High-Throughput Protein Crystallization via Microdialysis
Summary
The presented protocol describes a straightforward approach for screening protein crystallization conditions and crystal growth using a 96-well high-throughput dialysis plate. The use of dialyzer tubes for the large-scale growth of microcrystals is also demonstrated for serial crystallography and MicroED applications.
Abstract
Understanding the structure-function relationships of macromolecules, such as proteins, at the molecular level is vital for biomedicine and modern drug discovery. To date, X-ray crystallography remains the most successful method for solving three-dimensional protein structures at atomic resolution. With recent advances in serial crystallography, either using X-ray free electron lasers (XFELs) or synchrotron light sources, protein crystallography has progressed to the next frontier, where the ability to acquire time-resolved data provides important mechanistic insights into the behavior of biological molecules at room temperature. This protocol describes a straightforward high-throughput (HTP) workflow for screening crystallization conditions through the use of a 96-well dialysis plate. These plates follow the Society for Biomolecular Screening (SBS) standard and can be easily set up using any standard crystallization laboratory. Once optimal conditions are identified, large quantities of crystals (hundreds of microcrystals) can be produced using the dialyzer. To validate the robustness and versatility of this approach, four different proteins were crystallized, including two membrane proteins.
Introduction
Over the last century, X-ray crystallography has been critical in elucidating and understanding the structure-function paradigm of biological macromolecules. To date, it continues to be one of the most successful methods in elucidating atomic resolution structures of many uniquely different proteins that are crucial to the fundamental understanding of cell biochemistry, medicine, and early drug discovery1,2. However, protein crystallization remains a bottleneck in studying many protein targets, particularly membrane proteins and large protein complexes3. Consequently, protein crystallization is almost always considered an art due to the labor-intensive trial-and-error approaches employed4,5,6.
A precipitating agent is usually added to a protein solution at high concentration to form a well-ordered, regular, and repeating lattice arrangement of protein molecules, known as crystals. Under favorable conditions, such as temperature, pH, concentration, and precipitant agent, a supersaturated solution eventually forms, followed by crystal nucleation and growth7,8. Although there have been many advances in crystallization trial setups, predominantly with the development of high-throughput robotic systems and the availability of ready-made "sparse matrix" screens, the general approaches to protein crystallization have largely remained unchanged over the years. Common experimental protein crystallization techniques include vapor diffusion (hanging drop and sitting drop)9, microbatch (under oil)10,11, free-interface diffusion (microfluidic devices)12, and dialysis (using buttons and other techniques)13,14,15. However, other more specialized setups also exist, such as mesophase approaches for crystallizing membrane proteins16,17. While the majority of X-ray protein structures deposited in the Protein Data Bank have so far been solved through crystallization by vapor diffusion methods6,18,other approaches, such as crystallization by dialysis, seem to be underutilized, likely due to the practical aspects related to their experimental setup.
Crystallization by dialysis simply relies on the slow diffusion of solutes (precipitants, ions, additives, and buffers) through a semi-permeable membrane that simultaneously prevents protein molecules from circulating. This way, the protein solution is slowly brought into equilibrium, with the precipitant reaching the necessary concentration to crystallize. The system's kinetics depends on temperature, precipitant concentration, and the cellulose membrane molecular weight cut-off (MWCO)19. To date, the most popular crystallization setup by dialysis has been using microdialysis buttons made of transparent acrylic sheets. These are usually immersed in reservoirs (mostly using vapor diffusion hanging drop plates) containing the crystallization precipitant solutions. However, this lower-throughput method also requires specific assembly to seal the protein solution within the dialysis membrane placed over the button chamber, as illustrated in Figure 1. Moreover, air bubbles trapped between the dialysis membrane and the protein solution are a frequent problem that impair crystal growth. Another constraint of the method is the sample requirements, whereby much higher concentrations and volumes are necessary compared to vapor diffusion methods, to accommodate the dialysis buttons. Therefore, crystallization using microdialysis buttons has been perceived as an unappealing method, especially for difficult targets such as membrane proteins, whose purification yields are frustratingly low. Recently, microfluidic devices have been developed to facilitate protein crystallization by dialysis15. These chips have also been designed to have high X-ray transparency with low background, allowing the chips to be used for in situ data collection at room temperature, thus eliminating the inconvenience of harvesting and cryocooling crystals. Despite these advances, the approach is still very low-throughput and expensive.
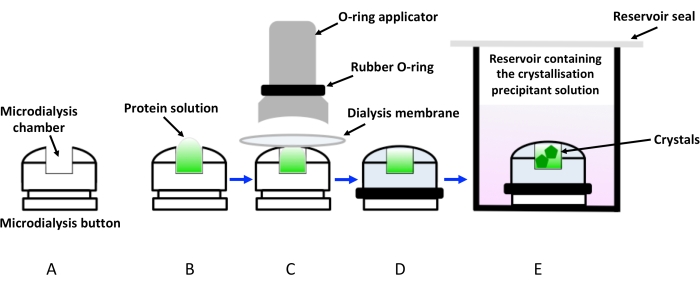
Figure 1: Schematic representation of crystallization by dialysis using dialysis buttons. (A) Schematic representation of a crystallization dialysis button. (B) The protein solution is added to the microdialysis button chamber. (C) The dialysis membrane is held to the microdialysis button with the help of a rubber ring (O-ring) applied via an applicator. (D) The dialysis button is ready to be immersed in the reservoir containing the crystallization solution (dialysis solution), as shown in (E). The vial containing the immersed dialysis button must be sealed to avoid evaporation. Please click here to view a larger version of this figure.
Here, a straightforward protocol is presented for screening protein crystallization conditions and crystal growth using the 96-well high-throughput dialysis plate. These disposable plates are designed to be used similarly to the vapor diffusion crystallization plates (pipette then seal), as shown in Figure 2. The plates can accommodate up to 3.2 µL of protein and 350 µL of dialysis solution. Each well features a separate regenerated cellulose membrane to prevent cross-contamination between the wells. The setup takes around 10 min to complete and does not require any specialized equipment besides what can be found in all standard crystallization laboratories. Four different proteins, including two membrane proteins, are used to demonstrate and validate this approach as an effective method for high-throughput (HTP) protein crystallography.
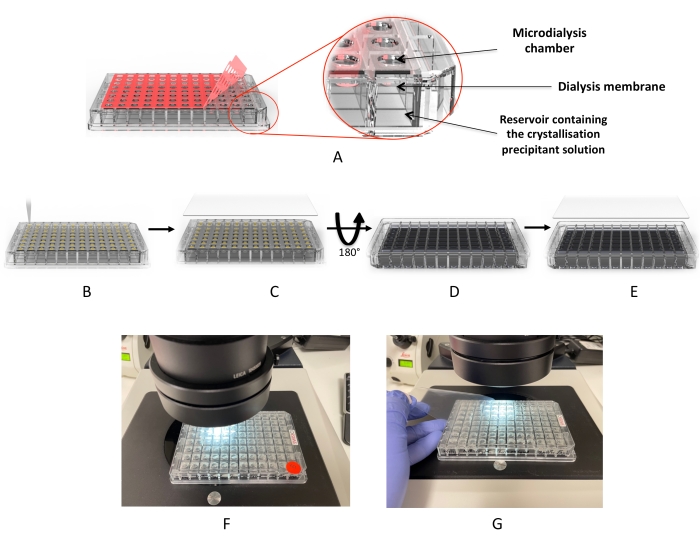
Figure 2: Crystallization workflow using the microdialysis plate. (A) Removal of the red adhesive "cover film". (B) Dispensing the protein droplets into each of the drop wells. (C) The wells are covered with the UV "cover film". (D) The plate is inverted to add the dialysis solutions (or crystallization screen). (E) The plate is sealed and incubated. (F,G) Microscope inspection of the drops. Please click here to view a larger version of this figure.
The use of this crystallization by dialysis protocol was demonstrated using the 0.5 mL dialyzer tube (Figure 3) for the large-scale (hundreds to thousands) production of microcrystals, suitable for state-of-the-art data collection methods such as serial crystallography at both XFEL facilities20,21,22,23,24 and synchrotrons25,26,27, as well as for MicroED28,29,30 approaches.
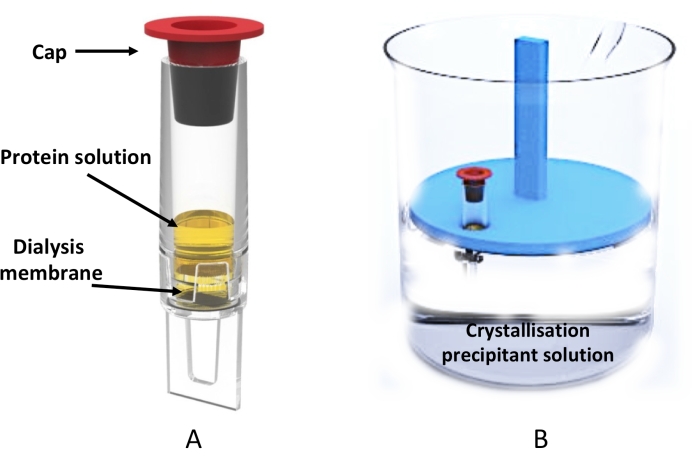
Figure 3: Large-scale microdialysis crystallization using the dialyzer tube. (A) Schematic representation of the 0.5 mL dialyzer tube. (B) Side view of a beaker containing the crystallization solution and the floating tube rack holding a dialyzer tube. Please click here to view a larger version of this figure.
Protocol
1. Protein sample preparation
- Prepare (through recombinant methods or otherwise) and purify the protein(s) of interest in a suitable buffer (i.e., one that is amenable for protein stability and crystallization) that has been filtered with a 0.22 µm filter (see Table of Materials). Evaluate the protein quality (in terms of purity, polydispersity, and stability) prior to crystallization31.
NOTE: Please see the representative results section for details on the proteins and buffers used in the present study. - Concentrate the sample up to 10-50 mg.mL-1 or higher (depending on the protein target) using the correct MWCO concentrator (see Table of Materials).
NOTE: The concentrated sample can be used immediately for crystallization or stored frozen at -80 °C. - If the protein has been frozen, centrifuge the sample for 10 min in a bench-top centrifuge (≥16,000 × g, at room temperature or 4 °C) to pellet and remove any unwanted material (e.g., precipitates).
- Aliquot the protein into clean 200 µL PCR tubes. Keep it on ice or at 4 °C if the protein is unstable at room temperature.
2. Setting up the microdialysis plate
NOTE: The commercially available microdialysis plate (see Table of Materials) consists of two sides ('protein side' and 'buffer side') with a regenerated cellulose membrane (available in different MWCOs), as shown in Figure 2.
- With the plate orientated protein side up, peel back and remove the adhesive cover tape (Figure 2A) from the 200 µm pressure adhesive spacer. Take note of the position of well A1.
- Using a multichannel pipette, load a maximum of 3.2 µL of protein (step 1.4) into each well (Figure 2B).
NOTE: The protein can also be loaded using a repeat dispensing pipette. Using a single-channel pipette is possible, but it will increase the likelihood of partial dehydration of the drops occurring due to the extended loading time. - Position the 200 µm UV cover film (see Table of Materials) over the 96-well plate and check its integrity (Figure 2C). Ensure that the protective film is facing up.
- Use a sealing paddle (see Table of Materials), press down the UV cover film to activate and seal the pressure adhesive.
- Invert the plate and take note of the position of well A1. Ensure that the plate is oriented buffer side up and that the well positions are mirrored (Figure 2D).
- Using a multichannel pipette, load a maximum of 350 µL of the dialysis solution into each of the wells (Figure 2D).
NOTE: In this step, either commercially available crystallization screens (sparse matrix screens) or laboratory-made customized screens (grid screens) can be used (see Table of Materials). - Carefully seal the wells with the 'reservoir cover film' (see Table of Materials) (Figure 2E).
- Place the plate (buffer side up) in a suitable temperature-controlled incubator (20 °C) for crystal growth.
NOTE: For the proteins selected for the present study, the crystals started to appear between 1-8 h at 20 °C. - To inspect for crystals under the microscope, invert the plate to bring the protein side up and remove the protective film from the 200 µm UV cover film (step 2.3), as shown in Figure 2F,G.
3. Large-scale crystallization using dialyzer tubes
NOTE: Once crystallization conditions have been explored using the microdialysis plate, large-scale crystallization of the protein (for serial crystallography or other purposes) can be performed using the dialyzer tube, as shown in Figure 3. Similar to the microdialysis plate, these devices are available with 3, 5, 10, and 30 kDa MWCO dialysis membranes.
- Prepare the optimized crystallization condition for the growth of microcrystals in large containers (adopting the conditions optimized using the microdialysis plate). Ensure the buffer has been previously filtered with a 0.2 µm filter.
- Pipette the protein into the dialyzer tube (maximum volume of 0.5 mL) and seal the end using the included red plastic cap (Figure 3A).
- Place the 500 µL dialyzer tube into a suitably sized container (depending on the total volume of dialysis solution) containing the floating rack (see Table of Materials), as shown in Figure 3B.
NOTE: The floating rack provided with the dialyzer kit holds up to 18 dialyzer tubes simultaneously. - Place the container stationary in a suitable temperature-controlled incubator (20 °C) for crystal growth.
- To inspect for crystals, pipette 1-2 µL of the solution onto a glass cover slide. Cover with a second glass cover slide on top (to prevent excess dehydration) and view under a microscope (see Table of Materials).
NOTE: Crystals may be damaged when sandwiched between two glass cover slides during the inspection. Crystals may also be viewed directly by placing the dialyzer tube under a stereo high-magnification microscope.
Representative Results
Four proteins were crystallized using the microdialysis plate (with 10 kDa MWCO membranes), including two membrane proteins. The chicken egg-white lysozyme from the lyophilized powder (see Table of Materials) was prepared at 50 mg.mL-1 in 20 mM NaOAc (pH 4.5), and the lyophilized thaumatin (see Table of Materials) was dissolved in water to a final concentration of 25 mg.mL-1. The two membrane proteins used in this study were the E. coli multidrug efflux pump AcrB and the E. coli lactose transporter LacY. AcrB was expressed using C43 (DE3) cells and purified by Ni-NTA affinity chromatography and size-exclusion chromatography in 10 mM Tris (pH 7.5), 300 mM NaCl, 0.03% (w/v) n-dodecyl-β-D-maltoside (DDM), and 5% (v/v) glycerol32. Subsequently, the protein was concentrated using a 100 kDa MWCO centrifugal concentrator to a final concentration of 6 mg.mL-1. LacY was also expressed in C43 (DE3) cells, purified by Ni-sepharose affinity chromatography followed by size-exclusion chromatography in 20 mM Tris (pH 7.5), 150 mM NaCl, 0.03% (w/v) DDM, and concentrated using a 100 kDa MWCO concentrator to 10 mg.mL-1 4,33.
For the lysozyme and thaumatin proteins, the 96-well microdialysis plate was filled with a crystallization grid screen prepared in-house as the dialysis solution, while for the membrane protein AcrB, a grid screen was prepared in-house from a previously published crystallization condition34. For LacY, initial hit conditions were found from a commercial screen (see Table of Materials) and further optimized with a grid screen made in-house. The crystallization drop ratio for lysozyme, thaumatin, and AcrB was 1:100, with 2 µL of protein to 200 µL of precipitant (dialysis solution). LacY crystallization drops were also at a 1:100 ratio, with 1 µL of protein to 100 µL of dialysis solution, due to a lower amount of available protein.
Crystals from all four proteins, including the membrane proteins, started to appear between 1-8 h at 20 °C following setup with the microdialysis plate. In this case, it was not necessary to supplement the dialysis solution with detergent for the membrane protein crystallization, typically done during the standard dialysis process to prevent membrane protein aggregation, as the detergent micelles were expected to be larger than the MWCO of the dialysis membranes35.
Following the successful crystallization of the proteins on the microdialysis plate (Figure 4), crystallization conditions were noted, and large-scale crystallization by dialysis was performed using the dialyzer tube with the same 10 kDa MWCO dialysis membranes. Thousands of microcrystals for each of the individual proteins grew inside the dialyzer, as shown in Figure 5. For thaumatin crystallization, 250 µL of protein was loaded onto the dialyzer and dialyzed against 50 mL (1:200 ratio). For AcrB, 250 µL of protein was dialyzed against 25 mL of dialysis solution (1:100). LacY crystallization was also set up at the same ratio with 100 µL of protein to 10 mL of dialysis solution.
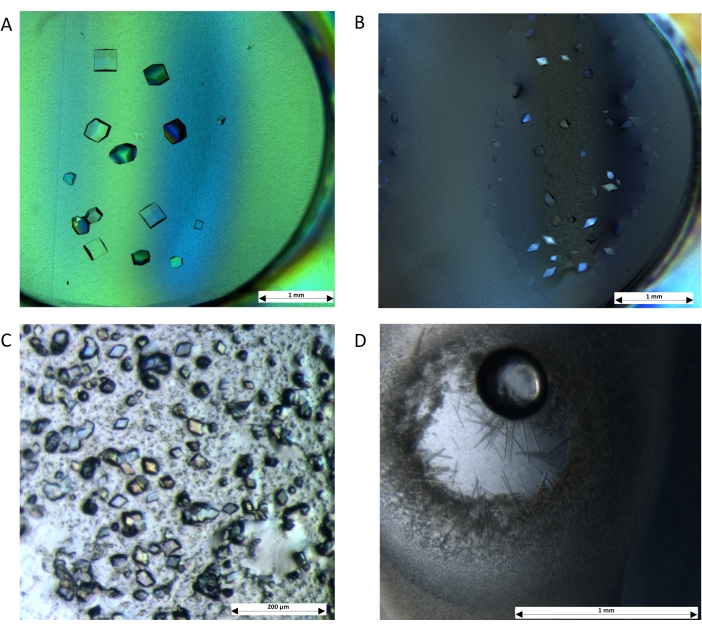
Figure 4: Crystals are grown by dialysis using the microdialysis plate. The protocol demonstrates the applicability of the setup with soluble proteins: (A) Lysozyme was dialyzed against 0.1 M NaOAc (pH 4.0), 0.5 M NaCl, and 25% (v/v) glycerol. (B) Thaumatin was dialyzed against 0.1 M Bis-tris propane (pH 6.6), 1 M K/Na tartrate, and 18% (v/v) ethylene glycol. Crystals were also obtained for membrane proteins: (C) AcrB was dialysed against 0.1 M MES (pH 5.5), 0.3 M NaCl, and 20% (v/v) PEG-400. (D) LacY was dialysed against 0.1 M MES (pH 6.5), 0.1 M NaCl, and 32% (v/v) PEG-300. Images were captured using a stereo high-magnification microscope with a cross polariser. Scale bars: (A,B,D) = 1 mm; (C) = 200 µm. Please click here to view a larger version of this figure.
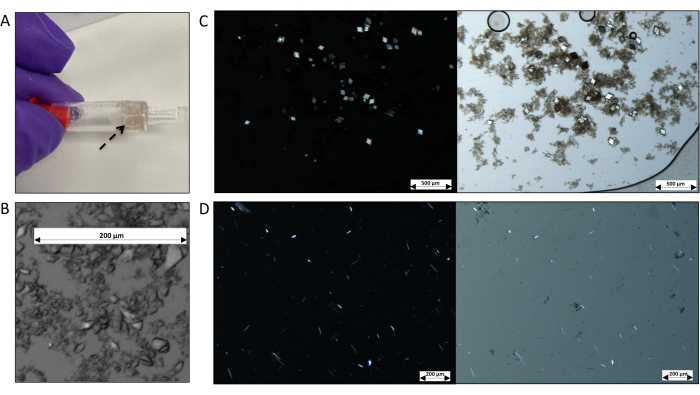
Figure 5: Representative images of membrane protein crystals grown by dialysis using the dialyzer tube. (A) Image showing the tube full of microcrystals (indicated by the black arrow). (B) Microscopic image of 2 µL from a slurry of thaumatin crystals grown by dialysis using the 0.5 mL dialyzer tube, dialyzed against 0.1 M Bis-Tris propane (pH 6.6), 1.4 M K/Na tartrate, and 18% (v/v) ethylene glycol. (C) Dark-field (left) and bright-field (right) images of AcrB microcrystals grown by dialysis against 0.1 M MES (pH 5.5), 0.3 M NaCl, and 20% (v/v) PEG-400. (D) Dark-field (left) and bright-field (right) images of LacY microcrystals grown by dialysis against 0.1 M MES (pH 6.5), 0.1 M NaCl, and 32% (v/v) PEG-300. Some precipitates are observed. Scale bars: (B,D) = 200 µm; (C) = 500 µm. Please click here to view a larger version of this figure.
Discussion
Currently, crystallization by dialysis is the most underutilized crystallization method, namely due to the low-throughput and tedious nature of the existing approaches, such as microdialysis with buttons. Here, a simple but robust protocol follows an HTP workflow for protein crystal growth by dialysis through a commercially available microdialysis plate and dialyzer tube. Depending on the target protein, the microdialysis plate and dialyzer tube used in the procedure come with a choice of a dialysis membrane with a 3, 5, 10, or 30 kDa MWCO. The protocol can be easily set up in any standard crystallization facility and has the great advantage of being applicable to both soluble and membrane proteins. However, protein-protein and protein-nucleic acid complexes were not tested during this protocol.
As in any crystallization approach by dialysis, the volume ratio between the protein sample and dialysis solution is critical. In this protocol, a ratio of 1:100 between the sample and dialysis solution is recommended, but since the microdialysis plate allows a maximum capacity of 350 µL of dialysis solution, these ratios can be explored to obtain crystal hits. A volume of 1-2 µL of protein is used in the presented protocol when setting up crystallization plates. This is to ensure that drops set accurately with a manual multichannel pipette. By using electronic pipettes (multichannel or repeat dispensing pipettes) or HTP liquid dispensing robots, drops of lower volumes can be achieved accurately, thus lowering the amount of protein required. Furthermore, due to the relatively low volumes of dialysis buffer required by the microdialysis plate (contrary to other conventional dialysis methods), it is possible (without the extensive use of resources) to explore large chemical spaces, not only using commercially available crystallization screens but also with optimization screens (designed around the initial crystallization hit condition).
A critical step in the presented HTP procedure is the timely application of small protein volumes (0.50-3.2 µL per condition) to the dialysis plate to limit dehydration and sample loss. This can be easily mitigated by using a multichannel pipette, a repeat dispensing pipette, or a robotic crystallization system. The long incubation time, such as more than 2 weeks, of the plates at 20 °C might lead to dehydration of the protein droplets or damage to the recently formed crystals. Keeping the dialysis plates inside a humidification chamber or a sealable bag can alleviate this effect. In addition, using sterile materials and techniques are recommended to avoid bacterial growth.
As mentioned in the introduction, recently, with the increased need to understand protein structural dynamics for disease mechanisms, protein-ligand binding interactions, and protein-protein interactions, the field of protein X-ray crystallography has been revolutionized through the development of new and existing crystallization techniques, modern approaches to crystal sample delivery, new generations of X-ray sources, and new sophisticated methods for data acquisition and processing36,37,38. Therefore, the advent of room temperature serial micro-crystallography, either performed using XFELs or synchrotron light sources, has emerged as a remarkable tool in structural biology, specifically in the field of membrane proteins39. However, thousands of microcrystals are required to generate enough data for a robust structure solution, which is not an easy task (at least by conventional crystallization methods). The dialysis crystallization method described here enables the production of a large number of microcrystals. Once the crystallization condition for the production of microcrystals (1-10 µm) has been determined through the use of a microdialysis plate, large quantities of high-density microcrystals can be produced using the 0.5 mL dialyzer device (Figure 5). These crystals are ideally suited for data collection using fixed targets or liquid jet sample delivery systems27,40. Crystals obtained through this method can also be appropriate for MicroED applications. However, these may need to be milled down to a suitable size and thickness for this specific application, as electrons interact much more strongly with crystals than X-ray photons41.
In conclusion, the crystallization by dialysis approach described here adds to the evolving strategies in protein crystallization for structure determination and expands the range of efforts than can be employed to determine novel protein targets that have previously been unsuccessful with other conventional methods.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We acknowledge funding from the United Kingdom's Department of Business, Energy, and Industrial Strategy (BEIS). We thank Alex R. Jones and Mike Shaw from the National Physical Laboratory for their feedback on the manuscript.
Materials
| 0.2 mL tubes | Thermo Scientific | AB0620 | For aliquoting protein solutions. |
| 0.2 µm syringe filter | Sartorius | 17823———-K | Surfactant-free cellulose acetate filters. For filtering dialysis solutions. |
| 0.22 µm membrane filters | Millipore | GSTF04700 | Membrane filters for filtering large volumes of buffers |
| 12-channel, variable 0.5 – 10 µL Research plus pipette | Eppendorf | 3125000028 | For dispensing protein drops onto the Diaplate. |
| 12-channel, variable 30 – 300 µL Eppendorf Research plus pipette | Eppendorf | 3125000060 | For dispensing dialysis solutions on the Diaplate reservoirs. |
| 20 mL syringe | Fisherbrand | 15889152 | For use with syringe filters. |
| 96 well 2.2 mL deep-well plates | Thermo Scientific | AB0788 | Polypropylene deep-well storage plates; for preparing screens using the Hamilton Microlab STARlet. |
| Centrifuge 5425 | Eppendorf | 5405000565 | With rotor FA-24×2 with a maximum g-force of 21,300 x g. |
| Diacon dialyser | SWISSCI | W72010 | Dialyzer tubes with a regenerated cellulose membrane with a molecular weight cut-off of 10 kDa. Ideal for protein solutions of up to 0.5 mL. |
| Diaplate 96-well plate | SWISSCI | W82010 | Microdialysis plate. The Diaplate consists of two sides with a regenerated cellulose membrane in-between with a molecular weight cut-off of 10 kDa. |
| Falcon 50 mL High Clarity PP Centrifuge Tube | Corning | 352070 | For holding dialysis solutions. |
| Floating rack | SWISSCI | n/a | Included in the Diacon kit |
| Floor-standing vibration-free incubator | Molecular Dimensions | MD5-01 | 400 L temperature-controlled incubator set to 20 °C. |
| Leica M205 C stereo microscope | Leica | Planapo 1.0x objective, 7.8x – 160x zoom range with DMC 4500 camera | |
| Lysozyme from chicken egg white | Sigma Aldrich | 62971 | Lyophilized protein |
| Memgold2 | Molecular Dimensions | MD1-64 | Sparse-matrix screen |
| Microlab STARlet | Hamilton | n/a | Liquid handler system. |
| Reservoir cover film | SWISSCI | n/a | Included in the Diaplate kit |
| Reusable bottle top filter | Thermo Scientific | DS0320-5045 | For fitering large volumes of buffers, for use with 0.22 µm membrane filters |
| Sealing paddle | SWISSCI | n/a | Included in the Diaplate kit |
| Thaumatin from Thaumatococcus daniellii | Sigma Aldrich | T7638 | Lyophilized protein |
| UV cover film | SWISSCI | n/a | Included in the Diaplate kit |
References
- Brooks-Bartlett, J. C., Garman, E. F. The nobel science: One hundred years of crystallography. Interdisciplinary Science Reviews. 40 (3), 244-264 (2015).
- Maveyraud, L., Mourey, L. Protein X-ray crystallography and drug discovery. Molecules. 25 (5), (2020).
- Kwan, T. O. C., Axford, D., Moraes, I. Membrane protein crystallography in the era of modern structural biology. Biochemical Society Transactions. 48 (6), 2505-2524 (2020).
- Birch, J., et al. The fine art of integral membrane protein crystallisation. Methods. 147, 150-162 (2018).
- Gorrec, F., Löwe, J. Automated protocols for macromolecular crystallization at the MRC laboratory of molecular biology. Journal of Visualized Experiments. 131 (131), (2018).
- Govada, L., Chayen, N. E. Choosing the method of crystallization to obtain optimal results. Crystals. 9 (2), 106 (2019).
- Chayen, N. E. Turning protein crystallisation from an art into science. Current Opinion in Structural Biology. 14 (5), 577-583 (2004).
- McPherson, A., Gavira, J. A. Introduction to protein crystallization. Acta Crystallographica Section F: Structural Biology Communications. 70 (1), 2-20 (2014).
- Gulbis, J. Protein crystallography: methods and protocols. Crystallography Reviews. 24 (2), 136-143 (2018).
- D’Arcy, A., Bergfors, T., Cowan-Jacob, S. W., Marsh, M. Microseed matrix screening for optimization in protein crystallization: What have we learned. Acta Crystallographica Section:F Structural Biology Communications. 70 (9), 1117-1126 (2014).
- Shaw Stewart, ., D, P., Kolek, S. A., Briggs, R. A., Chayen, N. E., Baldock, P. F. Random microseeding: a theoretical and practical exploration of seed stability and seeding techniques for successful protein crystallization. Crystal Growth & Design. 11 (8), 3432-3441 (2011).
- Junius, N., et al. A microfluidic device for both on-chip dialysis protein crystallization and in situ X-ray diffraction. Lab on a Chip. 20 (2), 296-310 (2020).
- Junius, N., Vahdatahar, E., Oksanen, E., Ferrer, J. L., Budayova-Spano, M. Optimization of crystallization of biological macromolecules using dialysis combined with temperature control. Journal of Applied Crystallography. 53 (3), 686-698 (2020).
- Vahdatahar, E., Junius, N., Budayova-Spano, M. Optimization of crystal growth for neutron macromolecular crystallography. Journal of Visualized Experiments. 169, (2021).
- Jaho, S., et al. Crystallization of proteins on chip by microdialysis for in situ X-ray diffraction studies. Journal of Visualized Experiments. 170, (2021).
- Liu, W., Cherezov, V. Crystallization of membrane proteins in lipidic mesophases. Journal of Visualized Experiments. 49, 2501 (2011).
- Ujwal, R., Abramson, J. High-throughput crystallization of membrane proteins using the lipidic bicelle method. Journal of Visualized Experiments. 59, (2012).
- Parker, J. L., Newstead, S. Membrane protein crystallisation: Current trends and future perspectives. Advances in Experimental Medicine and Biology. 922. , 61-72 (2016).
- Apostolopoulou, V., Junius, N., Sear, R. P., Budayova-Spano, M. Mixing salts and polyethylene glycol into protein solutions: The effects of diffusion across semi-permeable membranes and of convection. Crystal Growth & Design. 20 (6), 3927-3936 (2020).
- Neutze, R., Wouts, R., Vander Spoel, D., Weckert, E., Hajdu, J. Potential for biomolecular imaging with femtosecond X-ray pulses. Nature. 406 (6797), 752-757 (2000).
- Chapman, H. N., et al. Femtosecond X-ray protein nanocrystallography. Nature. 470 (7332), 73-78 (2011).
- Mizohata, E., Nakane, T., Fukuda, Y., Nango, E., Iwata, S. Serial femtosecond crystallography at the SACLA: breakthrough to dynamic structural biology. Biophysical Reviews. 10 (2), 209-218 (2018).
- Nogly, P., et al. Lipidic cubic phase serial millisecond crystallography using synchrotron radiation. IUCrJ. 2 (2), 168-176 (2015).
- Johansson, L. C., et al. XFEL structures of the human MT2 melatonin receptor reveal the basis of subtype selectivity. Nature. 569 (7755), 289-292 (2019).
- Owen, R. L., et al. Low-dose fixed-target serial synchrotron crystallography. Acta Crystallographica Section D: Structural Biology. 73 (4), 373-378 (2017).
- Weinert, T., et al. Serial millisecond crystallography for routine room-temperature structure determination at synchrotrons. Nature communications. 8 (1), 542 (2017).
- Axford, D., et al. Two states of a light-sensitive membrane protein captured at room temperature using thin-film sample mounts. Acta Crystallographica Section D: Structural Biology. 78 (1), 52-58 (2022).
- Nannenga, B. L., Gonen, T. The cryo-EM method microcrystal electron diffraction (MicroED). Nature Methods. 16 (5), 369-379 (2019).
- Nguyen, C., Gonen, T. Beyond protein structure determination with MicroED. Current Opinion in Structural Biology. 64, 51-58 (2020).
- Mu, X., Gillman, C., Nguyen, C., Gonen, T. An overview of microcrystal electron diffraction (MicroED). Annual Review of Biochemistry. 90, 431-450 (2021).
- Kwan, T. O. C., et al. Selection of biophysical methods for characterisation of membrane proteins. International Journal of Molecular Sciences. 22 (10), 2605 (2019).
- Pos, K. M., Purification Diederichs, K. crystallization and preliminary diffraction studies of AcrB, an inner-membrane multi-drug efflux protein. Acta Crystallographica Section D: Biological Crystallography. 58 (10), 1865-1867 (2002).
- Guan, L., Mirza, O., Verner, G., Iwata, S., Kaback, H. R. Structural determination of wild-type lactose permease. Proceedings of the National Academy of Sciences. 104 (39), 15294-15298 (2007).
- Kwan, T. O. C., Reis, R., Moraes, I. In situ measurements of polypeptide samples by dynamic light scattering: membrane proteins, a case study. Methods in Molecular Biology. , 189-202 (2021).
- Seddon, A. M., Curnow, P., Booth, P. J. Membrane proteins, lipids and detergents: not just a soap opera. Biochimica et Biophysica Acta (BBA)-Biomembranes. (1-2), 105-117 (2004).
- Wickstrand, C., et al. A tool for visualizing protein motions in time-resolved crystallography. Structural Dynamics. 7 (2), 024701 (2020).
- Orville, A. M. Recent results in time resolved serial femtosecond crystallography at XFELs. Current Opinion in Structural Biology. 65, 193-208 (2020).
- Schulz, E. C., Yorke, B. A., Pearson, A. R., Mehrabi, P. Best practices for time-resolved serial synchrotron crystallography. Acta Crystallographica Section D: Structural Biology. 78 (1), 14-29 (2022).
- Neutze, R., Brändén, G., Schertler, G. F. Membrane protein structural biology using X-ray free eletron lasers. Current Opinion in Structural Biology. 33, 115-125 (2015).
- Wierstall, U. Liquid sample delivery techniques for serial femtosecond crystallography. Philosophical Transactions of the Royal Society B: Biological Sciences. 369 (1647), 20130337 (2014).
- Shi, D., Nannenga, B. L., Iadanza, M. G., Gonen, T. Three-dimensional electron crystallography of protein microcrystals. eLife. 2, 01345 (2013).

