TRN and HFD/TRN groups throughout the training protocol. HIIT was performed on four different days every week for 8 weeks, resulting in 32 training sessions. The data are presented as mean ± SEM. Please click here to view a larger version of this figure.
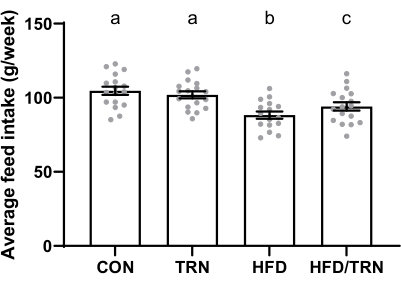
Figure 4: Average weekly feed intake of the CON, TRN, HFD, and HFD/TRN cohorts. Data are presented as mean ± standard error of the mean (SEM). a,b,cMeans with different letters differ (p < 0.05). Please click here to view a larger version of this figure.
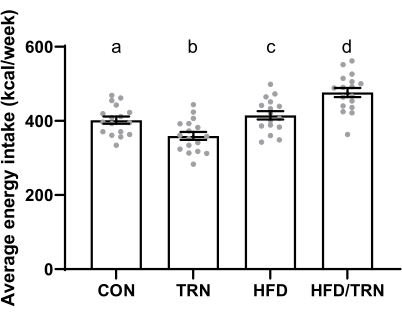
Figure 5: Weekly caloric intake of the CON, TRN, HFD, and HFD/TRN cohorts. Data are presented as mean ± SEM. a,b,c,dMeans with different letters differ (p < 0.05). Please click here to view a larger version of this figure.
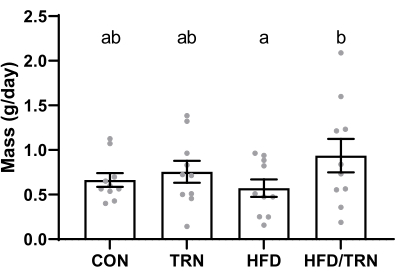
Figure 6: Average daily weight gain in the CON, TRN, HFD, and HFD/TRN cohorts. Data are presented as mean ± SEM. a,bGroups with different letters differ (p < 0.05). Please click here to view a larger version of this figure.
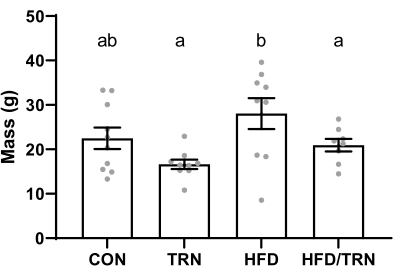
Figure 7: Average visceral fat mass at necropsy. Data are presented as mean ± SEM. a,bGroups with different letters differ (p < 0.05). Please click here to view a larger version of this figure.
Table 1: Compositions of the diets used in the protocol. Please click here to download this Table.
Discussion
This protocol provides an effective method for examining the effects of HIIT on several health markers in a diet-induced obesity model. The procedure draws from previous studies to allow for a more time-efficient method of examining multiple outcome variables, such as exercise training variables, appetite regulation markers, and invasive analyses of body composition3,7,8,18,23,24. The diet content, duration, and exercise intervention protocol were consistent with prior publications23,24. In this study, commercially available laboratory chow was purchased (see Table of Materials). The laboratory chow for the high-fat and control diets contained the same amount of protein and micronutrients. The carbohydrate and fat content of the diets were modified to provide a safe method of inducing obesity in the experimental group (see Table 1).
The 8 week obesity induction period used in the present study was modeled based on previous research showing significant changes in weight following the provision of commercial laboratory chow consisting of 45% kcal from fat (4.7 kcal/g), which represents the macronutrient breakdown found in the typical western diet23. Additionally, prior studies have demonstrated the effectiveness of an 8 week HIIT protocol on influencing food intake7,8, adipose profiles18,23, and muscle gain18. The results of the protocol described in this study were consistent with previous studies reporting that HIIT impacts appetite regulation, as well as compositional changes in adiposity and muscle mass.
A benefit of this protocol is that it maximizes the intensity of the exercise training in the animals and maintains maximum effort throughout the protocol. As the animals continuously learn how to use the treadmill proficiently and make fitness gains, the speed of the treadmill is increased accordingly relative to their performance. Furthermore, the use of the 5.0% inclination allows for the animals to reach maximum intensity in each session and throughout the protocol more quickly than would be accomplished without using inclination. As a result, the exercise performance is maximized for each workout and for the duration of the protocol.
During the study, one animal was unable to complete the experimental protocol due to illness, resulting in n = 39 animals completing the study, with only n = 9 rats in the HFD cohort. This protocol was initially designed to assess changes in cytokine profiles in response to exercise and diet, and the power analysis resulted greater than 90% power to identify a difference (p < 0.05) in the primary target cytokine (irisin). Future studies using this model should rely on unique power analyses to determine appropriate sample sizes.
This study was primarily designed to examine the physiologic outcomes of HIIT in a rodent model of diet-induced obesity and to maximize the intensity of exercise. This protocol was able to demonstrate variation in ADG and adiposity in response to diet and HIIT (Figure 6 and Figure 7). Future studies could specifically identify endocrine, myokine, and adipokine responses to HIIT. The elucidation of these mechanisms may prove beneficial in the treatment and prevention of obesity and its comorbidities.
This study also demonstrated the impact of diet and HIIT on feed intake. The results indicated that when the animals consumed a high-fat diet, the trained animals consumed more calories than the non-trained animals. In contrast, when the animals ate the control diet, the trained animals consumed less calories than the non-trained animals, demonstrating different appetite regulation responses depending on the composition of the diet. Therefore, strategies for weight loss that utilize HIIT may be less effective for those that simultaneously consume a high-fat diet, as they may be more likely to consume excess calories. In contrast, balanced macronutrient intake during HIIT may promote low calorie intake and, therefore, facilitate weight loss. This model can facilitate research efforts to develop a deeper understanding of the mechanisms behind energy balance and efforts to develop effective weight loss strategies.
Finally, this protocol demonstrated variation in cardiac tissue among the cohorts, reflecting adaptational changes in the body composition in response to diet and exercise training. These data suggest that obesity induction followed by HIIT may predispose individuals to myocardial hypertrophy without any accompanied alterations in hepatic size. Future analyses to determine the mechanisms behind these findings could be useful for investigating myocardial hypertrophy and the metabolic connections between obesity, HIIT, and cardiovascular disease.
The protocol described in this study has several limitations. First, the treadmill used in this study had five lanes, which allowed for five rats to be run at a single time. While this manner of executing the protocol was efficient, it was difficult for a single researcher to attend to each of the animals at once. There were occasions when it was difficult for the treadmill attendant to divide their attention among the multiple animals needing stimulation with bristle brushes. In the future, ensuring that more research personnel are available to assist with the training protocols will be a priority. Additionally, the five-lane treadmill model does not have the capability to measure gas exchange, and, therefore, the aerobic/anaerobic metabolism of the animals during the protocol could not be assessed. The company that provided the rodent treadmill (see Table of Materials) does offer a treadmill with the capability to measure gas exchange, but it is a single-lane treadmill and, therefore, would require significantly greater time and effort. That effort may be worthwhile, however, for investigators who need to measure or control for specific outcomes of indirect calorimetry. Additionally, there is very little evidence available regarding how the shock grid may impact exercise performance, which should be considered when interpreting the results from this model. Lastly, the exercise protocol described in this study was designed with young female Sprague-Dawley rats. Previous studies have shown sexually dimorphic effects, especially regarding HIIT and appetite regulation3,7. Although similar outcomes are anticipated, this protocol did not test animals of different species, ages, sexes, or health outcomes.
In comparison to prior models, this protocol demonstrates a more time-efficient method to evaluate a range of outcome variables. For instance, this protocol was able to identify interactions between HIIT and appetite regulation in a protocol that involved four training sessions per week for 8 weeks, in comparison to prior studies that involved five training sessions per week for 8 weeks24 or even 12 weeks of training8. Additionally, this study design allowed for the analysis of a variety of health markers, such as exercise data, markers of appetite regulation, and body composition. These markers, as well as the heart adaptations to exercise training, represent promising means of evaluating the training adaptations of the cardiovascular system as well. Measures of endothelial function, muscle fiber type composition, and cardiac myocyte hypertrophy could easily be added to further the understanding of these exercise-induced adaptations. Further, this protocol included performance-based escalations in intensity. This design allowed for the maximization of the training outcomes and ensured that the rats did not adapt to the exercise environment and approach a moderate-intensity continuous training model toward the end of the intervention. This is demonstrated in Figure 2; specifically, the sprint speeds of these animals were more than double the speeds achieved in previous publications, which went on to demonstrate many cardiovascular, skeletal muscle, and thermoregulatory adaptations consistent with HIIT interventions25.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Michael Pankey, Chris Butler, and the WVSOM staff for their assistance in the animal care and data collection.
Materials
| Commercial laboratory chow for control diet | Research Diets Inc., New Brunswick, NJ | D12450H | |
| Commercial laboratory chow for high-fat diet | Research Diets Inc., New Brunswick, NJ | D12451 | |
| GraphPad Prism software | GraphPad Software Inc., San Diego, CA | ||
| Precision Electronic Digital Scale | Ohaus Corporation, Pine Brook, NJ | V11P30 | |
| Rodent treadmill | Panlab, Barcelona, Spain | ||
| Sprague Dawley rats | Charles River, Durham, NC | ||
| Table top anesthesia machine | VetEquip Inc., Livermore, CA | V0557 |
References
- Overweight & obesity. Centers for Disease Control and Prevention Available from: https://www.cdc.gov/obesity/ (2019)
- Ylli, D., Sidhu, S., Parikh, T., Burman, K. D. Endocrine changes in obesity. Endotext. , (2017).
- Eckel, L. A., Moore, S. R. Diet-induced hyperphagia in the rat is influenced by sex and exercise. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 287 (5), R1080-R1085 (2004).
- Martins, C., Morgan, L., Truby, H. A review of the effects of exercise on appetite regulation: An obesity perspective. International Journal of Obesity. 32 (9), 1337-1347 (2008).
- Steinberg, G. R., et al. Endurance training partially reverses dietary-induced leptin resistance in rodent skeletal muscle. American Journal of Physiology, Endocrinology, and Metabolism. 286 (1), E57-E63 (2004).
- Blundell, J. E., Stubbs, R. J., Hughes, D. A., Whybrow, S., King, N. A. Cross talk between physical activity and appetite control: Does physical activity stimulate appetite. Proceedings of the Nutrition Society. 62 (3), 651-661 (2003).
- Nance, D. M., Bromley, B., Barnard, R. J., Gorski, R. A. Sexually dimorphic effects of forced exercise on food intake and body weight in the rat. Physiology and Behavior. 19 (1), 155-158 (1977).
- Sim, A. Y., Wallman, K. E., Fairchild, T. J., Guelfi, K. J. Effects of high-intensity intermittent exercise training on appetite regulation. Medicine & Science in Sports & Exercise. 47 (11), 2441-2449 (2015).
- Booth, F. W., Gordon, S. E., Carlson, C. J., Hamilton, M. T. Waging war on modern chronic diseases: primary prevention through exercise biology. Journal of Applied Physiology. 88 (2), 774-787 (1985).
- Görgens, S. W., Eckardt, K., Jensen, J., Drevon, C. A., Eckel, J. Exercise and regulation of adipokine and myokine production. Progress in Molecular Biology and Translation Science. 135, 313-336 (2015).
- Gleeson, M., et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nature Reviews Immunology. 11 (9), 607-615 (2011).
- Leal, L. G., Lopes, M. A., Batista, M. L. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Frontiers in Physiology. 9, 1307 (2018).
- Ilich, J. Z., et al. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. Ageing Research Reviews. 15, 51-60 (2014).
- Greenberg, A. S., Obin, M. S. Obesity and the role of adipose tissue in inflammation and metabolism. American Journal of Clinical Nutrition. 83 (2), 461 (2006).
- Sallam, N., Laher, I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxidative Medicine and Cellular Longevity. 2016, 7239639 (2016).
- Conroy, S. M., et al. Impact of aerobic exercise on levels of IL-4 and IL-10: Results from two randomized intervention trials. Cancer Medicine. 5 (9), 2385-2397 (2016).
- Dennett, A. Exercise has a positive effect on low-grade inflammation in women with breast cancer [commentary. Journal of Physiotherapy. 62 (4), 227 (2016).
- Wu, S., Park, K. S., McCormick, J. B. Effects of exercise training on fat loss and lean mass gain in Mexican-American and Korean premenopausal women. International Journal of Endocrinology. 2017, 5465869 (2017).
- Wang, Y., Wilsof, U., Kemi, O. J. Animal models in the study of exercise-induced cardiac hypertrophy. Physiology. 59 (5), 633-644 (2010).
- Shirvani, H., Arabzadeh, E. Metabolic cross-talk between skeletal muscle and adipose tissue in high-intensity interval training vs. moderate-intensity continuous training by regulation of PGC-1α. Eating and Weight Disorders. 25 (1), 17-24 (2020).
- Evans, C. C., et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 9 (3), e92193 (2014).
- Castro-Rodríguez, D. C., et al. Strengths and validity of three methods for assessing rat body fat across the life course. International Journal of Obesity. 44 (12), 2430-2435 (2020).
- Marques, C. M., Motta, V. F., Torres, T. S., Aguila, M. B., Mandarim-de-Lacerda, C. A. Beneficial effects of exercise training (treadmill) on insulin resistance and nonalcoholic fatty liver disease in high-fat fed C57BL/6 mice. Brazilian Journal of Medical and Biological Research. 43 (5), 467-475 (2010).
- Ferreira, J. C., et al. Maximal lactate steady state in running mice: effect of exercise training. Clinical and Experimental Pharmacology and Physiology. 34 (8), 760-765 (2007).
- Beleza, J., et al. Self-paced free-running wheel mimics high-intensity interval training impact on rats’ functional, physiological, biochemical, and morphological features. Frontiers in Physiology. 10, 593 (2019).

