One-step CRISPR-based Strategy for Endogenous Gene Tagging in Drosophila melanogaster
Summary
Here, we present a simplified endogenous gene tagging protocol for Drosophila, which utilizes a PCR-based technique for marker-free identification of successful genetic modifications, facilitating the development of stable knock-in lines.
Abstract
The study of protein subcellular localization, dynamics, and regulation in live cells has been profoundly transformed by the advent of techniques that allow the tagging of endogenous genes to produce fluorescent fusion proteins. These methods enable researchers to visualize protein behavior in real time, providing valuable insights into their functions and interactions within the cellular environment. Many current gene tagging studies employ a two-step process where visible markers, such as eye color changes, are used to identify genetically modified organisms in the first step, and the visible marker is excised in the second step. Here, we present a one-step protocol to perform precise and rapid endogenous gene tagging in Drosophila melanogaster, which enables screening for engineered lines without the visible eye marker, offering a significant advantage over past methods. To screen for successful gene-tagging events, we employ a PCR-based technique to genotype individual flies by analyzing a small segment from their middle leg. Flies that pass the screening criteria are then used to produce stable stocks. Here, we detail the design and construction of CRISPR editing plasmids and methods for screening and confirmation of engineered lines. Together, this protocol improves the efficiency of endogenous gene tagging in Drosophila significantly and enables studies of cellular processes in vivo.
Introduction
Fluorescent proteins have emerged as powerful tools for visualizing protein localization, dynamics, and protein-protein interactions in cells within living organisms1,2. Tagging endogenous genes with fluorescent proteins enables long-term live imaging of cellular processes with subcellular resolution. Furthermore, these endogenous gene-labeling techniques have several advantages compared to overexpression and immunostaining approaches. For example, overexpression of proteins might lead to protein misfolding, nonspecific protein-protein interactions, and mislocalization3. To date, researchers have been able to create vast libraries of endogenous genes fused to fluorescent proteins for a few model organisms, such as budding yeast, which can undergo efficient homologous recombination4. In the case of Drosophila melanogaster, various tools such as MiMICs5, transposon-based enhancer traps6, and fosmids7 have been devised to fluorescently tag genes.
The development of CRISPR-Cas9-based tools has made it possible to efficiently perform genome editing, including tagging endogenous proteins, in a wide range of model organism8,9. Cas9 is an RNA-guided enzyme that cleaves double-stranded DNA in a highly efficient and specific manner8, which can then be repaired by a nonhomologous end-joining (NHEJ) pathway leading to point mutations or deletions. Alternatively, the homology-directed repair (HDR) pathway allows the integration of heterologous DNA into the chromosome.
Past methods for CRISPR-based gene tagging in the model organism, Drosophila melanogaster, have relied on a two-step process10,11,12. This involves using a discernible eye-color marker, Dsred, to detect successful HDR events. Afterward, the Dsred marker would be excised using the Cre/loxP recombination system. To streamline and expedite this process, here, we present a simpler and more efficient method. This approach circumvents the need for lethal genotyping and allows for the direct screening of individual flies. Specifically, we employ a PCR-based approach to extract DNA from a small segment of the fly's middle leg, allowing us to genotype individual flies. Notably, this procedure does not compromise the vitality, movement, or reproductive capabilities of the sampled fly. Only the appropriate individuals, identified through this method, are further developed into stable stocks.
Here, we present a detailed protocol for generating fluorescent protein tagged flies in which endogenous genes are labeled with fluorescent reporters. This technique uses CRISPR-Cas9 genome editing to fuse any desired fluorophore tag to the N- or C- terminal of an endogenous gene. Below, we describe the design and construction of gRNA plasmids and a donor plasmid, one-step screening strategy to select CRISPR-positive flies, and steps to establish stable lines. Specifically, we describe strategies to tag an endogenous core clock Drosophila gene, period, with a fluorophore at the C-terminal. We also briefly describe the control experiments that need to be performed to confirm that the tagged protein is functional and live-imaging techniques to visualize the Period protein in live clock neurons within intact Drosophila brains13. The protocol described here is also broadly applicable to screen candidates for deletions and insertions of specific bits of DNA by CRISPR-Cas9 genome editing.
Protocol
1. Design and construction of gene editing reagents
NOTE: To carry out endogenous tagging, it is essential to identify the various isoforms of the desired gene. Depending on the specific goals of the experiment, a fluorescent tag can be incorporated either internally or at the N- or C-termini of the selected protein isoform(s). For optimal CRISPR-mediated editing, it's crucial to position the two sgRNAs suitably close to the intended knock-in site. Subsequently, to enable editing through homologous recombination, a donor vector encompassing sequences homologous to the target gene, along with the fluorescent tag, should be prepared. Below is a step-by-step guide to these processes (Figure 1).
- Design sgRNA as described below.
- Design sgRNAs for Drosophila using these websites: http://tools.flycrispr.molbio.wisc.edu/targetFinder/ or http://www.flyrnai.org/crispr2/.
- For the flycrispr website, enter the sequence ±100 bp surrounding the desired knock-in location(N terminal, C terminal, or any genomic locus of interest). If using the nos-Cas9 attP2 as the injection background, choose the genome option Drosophila melanogaster (nos-Cas9 III, BDSC #78782). The Nos-Cas9 system ensures germline-specific Cas9 expression.
- To improve sgRNA expression efficiency, select the CRISPR targets with 5' G option, as sgRNAs that commence with a guanine will enhance editing activity. Then, click the Find CRISPR Targets button. A list of possible sgRNA sequences will be displayed.
- For each sequence, click the Evaluate button. This action checks for any potential off targets for each sequence based on the previously selected genome. From the provided list, choose 2 sgRNAs that have 0 off targets. Ensure one sgRNA is upstream and the other is downstream relative to the knock-in location. Ideally, their positions should be as near to the knock-in site as possible (<200 bp), but they must not overlap with one another (Figure 2).
NOTE: It might sometimes be difficult to find appropriate sgRNAs for a given target gene. The gap between sgRNAs and the knock-in location can be increased to ± 200 bp. Using sgRNAs not initiating with G might slightly decrease the positive rate.
- Perform fluorescent tag selection as described below.
- Fluorescent tags offer a diverse range of characteristics, each having their unique advantages and drawbacks1,2. When determining the ideal fluorescent tag for labeling a specific protein, take care of the key considerations described in the following steps that come into play.
- Given that the natural protein expression levels are typically much lower than those seen in overexpression experiments1,2, start with the tags that are bright and photostable and that fit the experimental setup. Some fluorescent tags, specifically certain RFPs like tdTomato and DsRed, tend to form multimers14 and may lead to protein aggregation.
- For enhanced versatility, link epitope tags (e.g., FLAG or V5) to the fluorescent marker using a flexible linker, which allows for biochemical analyses such as Western Blot or Co-Immunoprecipitation.
- For linker design, follow the steps described below.
- For optimal functionality, ensure that both the endogenous protein of interest and the fluorescent tag fold separately into their respective functional forms. To achieve this, use a linker peptide ranging from 2-20 amino acids in length. For example, place two glycine residues between the last amino acid of the Period protein and the first amino acid of the knock-in fluorescent protein mNeonGreen.
- Ensure that these linker peptide sequences comprise amino acids like glycine and serine, which impart flexibility to the structure15. This ensures that the natural behavior and location of the endogenous protein remain unaltered.
- Design donor vector as described below.
NOTE: The donor vector introduces the fluorescent tag to the desired endogenous location through the process of homology-directed repair, which is initiated by CRISPR cleavage. As such, besides the fluorescent tag, the donor vector must possess sequences that are homologous on both sides of the tag.- Begin the donor vector design by organizing the DNA sequence of the linker peptide and the fluorescent tag and ensure there are homologous sequences flanking both ends (homology arms). For optimal results, ensure the homology arms encompass at least 800 nucleotides on each side of the intended knock-in site (Figure 2).
NOTE: Recent reports suggest that homology arms can be shorter (100-200 bp)16. - Ensure that any PAM sequences within the donor plasmid are modified in such a way that the resulting protein's amino acid sequence remains unchanged. If the PAM sequence is located in the UTR, remove it entirely.
- The positioning of START and STOP codon depends on the location of the tag. For internal tagging, ensure the fluorescent tag is in frame with the coding sequence of the target gene. For N-terminus tagging, position the fluorescent tag before the endogenous start codon. For C-terminus tagging, situate the fluorescent tag before the natural stop codon and make sure to retain only a single start codon to avoid potential translation issues.
- Begin the donor vector design by organizing the DNA sequence of the linker peptide and the fluorescent tag and ensure there are homologous sequences flanking both ends (homology arms). For optimal results, ensure the homology arms encompass at least 800 nucleotides on each side of the intended knock-in site (Figure 2).
- Synthesize sgRNA and donor plasmids as described below.
- For sgRNA plasmid creation, follow the U6-gRNA cloning guidelines, which can be found at flycrispr.org/protocols/grna/. Employ the alternative restriction enzyme-based cloning approach (referred to as strategy C in the provided resource) to insert gRNA sequence into the pU6-BbsI-chiRNA plasmid (Addgene, #45946).
- Transform in DH5a E. coli (NEB, C2987H) following standard transformation protocols provided by the manufacturer. For verification, conduct Sanger sequencing on the resulting clones using T3 or T7 standard sequencing primers.
- For the creation of donor plasmid, acquire double-stranded DNA with the desired donor sequence from commercial suppliers (e.g., IDT). Integrate it into the pBlueScriptII SK(-) multiple cloning site. Sequence the donor plasmid to ensure sgRNA or PAM sequences are correctly modified and there are no SNPs present in the plasmid.
NOTE: An alternative to the integration using pBlueScriptII is to PCR the homology arms and the fluorescent tag segments separately and consolidate them into the backbone using techniques like Golden Gate Assembly or Gibson Assembly.
- Use the following injection and crossing scheme.
- To acquire germline-edited flies, co-inject gRNA plasmids and the donor plasmid into the appropriate Cas9 background (e.g., nos-Cas9 III, BDSC 78782). If utilizing commercial injection services, follow instructions from the vendor to perform plasmid preparation.
NOTE: Typically, the gRNA plasmid concentration should exceed 0.5 µg/µL, and the donor plasmid concentration should be above 1.0 µg/µL. A common timeline from sending out plasmid samples to receiving injected embryos is approximately 2 weeks. A strategy to screen for gene editing events on the X chromosome employing the FM7/L balancer strain is presented here. For genes on the second or third chromosome, employ the appropriate balancer strains. - For the collection of injected flies (G0), anesthetize with carbon dioxide and separate male flies and virgin females. Establish fly crosses by pairing each G0 male with at least two FM7/L virgin females in individual narrow CT food vials. Place crossed vials in a vented incubator maintained at 25°C and ~60% humidity until F1 flies eclose (FM7/L is the most commonly used X chromosome balancer). Similarly, pair each G0 female with FM7/Y male flies.
- Anesthetize and collect at least 10 virgin female offspring (F1) from both G0 male and female crosses. From G0 female crosses, gather at least 10 male F1 flies (Figure 3A). Ensure each F1 fly is tagged with the details of its parentage and stored separately. Proceed to the screening step.
- To acquire germline-edited flies, co-inject gRNA plasmids and the donor plasmid into the appropriate Cas9 background (e.g., nos-Cas9 III, BDSC 78782). If utilizing commercial injection services, follow instructions from the vendor to perform plasmid preparation.
- Use the following single-leg PCR protocol.
- Primer creation: Design a primer pair encompassing the editing site of the target gene. Ideally, the melting temperatures of the primers should be similar, around 55°C.
- Lysis buffer preparation: Prepare 10 µL of lysis buffer for every fly sample. The buffer consists of 10 mM Tris (pH 8.2), 1 mM EDTA (pH 8.0), 25 mM NaCl, and 400 µg/mL Proteinase K. Prepare the lysis buffer as a master mix, then distribute 10 µL into each well of a 96-well PCR plate. Ensure the buffer stays cold throughout the leg dissection process (Figure 3B).
- Tube setup: Prepare glass capillary tubes, filling one end with sucrose-agar food (2% agar and 4% sucrose), and place these in a 96-well deep-well plate, which will be referred to as the fly hotel (Figure 3C).
- Leg dissection: Place the carbon dioxide anesthetization pad under a stereo microscope, anesthetize one candidate fly and sever one of the middle legs. Place the leg into the lysis buffer, ensuring complete immersion. It is not necessary to macerate the leg, directly proceed to the lysis protocol step (Figure 3B). Hold the fly wing with a pair of fine metal forceps, move the fly to the fly hotel, and seal the tube with yarn immediately.
- Control samples: Two wild-type flies may be used as negative controls following the same leg dissection procedure. A diluted donor plasmid serves as the positive control.
- Post-dissection care: House the fly hotel in a vented incubator maintained at 25 °C and ~60% humidity. While flies can survive overnight, it's recommended to complete subsequent steps and mating within the same day.
- Lysis protocol: Place the severed legs in the lysis buffer solution in a thermocycler set at 37 °C for 90 min, followed by 95 °C for 5 min, finally held at 4 °C. This will extract sufficient DNA from the single leg of each fly (Figure 3B).
- PCR reaction: Organize the PCR reaction as instructed by the manufacturer, utilizing 1.5 µL of the fly leg lysate as the DNA source.
- Gel electrophoresis: Subject the PCR products to agarose gel electrophoresis. Interpret the results from the gel based on band size. Typically, negative control flies will exhibit a lower band, and positive plasmid controls display a higher band. Ensure specific band sizes are consistent with the original donor plasmid design. Female positive flies will display two bands as they are heterozygous, and male positives will present a single band (Figure 3B).
NOTE: Usually, screening of 70 flies is achievable in a single day. Ensure to identify a minimum of 10 positive F1 flies across 4 to 5 distinct G0 crosses, as certain F1s might be sterile due to off-target mutations.
- Carry out the following post-PCR cross set-up.
- Based on the gel results, select the positive flies from the fly hotel and individually cross them with balancer flies (FM7/L for tagging X chromosome genes) to produce the F2 generation. Once the F2 generation emerges, establish sibling crosses to create homozygous stable stocks (Figure 3D).
- Use homozygous stocks for Sanger sequencing of the editing site to ensure accuracy. Backcross validated fly lines with wild-type flies to remove background mutations. Screen each generation using the single-leg PCR protocol.
Representative Results
In our experiments, we typically found a success rate of ~20%-30% in screening for various endogenous tagged genes on all chromosomes (X, II, III). The efficiency of this process can vary based on several factors, such as gRNA selection, the nature of the gene, and injection quality. Here, we illustrate the results from our strategy to tag the endogenous period gene with the mNeonGreen fluorophore13. The period gene, positioned on the X chromosome of D. melanogaster, plays a pivotal role in the circadian clock. Drosophila has a well characterized clock network consisting of approximately 150 clock neurons that express clock proteins17.
We tagged the Period protein with the green fluorescent protein mNeonGreen on its C-terminal end, connected by a dual-glycine linker13. Post-plasmid injection, we crossed the G0 flies with FM7/L balancer and subsequently harvested the F1 generation. Out of 126 F1 flies screened using specialized PCR primers (Table 1), we identified 47 positive cases, yielding a 37.3% success rate. From these, we selected 5 F1 individuals to set up additional crosses to produce stable stocks. Gender did not impact the positive outcome rate during this screening. After verifying the sequence accuracy of the tagged gene, we executed control tests, including behavioral assessments and qPCR evaluations to ensure mRNA oscillations to confirm the functionality of the tagged protein (PERIOD-mNeonGreen)13.
To perform live-imaging experiments, we crossed these PERIOD-mNeonGreen flies with Clock856-GAL4;UAS-CD4-tdTomato flies, where Clock856-GAL4 labels all clock neurons19 and CD4 is a transmembrane protein that labels cell membranes. For live-imaging experiments, we dissected 3-4 adult brains using ice-cold Schneider's Drosophila medium. The brains were mounted and imaged using an Airyscan confocal microscope. Figure 4 shows the representative images displaying the PERIOD protein within clock neurons in Drosophila brains.
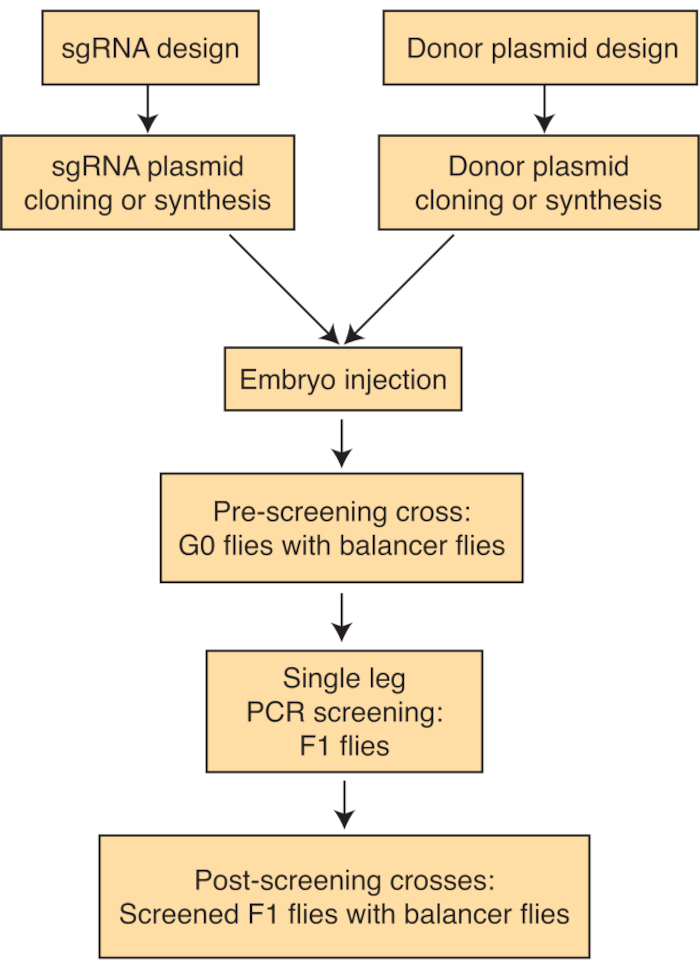
Figure 1: Schematic of the CRISPR/Cas9-based gene editing protocol. The protocol outlines a series of steps to design and assemble sgRNA and donor plasmids, followed by screening through the single-leg PCR method and production of stable engineered lines. Please click here to view a larger version of this figure.
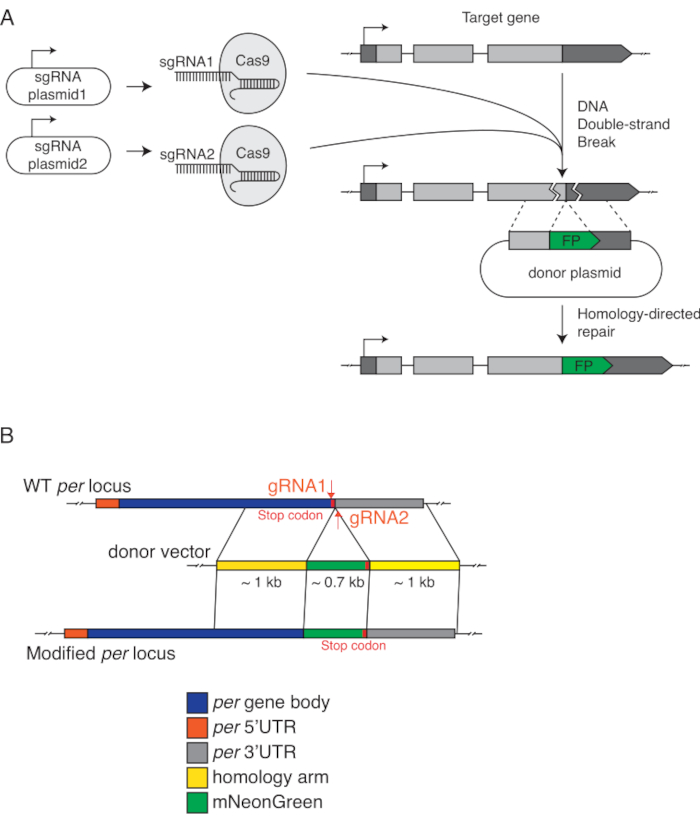
Figure 2: Schematic of CRISPR/Cas9 genome editing to generate period-mNeonGreen flies. (A) Schema illustrating the addition of a fluorescent protein tag to the C-terminal of an endogenous gene. (B) The endogenous period (per) gene is tagged with mNeonGreen, a bright, monomeric green fluorescent protein at the carboxyl terminus. Two sgRNAs are in the last exon and the 3'UTR, respectively. The donor vector contains two ~1 kb homology sequences flanking the mNeonGreen tag. Stop codon is added to the end of the tag for proper translation termination. Please click here to view a larger version of this figure.
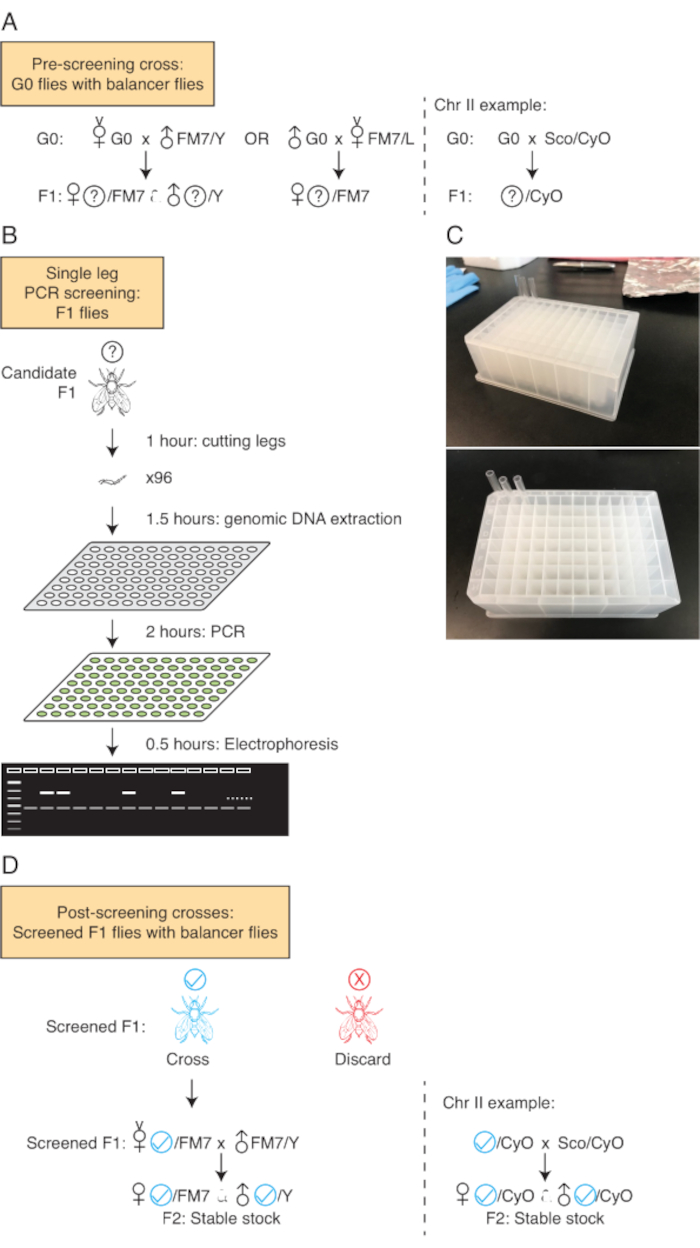
Figure 3: Strategy to screen for CRISPR-positive flies. (A) Approach to set up pre-screening genetic crosses for genes intended for CRISPR tagging on X and II chromosomes. G0 denotes the initially injected flies, while F1 represents their first-generation offspring that require screening. (B) The single-leg PCR screening method and the duration for each procedure step are outlined. (C) Shown here is a picture of the Fly Hotel. Glass tubes are filled with fly food and sealed at one end. Single flies are loaded into individual tubes. After the DNA extracted from the middle led of individual flies is tested, the appropriate flies are taken from the fly hotel and the stable lines are established. (D) Approach to set up post-screening crosses of flies (shown here in blue) which passed the screening test. Please click here to view a larger version of this figure.
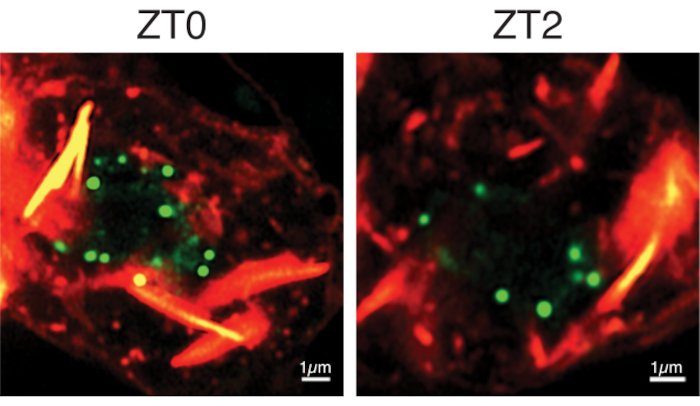
Figure 4: Representative images of PERIOD-mNeonGreen protein in clock neurons. Representative images from period-mNeonGreen, Clock-GAL4, UAS-CD4-tdTomato flies entrained to Light-Dark cycles. ZT refers to Zeitgeber Time and ZT0 refers to time of lights on and ZT12 refers to time of lights off. Clock neuron's nuclear envelope is labeled with tdTomato, shown here in red, and PERIOD protein is tagged with mNeonGreen and shown in green. PERIOD protein is organized into discrete nuclear foci throughout the late night and early morning timepoints. Scale bars, 1 μm. Please click here to view a larger version of this figure.
| Name | DNA sequence(5' to 3') |
| Period screening primer forward | ATACTCGTCCATAGACCACG |
| Period screening primer reverse | ACATTATCCTCGGCTTGCAT |
| per-gRNA1-sense | cttcACCAGACACAGCACGGGGAT |
| per-gRNA1-antisense | aaacATCCCCGTGCTGTGTCTGGT |
| per-gRNA2-sense | cttcGTCAGCAGCAACTGCGGGTG |
| per-gRNA2-antisense | aaacCACCCGCAGTTGCTGCTGAC |
| period homology arms and mNeonGreen insert | GAATTCCAAGCTGCTCATCCTGCCCATTGTCAGCAA GGGCGACCTGACCATTCGGTTGAACGATGTGCACA CAAAGGTTTGGATTACCGCCGAGCCAGTGAAGCGC TCCGATGGCCACACCTATCTCAACATCACCGACTAC AAGACGGCCACCAAGATCAAGGGgtgagcatggcctgcat gtctagccaactgaatggtgacctcaaacctcttctcgtagTGGCCACT TTGACCTGTCGAATCTGTTCAACGACAACAAGGAGC GCGCGACAGCACGCTGAAGGTGCTGAACCAGGAGT GGAGCACCCTGGCCCTCGATGTCCAGCCGAAGATC AACGAGGCCTGCGCCAAGGCCTTCAGTGCCATCGT ACAGAGTCTGTGGGCCAACATTCCCTACGACGAGTT CTTCGAAAAGGAATGAACGCATATGTATCTAACAAAGT CCGGTCTAACTAGCCACTCTAGCTAATTACTGATTAAA CTACCTAATTGCAGCTAAATCCAATCCATCTTCGATTAA AACAACTACTACAAGTGTTGGCGTTGGCTTTTCGATAT TTATTGTACAATAAATAACTAAAAAAGATTCGGATATATA AACCTTAGGGCTGAGAAGGGTGGTTCGATGTTCGAAC CCTCTCTAGTTTTCAATTCACTTAATATTCTGATTAAACAT AGGATAGATATCATCTAAAAGCTTTGCTTGGCTTGAGAT CTACATTATCCTCGGCTTGCATGGGTTCTGGGCATCCTT CCACGTCAGTTCGTCTGATGCTTTCGTTACTTGTTCGGA TACTGAATGGTGACATCCCACGGAAGCGTTCGCGTTGAT TCGAAGAActtgaagggaatggaagggggagttaggaataggaactggtgg gactggctggtactcggtgcccagaccggaggcaattgctcacTCGTTTCCA GGACCCTGCTGCTCCTCGGTGTCTGCTGCTCGGATCATC CCAGGGATCGACATTGTGCACTCGGTTGTGTACGTCGGT CAGCAGCAACTGCGGGTGTCATTACTTGTACAGTTCGT CCATACCCATGACATCGGTGAAAGCTTTTTGCCATTCTT TGAAATTCAGCTCTGTTTTCGAGTGCTTCAGTTCGGTTT TTCGAAAGACATACATCGGTTGGTTCTTGAGATAGTTAG CCGCCATCGGCTTGGCAAAAGTGTACGTTGTTCGCGCT GTGGAGCGATACCTTTTTCCATTTCCTGTTGTATAGGAC CATTTGAAAGTGGAAATAATAGTCTTATCATTGGGGTAG GTCTTTTTGCTGCGGCACCAGTCAGCGGCAGTGAGC GAATTTGTCATAACTGGGCCATCAGCGGGGAAACCCG TTCCCTTGACTTGGGCTTCGCCTTTAATATGGCTACCT TCGTAAGTGTACCGATAGTTCACCGTGAGCGAGGCTC CGTCTTCAAATTGCATTGTTCGATGCACCTGGTAGCCC GAGCCATCTACCATCGCGGCTTGGAAGGGCGACATGC CATCTGGATACGGCAAATACTGATGGAATCCGTAACCG ATGTGTGGAACCAGGATCCAAGGGGAAAATTGGAGAT CGCCTTTAGTCGATTTCAGATTGAGCTCTTCGTAGCC GTCGTTGGGGTTTCCCGTACCCTGTCCGACCATATC GAAGTCGACTCCATTGATCGAACCAAAGATATGCAG CTCGTGTGTAGCCGGCAACGAAGCCATATTATCTTC TTCACCCTTCGACACACCTCCGCCGTCGCCGTGCT GTGTCTGGTCCTCCTCCGGGTGCTCCATGATCTTGC TCTCAGATGTGCTCATGCTctgcaaaagaacggaaatggatta cattgaatcgcatcgtggactgaactgtacgtacCTTCAGCTTTCGG TGCTTGGGGTCCTTTTCGGTGTCCGGCGGACTCTC CGATCCGTCCGTGGTCTTGATGAAGGACGAGTAGA AGGAGGAGAAGCTGGAGCCATCCATGTCGTCGCTA TTCCCATTGCTGTCCGTGTATTTctgtggatgagaccatgttc ttcataatgaccaatcaccaatggacctcattcgtatagcatacCTTGTT GTTGGCGGGATTGCTGCTGCTGCAGGGCGGGTCG GAGTTGTAGTCGCCCATCACGGAGGGAATGGGCGA GGAGTCCGGCACCTCCTTCTTGCAGGGATCGGATA CCGCTGCACTGGAGCCCGGCTCCGTCTTGACGGA TGCGCTCTGCGAGGAGGGACGCTGCACCTGGGC AGGAGTGGTGACCGAGTGGAATGCACCCGGCACC TTCTTCGTCATGGACGCCGGCGTGGTctatggacgagta tggagttggagttggagttaagacagttcggtgaggcaccaccggccactg acttacCGTGTACACCGACTTGTTGTACGCGGATTGGG AGCCCAACGGACGTTCGGGAATCTGGAGAGCGTTG GCCATTCCCGGAAAGGGCATCGGCTGGTACATCAT GGCCGTGGCCGCGGCCGCCGCCGGGTGTGTGTAG AAAAGCGAAGGATGCGGGTACATCACGCCGGCCAT GTACTGCAGCGGCATGGCCTGGGCGGCCGCGGCA GCCGCCTGCTGCGAGGTGGTGGGCATGTCCGTGC CACCCTTGTGCGGATGCTTGTGCATCCGGGGAGAG CGCGTGGGACTGGTGGGCGTCAAGGAGGCGGGGA TGTAGTAGAAGGTCGGGAAGAGGCCGGCGGAGGA GAAGCTGCTCTGGGCCATGGCCGTGTGCGTGGAG TGAACGGGCGGTGTGATGCCCACCGAGAACGGTG GCCAGAGGTTTATGTTCTAGA |
Table 1: List of sgRNA sequences to tag endogenous Period gene at the C terminal and primers used to perform single-leg PCR screening.
Discussion
The results demonstrate a streamlined, simple one-step CRISPR-based strategy for tagging endogenous genes in Drosophila. In the protocol, we use two gRNAs to induce DNA double-strand break and homologous recombination more efficiently. The gRNAs and the donor plasmid are injected into fruit fly embryos, which express Cas9 enzyme in their germline. These embryos are subsequently cultivated under standard conditions until adulthood, at which point they are crossed with balancer flies to produce the first generation (F1) offspring. PCR genotyping is performed on the middle leg of each F1 specimen to screen for genetically modified flies. This method provides a significant advantage over the current screening methods which use two steps of screening, where the first step relies on using a visible marker such as eye color to identify the appropriate genotype and the second step involves removal of the eye color marker10. Here, we present a method employing PCR on DNA extracted from a small segment of the middle leg of an individual fly allowing non-lethal genotyping of individual flies, offering a more efficient and cost-effective alternative to existing methods. This method allows screening of candidate animals one by one or in batches until the appropriate genotype is isolated. The number of animals screened before appropriate stocks are isolated varies from experiment to experiment, depending on many factors including efficiency of sgRNAs selected, efficiency of injection, off-target insertions which can affect viability. Alternatively, DNA from single wings from individual flies can also be used for genotyping purposes18.
It is possible the addition of a tag to the N- or C-terminal of a gene can adversely affect the function of the gene, which can potentially affect the viability of the flies. If protein structure information is available, it can be used to determine whether N- or C-terminal tagging may better preserve function and localization of the native protein. It is always recommended to perform control experiments, including testing whether the tagged protein shows similar localization pattern as the wildtype protein by antibody staining methods. The same screening strategy can be used to screen other kinds of CRISPR-based genetic insertions or deletions. In conclusion, the one-step screening strategy makes it much easier to generate CRISPR-edited flies and opens many possibilities to study the subcellular localization and dynamics of endogenous genes in their native context.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank George Watase and Josie Clowney for discussions during the initial stages of the protocol development. The work was supported by funds from the NIH (grant no. R35GM133737 to S.Y.), Alfred P. Sloan Fellowship (to S. Y.) and McKnight Scholar Award (to S. Y.).
Materials
| 0.5M EDTA pH8.0 | Invitrogen | AM9260G | |
| 5-alpha Competent E. coli (High Efficiency) | NEB | C2987H | |
| 96-well deep well plate | BRAND | 701354 | |
| Agar | Fisher Scientific | BP1423-500 | |
| BbsI-HF | NEB | R3539S | |
| DreamTaq Green PCR Master Mix (2X) | Thermo Scientific | K1081 | |
| D-Sucrose | Fisher Scientific | BP220-1 | |
| EcoRI-HF | NEB | R3101S | |
| Hydrochloric Acid | Fisher Scientific | A142-212 | |
| Prolong Glass Antifade Mounting Medium | Invitrogen | P36982 | |
| Proteinase K | QIAGEN | 19131 | |
| Schneider's Drosophila Medium | Gibco | 21720001 | |
| Sodium Chloride | Fisher Scientific | S271-500 | |
| T4 DNA ligase | NEB | M0202S | |
| Tris Base | Fisher Scientific | BP152-500 | |
| XbaI | NEB | R0145S |
References
- Chudakov, D. M., Matz, M. V., Lukyanov, S., Lukyanov, K. A. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol Rev. 90 (3), 1103-1163 (2010).
- Costantini, L. M., et al. A palette of fluorescent proteins optimized for diverse cellular environments. Nat Commun. 6, 7670 (2015).
- Gibson, T. J., Seiler, M., Veitia, R. A. The transience of transient overexpression. Nat Methods. 10 (8), 715-721 (2013).
- Huh, W. K., et al. Global analysis of protein localization in budding yeast. Nature. 425 (6959), 686-691 (2003).
- Venken, K. J., et al. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat Methods. 8 (9), 737-743 (2011).
- Quinones-Coello, A. T., et al. Exploring strategies for protein trapping in Drosophila. Génétique. 175 (3), 1089-1104 (2007).
- Sarov, M., et al. A genome-wide resource for the analysis of protein localisation in Drosophila. Elife. 5, e12068 (2016).
- Ran, F. A., et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 8, 2281-2308 (2013).
- Port, F., Chen, H. M., Lee, T., Bullock, S. L. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc Natl Acad Sci U S A. 111 (29), E2967-E2976 (2014).
- Gratz, S. J., et al. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Génétique. 196 (4), 961-971 (2014).
- Kanca, O., et al. An efficient CRISPR-based strategy to insert small and large fragments of DNA using short homology arms. Elife. 8, e51539 (2019).
- Lamb, A. M., Walker, E. A., Wittkopp, P. J. Tools and strategies for scarless allele replacement in Drosophila using CRISPR/Cas9. Fly (Austin). 11 (1), 53-64 (2017).
- Xiao, Y., Yuan, Y., Jimenez, M., Soni, N., Yadlapalli, S. Clock proteins regulate spatiotemporal organization of clock genes to control circadian rhythms. Proc Natl Acad Sci U S A. 118 (28), e2019756118 (2021).
- Costantini, L. M., Fossati, M., Francolini, M., Snapp, E. L. Assessing the tendency of fluorescent proteins to oligomerize under physiologic conditions. Traffic. 13 (5), 643-649 (2012).
- Snapp, E. L. Fluorescent proteins: a cell biologist’s user guide. Trends Cell Biol. 19 (11), 649-655 (2009).
- Kanca, O., et al. An expanded toolkit for Drosophila gene tagging using synthesized homology donor constructs for CRISPR-mediated homologous recombination. Elife. 11, e76077 (2022).
- Nitabach, M. N., Taghert, P. H. Organization of the Drosophila circadian control circuit. Curr Biol. 18 (2), R84-R93 (2008).
- Carvalho, G. B., Ja, W. W., Benzer, S. Non-lethal PCR genotyping of single Drosophila. Biotechniques. 46 (4), 312-314 (2009).
- Gummadova, J. O., Coutts, G. A., Glossop, N. R. J. Analysis of the Drosophila Clock promoter reveals heterogeneity in expression between subgroups of central oscillator cells and identifies a novel enhancer region. J Biol Rhythms. 24 (5), 353-367 (2009).

