Collection of Alfalfa Root Exudates to Study the Impact of Di(2-ethylhexyl) Phthalate on Metabolite Production
Summary
The secretion of root exudates is usually an external detoxification strategy for plants under stress conditions. This protocol describes how to assess the impact of xenobiotics on alfalfa via nontargeted metabolomic analysis.
Abstract
Root exudates are the main media of information communication and energy transfer between plant roots and the surrounding environment. The change in secretion of root exudates is usually an external detoxification strategy for plants under stress conditions. This protocol aims to introduce general guidelines for the collection of alfalfa root exudates to study the impact of di(2-ethylhexyl) phthalate (DEHP) on metabolite production. First, alfalfa seedlings are grown under DEHP stress in a hydroponic culture experiment. Second, the plants are transferred to centrifuge tubes containing 50 mL of sterilized ultrapure water for 6 h to collect root exudates. The solutions are then freeze-dried in a vacuum freeze dryer. The frozen samples are extracted and derivatized with bis(trimethylsilyl)) trifluoroacetamide (BSTFA) reagent. Subsequently, the derivatized extracts are measured using a gas chromatograph system coupled with a time-of-flight mass spectrometer (GC-TOF-MS). The acquired metabolite data are then analyzed based on bioinformatic methods. Differential metabolites and significantly changed metabolism pathways should be deeply explored to reveal the impact of DEHP on alfalfa in view of root exudates.
Introduction
Di(2-ethylhexyl) phthalate (DEHP) is a synthetic chemical compound that is widely used in various plastics and polymers as a plasticizer to improve their plasticity and strength. In the past few years, an increasing number of studies have suggested that DEHP is an endocrine disruptor and has adverse effect on the respiratory, nervous, and reproductive systems of humans and other animals1,2,3. Considering its health risk, the United States Environmental Protection Agency, European Union, and Environmental Monitoring Center of China have all classified DEHP in the list of priority pollutants. Soil has been considered as an important sink of DEHP in the environment, due to the application of plastic mulching and organic fertilizers, irrigation with wastewater, and sludge farm application4. As expected, DEHP has been ubiquitously detected in farmland soils, the content of which even reaches up to milligrams per kilogram of dried soil in some regions in China5,6. DEHP can enter plants mainly via the roots and undergo biomagnification at different trophic levels in soil ecosystems7. Therefore, significant concern has been raised about DEHP-induced stress in plants over recent decades.
Plants are usually vulnerable to DEHP exposure. DEHP stress has been observed to exert an adverse effect on seed germination and normal metabolism, thereby inhibiting plant growth and development8,9. For example, DEHP can induce oxidative damage to mesophyll cells, decrease the contents of chlorophyll and osmolytes, and elevate antioxidative enzyme activities, eventually resulting in a decline in the yield and quality of edible plants10,11. However, most of the previous studies on the response of plants to DEHP stress have focused on oxidative stress and physiological and biochemical characteristics. The corresponding mechanisms associated with plant metabolism are less-studied. Root exudates is a generic term describing compounds that plant roots secrete and release into the environment. They have been considered as the interaction media between plants and rhizosphere soil, playing an important role in supporting plant growth and development12. It has been well known that root exudates account for approximately 30%-40% of all photosynthetic carbon13. In polluted environments, root exudates are involved in improving the tolerance of plants to the stress of pollutants through metabolism or external exclusion14. As a consequence, a deep understanding of the response of plant root exudates to pollution stress may help reveal the underlying mechanisms associated with cell biochemistry and biological phenomena15.
Metabolomics technology provides an efficient strategy for measuring a large number of small molecule metabolites simultaneously within cells16,17, tissues18, and even exudates of organisms19, including sugars, organic acids, amino acids, and lipids. Compared with traditional or classical chemical analysis methods, the metabolomics approach greatly increases the number of metabolites that can be detected20, which can help identify metabolites in a higher-throughput way and identify key metabolic pathways. Metabolomics has been widely used in the research field of biological response in stress environments, such as heavy metals21, emerging pollutants22, and nanoparticles19. Most of these studies on plants have focused on the metabolic changes in interior plant tissues, whereas few have been reported on the response of root exudates to environmental stress. Therefore, the aim of this study is to introduce general guidelines for the collection of alfalfa root exudates to study the impact of DEHP on metabolite production. The results will provide a method guidance for the follow-up study of plant metabolomics by DEHP.
Protocol
The aim of this protocol is to provide a general pipeline, from a hydroponic culture experiment to metabolomic analysis, quantifying the effect of DEHP on alfalfa root exudates.
1. Hydroponic culture experiment
NOTE: This protocol presents an example of an alfalfa hydroponic culture experiment designed to obtain alfalfa (Medicago sativa) seedlings under the stress of different concentrations of DEHP. Three treatments were set up: the control without any additions, and the nutrient solution spiked with 1 mg kg-1 and 10 mg kg-1 of di(DEHP. The concentrations of DEHP were set according to the real content of DEHP in soil23. Each treatment had six replicates.
- Sterilize alfalfa seeds with 0.1% sodium hypochlorite for 10 min and 75% ethyl alcohol for 30 min.
- Rinse the sterilized seeds several times with distilled water and then germinate on moist filter paper in a sterile Petri dish at 30 °C in the dark.
- Transfer 20 uniform, germinated, big-plump seeds onto an engraftment basket in a culture bottle filled with nutrient solution, composed of (in µM): Ca(NO3)2, 3,500; NH4H2PO4, 1,000; KNO3, 6,000; MgSO4, 2,000; Na2Fe-ethylenediaminetetraacetic acid (EDTA), 75; H3BO3, 46; MnSO4, 9.1; ZnSO4, 0.8; CuSO4, 0.3; and (NH4 )6Mo7O24, 0.02. Adjust the solution pH to 7.0 using 0.1 M KOH. Renew all solutions weekly.
- Place all the culture bottles in a controlled growth chamber with a light intensity of 150-180 µmol m-2 s-1 with a photoperiod of 16 h each day, at 27 °C and 20 °C representing day (16 h) and night (8 h), respectively.
- Transfer 15 uniform alfalfa seedlings to a new glass bottle for culture experiments under 1 mg kg-1 and 10 mg kg-1 DEHP stress after 2 weeks. Wrap the glass bottles with aluminum foil and parafilm to prevent photolysis and volatilization of the DEHP. To apply the same conditions, also wrap the control bottles with aluminum foil and parafilm. Supplement the nutrient solution daily to maintain the liquid level.
- Randomly place and rotate the bottles every 2 days to ensure consistent growth conditions for the alfalfa seedlings.
- After 7 days of cultivation, remove the alfalfa seedlings from the bottles and wash with ultrapure water several times, preparing for the collection of root exudates.
2. Collection, extraction, and metabolomic analysis of root exudates
NOTE: This protocol is divided into three parts: a collection experiment, an extraction experiment, and metabolomic analysis of the root exudates. The goal of the collection experiment is to transfer the metabolites secreted in plant samples to the solution system for subsequent extraction.
- Collection experiment
- Transfer 10 uniform alfalfa seedlings to centrifuge tubes filled with 50 mL of sterilized deionized water. Submerge the roots in water to collect root exudates for 6 h; keep the tubes upright. Perform at least six replicates for each treatment.
- Wrap the centrifuge tubes with aluminum foil to protect the roots from light.
- Remove the plants and freeze-dry the collected liquid for metabolite profiling.
- Extraction experiment
- Add 1.8 mL of extraction solution (methanol:H2O = 3:1, V/V) to the tubes and vortex for 30 s.
- Apply ultrasound waves to the tubes for 10 min in an ice water bath.
- Centrifuge the samples at 4 °C and 11,000 × g for 15 min.
- Carefully transfer 200 µL of supernatant into a 1.5 mL microcentrifuge tube. Take 45 µL of supernatant from each sample and mix it into quality control (QC) samples at a final volume of 270 µL, which is used for calibration of the metabolome data of samples.
- Freeze-dry the extracts in a vacuum concentrator. Continue drying with 5 µL of the internal standard (ribonucleol).
- After evaporation in a vacuum concentrator, add 30 µL of methoxyamination hydrochloride (dissolved in pyridine at a concentration of 20 mg mL-1) to the tubes and incubate the tubes at 80 °C for 30 min. Then, add 40 µL of bis(trimethylsilyl)trifluoroacetamide (BSTFA) reagent (with 1% trimethylchlorosilane [TMC], V/V) to the samples and place the tubes at 70 °C for 1.5 h for derivatization.
- Cool the samples to room temperature and add 5 µL of fatty acid methyl esters (FAMEs) (in chloroform) to the QC samples.
- Metabolomic analysis
- Inject 1.0 µL of the derivatized extracts into a gas chromatograph system coupled to a time-of-flight mass spectrometer (GC-TOF-MS) for metabolomic profiling analysis using a splitless mode.
- Use a capillary column (30 m x 250 µm x 0.25 µm) for the separation of root exudates, with helium as a carrier gas at a flow rate of 1.0 mL min-1. Set the injection temperature to 280 °C, and maintain the transfer line temperature and ion source temperature at 280 °C and 250 °C, respectively.
- For separation, use the following oven program: 1 min isothermal heating at 50 °C, a 10 °C/min-1 oven ramp to 310 °C, and a final isothermal heating at 310 °C for 8 min.
- Perform electron collision mode with -70 eV of energy. Obtain mass spectra using full scan monitoring mode with a mass scan range of 50-500 m/z at a rate of 12.5 spectra/s.
- Filter individual peaks to remove noise. The deviation value is filtered based on the interquartile range.
- Fill the missing values with half of the minimum values, standardize, and normalize the data.
- Import the final data in .csv format into statistical analysis software for multivariate analysis.
- Look up the metabolites in Kyoto Encyclopedia of Genes and Genomes (KEGG) database (a database resource for understanding high-level functions and utilities of the biological system), and classify the metabolites into different categories, such as carbohydrates, acids, lipids, alcohols, and amines. Use statistical analysis software to construct a pie chart to indicate the percentage of each category in all the root exudates.
- Apply supervised orthogonal projections to latent structures-discriminate analysis (OPLS-DA) to demonstrate the differences among groups.
- Screen significantly changed metabolites as differential metabolites based on a variable importance in projection (VIP) > 1 and p < 0.05 (Student's t test).
- Use the metabolome data to construct heat maps with the statistical analysis software and use the fold changes under different treatments to construct histograms.
- Look up the differential metabolites in the KEGG database and Pubchem and compile the metabolic pathways containing the differential metabolites. Perform pathway enrichment analysis or topology analysis.
- Inject 1.0 µL of the derivatized extracts into a gas chromatograph system coupled to a time-of-flight mass spectrometer (GC-TOF-MS) for metabolomic profiling analysis using a splitless mode.
Representative Results
In this experiment, alfalfa root exudates were collected, extracted, and analyzed according to the above methods (Figure 1). Three treatment groups were set up: control, low concentration of DEHP (1 mg L−1), and high concentration of DEHP (10 mg L−1).
A total of 778 peaks were detected in the chromatograph of the control, of which 314 metabolites could be identified according to the mass spectra. As shown in Figure 2, these metabolites could be classified into six types based on the relative abundance: carbohydrates (28.6%), acids (15.58%), lipids (13.87%), alcohols (3.91%), amines (0.92%), and others (37.12%). Metabolites that accounted for less than 0.5% were grouped as other substances (Figure 2A). The acids were further subdivided into fatty acids (56.09%), amino acids (26.62%), organic acids (13.95%), and phenolic acids (3.34%) (Figure 2B). In addition, some common substances in the root exudates of most plants could also be detected in alfalfa root exudates, including pyrimidines, hydroxy pyridines, flavonoids, phenols, ketones, pyrimidines, flavonoids, and diterpenes.
A heat map was plotted to visualize the variation in differential metabolites among different DEHP treatments, based on the VIP score (Figure 3). Compared with the control, the exposure to DEHP significantly changed the content of 50 metabolites in alfalfa root exudates, mainly including some carbohydrates and low-molecular weight organic acids. Five types of carbohydrates (lyxose, digitoxose, erythrose, trehalose, and fructose 2, 6-bihosphate) were upregulated in the presence of DEHP, and two of these (lyxose and digitoxose) were significantly increased as the concentration of DEHP increased. In addition, five metabolites were downregulated in the presence of DEHP, including monosaccharides such as D-talose and glucose, disaccharides such as maltose, cellobiose, and trehalose, and sugar alcohols such as D-arabitol. Carbohydrate content has been considered as an indicator of plant physiological status24. Therefore, the decrease in monosaccharide and disaccharide levels herein indicated physiological stress caused by DEHP stress. Compared with carbohydrates, DEHP exerted a greater effect on acid metabolism in alfalfa seedlings. Under exposure to DEHP, the contents of 11 acid metabolites were significantly increased, mainly including 2-amino-2-norbornanecarboxylic acid, 5-hydroxyindole-2-carboxylic acid, 3-hydroxy-L-proline, pelargonic acid, and palmitic acid. At the same time, DEHP also inhibited the metabolism of some flavonoids in alfalfa seedlings, including 4', 5-dihyrroxy-7-methoxyisoflavone and neohesperidin.
The metabolic pathways influenced by DEHP are described in Figure 4. DEHP significantly inhibited the metabolism of carbohydrates, such as some monosaccharides and disaccharides, which are products of photosynthesis. Therefore, DEHP can suppress the photosynthesis of alfalfa to a certain extent. Moreover, DEHP can promote the metabolism of fatty acids, which are helpful for plants to resist stress from DEHP. The major metabolic pathways influenced by DEHP were carbohydrate metabolism and fatty acid metabolism, while amino acid metabolism, lipid metabolism, and the tricarboxylic acid (TCA) cycle were affected to a much lesser extent.
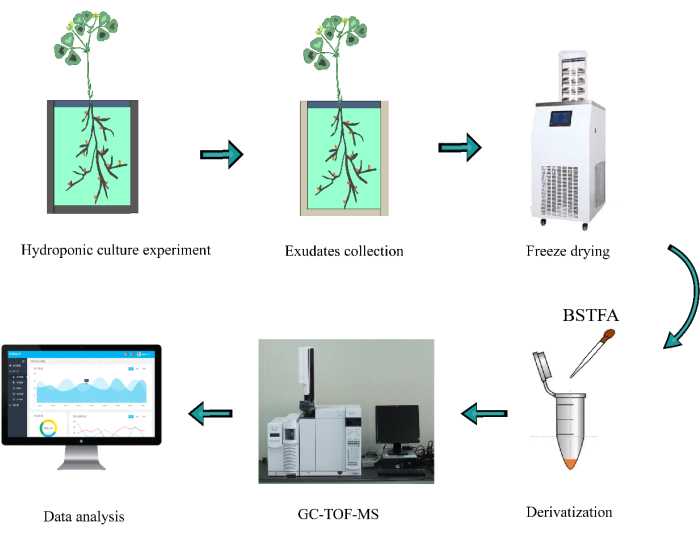
Figure 1: A flow chart of nontargeted metabolomic analysis for alfalfa root exudates. BSTFA represents bis(trimethylsilyl)trifluoroacetamide (BSTFA) reagent (with 1% trimethylchlorosilane [TMC], V/V). Please click here to view a larger version of this figure.
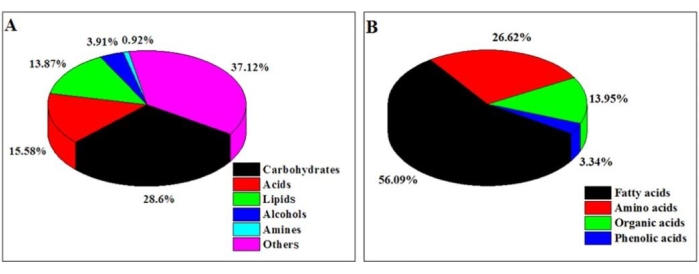
Figure 2: Classification of metabolites. (A) Classification of the known metabolites and (B) acids. The percentage of each type of material is divided by the sum of the peak area of each category by the sum of the peak area of all the substances in the control. Other substances were those at <0.5%. Other acids were those at <0.5%. This figure has been modified from Wang et al.25. Please click here to view a larger version of this figure.
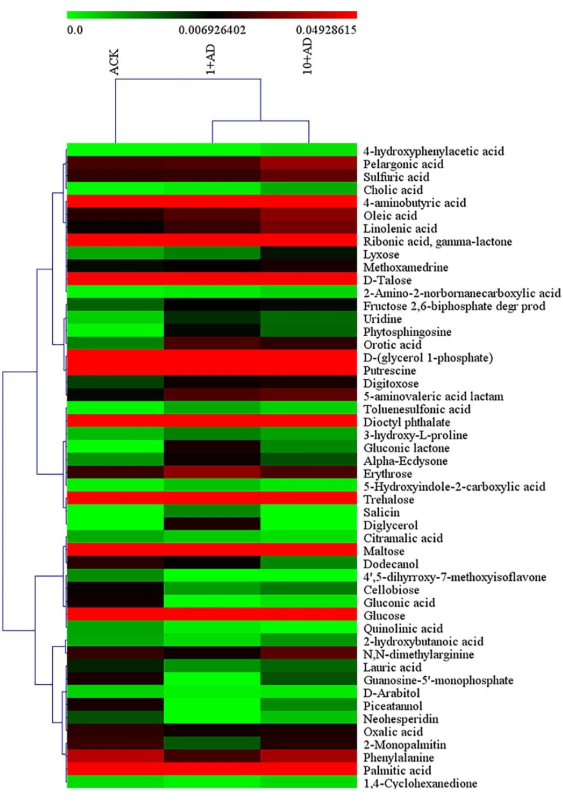
Figure 3: Heatmap of hierarchical clustering analysis for root exudates (VIP > 1, p < 0.05) of alfalfa seedlings with different DEHP treatments. Red and green represent high and low abundance, respectively. ACK represents the control; 1+AD represents the treatment with 1 mg L−1 DEHP; 10+AD represents the treatment with 10 mg L−1 DEHP. This figure has been modified from Wang et al.25. Please click here to view a larger version of this figure.
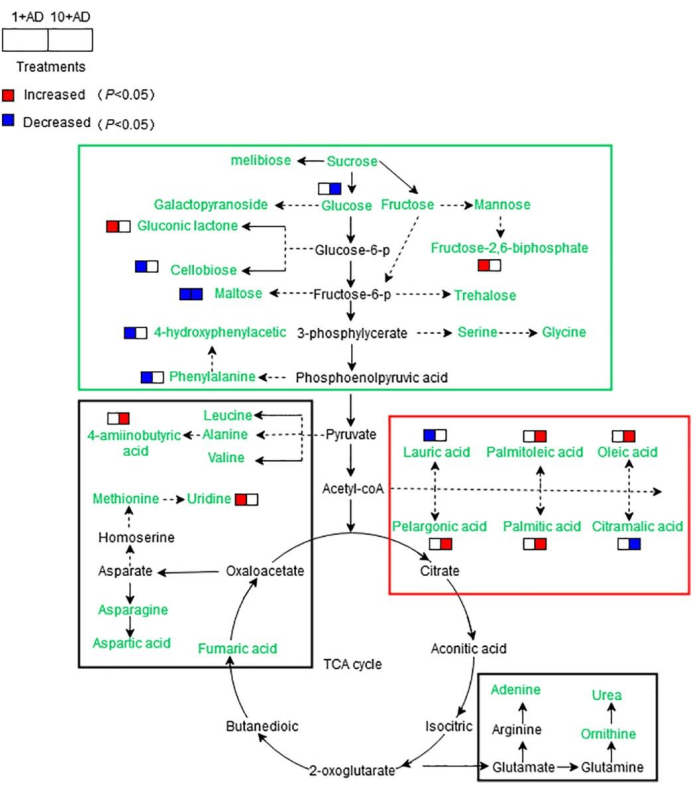
Figure 4: Relationships between the disturbance of metabolic pathways and the alterations in biological endpoints (1 mg L−1 DEHP, 10 mg L−1 DEHP). The metabolic pathways were established based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. The metabolites in green text were the metabolites detected in the present work. The signs "red boxes" and "blue boxes" in parentheses indicate that metabolites increased (p < 0.05) or decreased (p < 0.05) contributions to the biological endpoints, respectively. The figure has been made readable by separating the metabolites roughly into carbohydrate, fatty acid, and protein metabolism, as shown by the green, red, and black rectangular boxes, respectively. ACK represents the control; 1+AD represents the treatment with 1 mg L−1 DEHP; 10+AD represents the treatment with 10 mg L−1 DEHP. This figure has been modified from Wang et al.25. Please click here to view a larger version of this figure.
Discussion
This protocol provides general guidance on how to collect and measure the root exudates of alfalfa under DEHP stress, as well as how to analyze the metabolome data. Close attention needs to be paid to some critical steps in this protocol. In hydroponic culture experiments, alfalfa seedlings were hydroponically cultured in glass bottles filled with nutrient solutions with different concentrations of DEHP. The glass bottles should be protected from light by covering them with aluminum foil throughout the culture period, in order to prevent DEHP from photolysis and assure the uniformity of DEHP concentrations in all culture solutions25,26. DEHP concentrations were set as 1 mg L-1 and 10 mg L-1, according to the concentrations usually found in soils contaminated with DEHP27,28. During the collection of root exudates, the centrifuge tubes still need to be wrapped with aluminum foil to protect the roots from light. Here, the collection time was set to be 6 h. If the collection time is too short, the concentration of some low-abundance root exudates might be too low to be detected, so the composition of the root exudates might not reflect the real response of alfalfa seedlings under DEHP stress. If the collection time is too long, the collected root exudates might be degraded to a certain extent by microbes in the culture systems. In this case, some microbial metabolites might also be detected, changing the composition of the root exudates29. Moreover, at least six replicates should be performed for each treatment so as to ensure accurate metabolome data analysis.
Only some certain types of root exudates, such as amino acids, fatty acids, phenolic compounds, and other organic acids, can be determined using conventional targeted analytical methods (i.e., searching for known compounds)30,31,32, using standard analytical methods, such as spectrophotometry, high-pressure liquid chromatography (HPLC), ion chromatography (IC), gas chromatography-tandem mass spectrometry (GC-MS/MS), or liquid chromatography combined with tandem mass spectrometry (LC-MS/MS). With the development of instrumental analysis techniques, nontargeted metabolomic analysis has emerged based on GC-TOF-MS, liquid chromatography-high resolution mass spectrometry (LC-HR-MS), and nuclear magnetic resonance (NMR)33. Compared with other conventional analytical methods of root exudates, nontargeted metabolomic analysis greatly expands the number of detected root exudates, which facilitates deeply understanding plant metabolic responses to environmental stress.
The technique in the current study has some limitations, especially in the quantitative analysis of metabolites. Nontargeted metabolomic analysis only demonstrates the relative quantitative relationships among different metabolites, rather than accurate concentrations. This is unfavorable for investigations into the environmental behaviors or ecological effects of root exudates34. For the growth media of alfalfa seedlings, hydroponic culture experiments were performed in the current study, which have the advantages of easy control and easy operation. However, the artificial hydroponic growth environment is different from the real soil environment, leading to possible underestimation of the gross exudation rates due to metabolite reuptake by roots35. Compared with hydroponic culture, sand culture is a relatively more convincing method because it is closer to the real soil environment, facilitating the collection of root exudates in a realistic environment36. Therefore, work is under progress to establish a method of sand culture or even real soil culture to make the experimental results more convincing, which will help us conduct more in-depth research, showing better results of practical significance for explaining the toxicity response.
Based on the nontargeted metabolic analysis, we can not only explore the metabolic response of plants to environmental stress37, but also determine differential metabolites and further investigate the important role of the differential metabolites in the environmental behavior of contaminants38. Previous studies have shown that pelargonic acid level can be used as an indicator of root membrane damage when plants are under stress39. Unsaturated fatty acids (palmitic acid) were also found to increase membrane fluidity40, which may protect alfalfa root membranes from DEHP damage. Some flavonoids inhibited by DEHP can participate in the interaction between legumes and microorganisms. Moreover, the interactions between plants and the rhizosphere biological community may be more deeply deciphered with nontargeted metabolic analysis, especially under the stress of contaminants41. More types of root exudates will be identified42 with the development of analytical instruments for nontargeted metabolic analysis, which is helpful for constructing the metabolomic fingerprinting of plant root exudates43.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (41877139), the Major Projects of the National Natural Science Foundation of China (41991335), the National Key Research and Development Program of China (2016YFD0800204), the Natural Science Foundation of Jiangsu Province (No. BK20161616), the "135" Plan, and the Frontiers Program of the Chinese Academy of Sciences (ISSASIP1615).
Materials
| Adonitol | SIGMA | ≥99% | |
| Alfalfa seeds | Jiangsu Academy of Agricultural Sciences (Nanjing, China) | ||
| Analytical balance | Sartorius | BSA124S-CW | |
| BSTFA | REGIS Technologies | with 1% TMCS, v/v | |
| Centrifuge | Thermo Fisher Scientific | Heraeus Fresco17 | |
| Chromatographic column | Agilent | DB-5MS (30 m × 250 μm × 0.25 μm) | |
| Di(2-ethylhexyl) phthalate | Dr. Ehrenstorfer | ||
| FAMEs | Dr. Ehrenstorfer | ||
| Gas chromatography(GC) | Agilent | 7890A | |
| Grinding instrument | Shanghai Jingxin Technology Co., Ltd | JXFSTPRP-24 | |
| Mass spectrometer(MS) | LECO | PEGASUS HT | |
| Methanol | CNW Technologies | HPLC | |
| Methoxyaminatio hydrochloride | TCI | AR | |
| Microcentrifuge tube | Eppendorf | Eppendorf Quality | 1.5 mL |
| Oven | Shanghai Yiheng Scientific Instrument Co., Ltd | DHG-9023A | |
| Pyridine | Adamas | HPLC | |
| R software | statistical analysis software (pathway enrichment, topology) | ||
| SIMCA16.0.2 | statistical analysis software (OPLS-DA etc) | ||
| Ultra low temperature freezer | Thermo Fisher Scientific | Forma 900 series | |
| Ultrasound | Shenzhen Fangao Microelectronics Co., Ltd | YM-080S | |
| Vacuum dryer | Taicang Huamei biochemical instrument factory | LNG-T98 |
References
- Yin, X. H., Zeb, R., Wei, H., Cai, L. Acute exposure of di(2-ethylhexyl) phthalate (DEHP) induces immune signal regulation and ferroptosis in oryzias melastigma. Chemosphere. 265, 129053 (2021).
- Seyoum, A., Pradhan, A. Effect of phthalates on development, reproduction, fat metabolism and lifespan in Daphnia magna. The Science of the Total Environment. 654, 969-977 (2019).
- van T Erve, T. J., et al. Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environmental Science & Technology. 53 (6), 3258-3267 (2019).
- He, L., et al. Contamination and remediation of phthalic acid esters in agricultural soils in China: a review. Agronomy for Sustainable Development. 35 (2), 519-534 (2015).
- Liu, S. S., et al. Di-(2-ethylhexyl) phthalate as a chemical indicator for phthalic acid esters: an investigation into phthalic acid esters in cultivated fields and e-waste dismantling sites. Environmental Toxicology and Chemistry. 38 (5), 1132-1141 (2019).
- Li, K. K., Ma, D., Wu, J., Chai, C., Shi, Y. X. Distribution of phthalate esters in agricultural soil with plastic film mulching in Shandong Peninsula, East China. Chemosphere. 164, 314-321 (2016).
- Sun, J., Wu, X., Gan, J. Uptake and metabolism of phthalate esters by edible plants. Environmental Science & Technology. 49 (14), 8471-8478 (2015).
- Kim, D., Cui, R., Moon, J., Kwak, J. I., An, Y. J. Soil ecotoxicity study of DEHP with respect to multiple soil species. Chemosphere. 216, 387-395 (2019).
- Gao, M. L., Qi, Y., Song, W. H., Xu, H. R. Effects of di-n-butyl phthalate and di (2-ethylhexyl) phthalate on the growth, photosynthesis, and chlorophyll fluorescence of wheat seedlings. Chemosphere. 151, 76-83 (2016).
- Zhang, Y., et al. Effects of diethylphthalate and di-(2-ethyl)hexylphthalate on the physiology and ultrastructure of cucumber seedlings. Environmental Science and Pollution Research. 21 (2), 1020-1028 (2014).
- Gao, M. L., Liu, Y., Dong, Y. M., Song, Z. G. Physiological responses of wheat planted in fluvo-aquic soils to di (2-ethylhexyl) and di-n-butyl phthalates. Environmental Pollution. 244, 774-782 (2019).
- Lundberg, D. S., Teixeira, P. J. P. L. Root-exuded coumarin shapes the root microbiome. Proceedings of the National Academy of Sciences. 115 (22), 5629-5631 (2018).
- Canarini, A., Kaiser, C., Merchant, A., Richter, A., Wanek, W. Root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli. Frontiers in Plant Science. 10, 157 (2019).
- Chai, Y. N., Schachtman, D. P. Root exudates impact plant performance under abiotic stress. Trends in Plant Science. 27 (1), 80-91 (2022).
- Olanrewaju, O. S., Ayangbenro, A. S., Glick, B. R., Babalola, O. O. Plant health: feedback effect of root exudates-rhizobiome interactions. Applied Microbiology and Biotechnology. 103 (3), 1155-1166 (2019).
- Chamberlain, C. A., Hatch, M., Garrett, T. J. Metabolomic and lipidomic characterization of Oxalobacter formigenes strains HC1 and OxWR by UHPLC-HRMS. Analytical and Bioanalytical Chemistry. 411 (19), 4807-4818 (2019).
- vander Hooft, J. J. J., Goldstone, R. J., Harris, S., Burgess, K. E. V., Smith, D. G. E. Substantial extracellular metabolic differences found between phylogenetically closely related probiotic and pathogenic strains of Escherichia coli. Frontiers in Microbiology. 10, 252 (2019).
- Liu, N., Zhu, L. Metabolomic and transcriptomic investigation of metabolic perturbations in Oryza sativa L. triggered by three pesticides. Environmental Science & Technology. 54 (10), 6115-6124 (2020).
- Zhao, L., et al. H-1 NMR and GC-MS based metabolomics reveal defense and detoxification mechanism of cucumber plant under nano-Cu stress. Environmental Science & Technology. 50 (4), 2000-2010 (2016).
- Llanes, A., Arbona, V., Gómez-Cadenas, A., Luna, V. Metabolomic profiling of the halophyte Prosopis strombulifera shows sodium salt- specific response. Plant Physiology and Biochemistry. 108, 145-157 (2016).
- Zhang, Y., et al. Zinc stress affects ionome and metabolome in tea plants. Plant Physiology and Biochemistry. 111, 318-328 (2017).
- Wright, R. J., Bosch, R., Gibson, M. I., Christie-Oleza, J. A. Plasticizer degradation by marine bacterial isolates: a proteogenomic and metabolomic characterization. Environmental Science & Technology. 54 (4), 2244-2256 (2020).
- He, L., et al. Contamination and remediation of phthalic acid esters in agricultural soils in China: a review. Agronomy for Sustainable Development. 35 (2), 519-534 (2015).
- Koch, K. E. Carbohydrate-modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 47, 509-540 (1996).
- Wang, Y. T., et al. Nontargeted metabolomic analysis to unravel the impact of di (2-ethylhexyl) phthalate stress on root exudates of alfalfa (Medicago sativa). The Science of the Total Environment. 646, 212-219 (2019).
- Yu, Q., et al. Photolysis of bis(2-ethylhexyl) phthalate in aqueous solutions at the presence of natural water photoreactive constituents under simulated sunlight irradiation. Environmental Science and Pollution Research International. 26 (26), 26797-26806 (2019).
- Zhang, S. H., Guo, A. J., Fan, T. T., Zhang, R., Niu, Y. J. Phthalates in residential and agricultural soils from an electronic waste-polluted region in South China: distribution, compositional profile and sources. Environmental Science and Pollution Research. 26 (12), 12227-12236 (2019).
- Liu, S. S., et al. Di-(2-ethylhexyl) phthalate as a chemical indicator for phthalic acid esters: An investigation into phthalic acid esters in cultivated fields and e-waste dismantling sites. Environmental Toxicology and Chemistry. 38 (5), 1132-1141 (2019).
- Fan, T. W., Lane, A. N., Pedler, J., Crowley, D., Higashi, R. M. Comprehensive analysis of organic ligands in whole root exudates using nuclear magnetic resonance and gas chromatography-mass spectrometry. Analytical Biochemistry. 251 (1), 57-68 (1997).
- Bobille, H., et al. Evolution of the amino acid fingerprint in the unsterilized rhizosphere of a legume in relation to plant maturity. Soil Biology and Biochemistry. 101, 226-236 (2016).
- Zhang, Z., et al. Effects of two root-secreted phenolic compounds from a subalpine coniferous species on soil enzyme activity and microbial biomass. Chemistry and Ecology. 31 (7), 636-649 (2015).
- Yuan, J., et al. Organic acids from root exudates of banana help root colonization of PGPR strain Bacillus amyloliquefaciens NJN-6. Scientific Reports. 5, 13438 (2015).
- van Dam, N. M., Bouwmeester, H. J. Metabolomics in the rhizosphere: tapping into belowground chemical communication. Trends in Plant Science. 21 (3), 256-265 (2016).
- Shen, X., Yang, F., Xiao, C., Zhou, Y. Increased contribution of root exudates to soil carbon input during grassland degradation. Soil Biology & Biochemistry. 146, 107817 (2020).
- Oburger, E., Jones, D. L. Sampling root exudates-mission impossible. Rhizosphere. 6, 116-133 (2018).
- Zhang, L. Effects of root exudates of wheat stressed by Cd on the germination of crop seeds. International Symposium on Water Resources and the Urban Environment. , 319-321 (2003).
- Shinano, T., et al. Metabolomic analysis of night-released soybean root exudates under high- and low-K conditions. Plant and Soil. 456, 259-276 (2020).
- Adeleke, R., Nwangburuka, C., Oboirien, B. Origins, roles and fate of organic acids in soils: A review. South African Journal of Botany. 108, 393-406 (2017).
- Rico, C. M., et al. Cerium oxide nanoparticles modify the antioxidative stress enzyme activities and macromolecule composition in rice seedlings. Environmental Science & Technology. 47 (24), 14110-14118 (2013).
- Mortimer, M., Kasemets, K., Vodovnik, M., Marinsek-Logar, R., Kahru, A. Exposure to CuO nanoparticles changes the fatty acid composition of protozoa Tetrahymena thermophila. Environmental Science & Technology. 45 (15), 6617-6624 (2011).
- Liao, Q. H., et al. Root exudates enhance the PAH degradation and degrading gene abundance in soils. Science of the Total Environment. 764, 144436 (2021).
- Ding, Y., et al. Adaptive defence and sensing responses of host plant roots to fungal pathogen attack revealed by transcriptome and metabolome analyses. Plant, Cell & Environment. 44 (12), 3526-3544 (2021).
- Rugova, A., Puschenreiter, M., Koellensperger, G., Hann, S. Elucidating rhizosphere processes by mass spectrometry-A review. Analytica Chimica Acta. 956, 1-13 (2017).

