Non-Aqueous Isolation and Enrichment of Glandular Capitate Stalked and Sessile Trichomes from Cannabis sativa
Summary
A protocol is presented for the convenient and high-throughput isolation and enrichment of glandular capitate stalked and sessile trichomes from Cannabis sativa. The protocol is based on a dry, non-buffer extraction of trichomes using only liquid nitrogen, dry ice, and nylon sieves and is suitable for RNA extraction and transcriptomic analysis.
Abstract
This paper presents a protocol for the convenient and high-throughput isolation and enrichment of glandular capitate stalked and sessile trichomes from Cannabis sativa. The biosynthetic pathways for cannabinoid and volatile terpene metabolism are localized primarily in the Cannabis trichomes, and isolated trichomes are beneficial for transcriptome analysis. The existing protocols for isolating glandular trichomes for transcriptomic characterization are inconvenient and deliver compromised trichome heads and a relatively low amount of isolated trichomes. Furthermore, they rely on expensive apparatus and isolation media containing protein inhibitors to avoid RNA degradation. The present protocol suggests combining three individual modifications to obtain a large amount of isolated glandular capitate stalked and sessile trichomes from C. sativa mature female inflorescences and fan leaves, respectively. The first modification involves substituting liquid nitrogen for the conventional isolation medium to facilitate the passage of trichomes through the micro-sieves. The second modification involves using dry ice to detach the trichomes from the plant source. The third modification involves passing the plant material consecutively through five micro-sieves of diminishing pore sizes. Microscopic imaging demonstrated the effectiveness of the isolation technique for both trichome types. In addition, the quality of RNA extracted from the isolated trichomes was appropriate for downstream transcriptomic analysis.
Introduction
Glandular trichomes are hair-like structures present in plants that contain many secondary metabolites1 and represent a valuable bank of novel biosynthetic genes and enzymes2. In Cannabis, the biosynthesis of the important secondary metabolites, cannabinoids3 and terpenes4, is localized in the trichomes. Considering the role of trichomes in determining the quality of Cannabis both for medicinal and recreational uses, the study of trichome gene expression is of interest. To characterize the expression of trichome-specific genes, the trichomes of interest must first be isolated. Trichome isolation protocols were first described as early as 19925, and their latest developments have been recently reviewed2. In general, protocols for extracting glandular trichomes for transcriptomic characterization can be divided into two distinct sequential steps. The first step involves a thorough physical separation of the trichomes from the plant tissue. This step can be performed by using dry ice5, glass beads with a commercial apparatus6,7, grinding the plant material against a mesh sieve8, or vortexing the plant tissue in an isolation buffer9. The second step involves a more refined separation of the trichomes of interest from the microscopic plant residue and/or other trichome types. This step can be executed using density gradient centrifugation8,10 or sieves of various sizes7,9. Due to the extreme sensitivity of RNA in processed tissues to degrading agents, these two sequential steps are usually conducted in the ice-cold isolation medium, often in the presence of protein inhibitors4.
Conventional trichome isolation protocols require, in addition to the ice-cold temperatures, large amounts of isolation medium to ensure an efficient extraction procedure. The combination of these components results in an arduous, time-consuming isolation process that hinders high throughput. Presenting a straightforward, user-friendly alternative trichome isolation protocol is, therefore, likely to be beneficial for various aspects associated with trichome characterization. The present paper aims to offer an alternative protocol for isolating stalked and sessile glandular capitate trichomes from Cannabis sativa by combining and integrating several elements from the conventional protocols. These elements include dry ice5, the passing of the trichomes through several micro-sieves with decreasing pore sizes7,9, and substituting liquid nitrogen (LN) for the isolation medium8.
The novelty of the present trichome isolation protocol, as compared to conventional protocols, presents in a number of ways. This protocol is convenient, as it does not require hazardous components. The procedure can be conducted in the lab with minimal precautions and facilitates high throughput. Substituting LN for the standard liquid isolation medium ensures the integrity of the trichomes throughout the isolation process, enabling subsequent transcriptomic analysis. Upon sublimation of the LN and dry ice, the isolated trichomes are left free of harmful residuals. Further, the propensity of LN to sublimate at room temperature allows its generous use throughout the protocol. In contrast, using large volumes of conventional isolation medium generates practical difficulties in its handling. Finally, the protocol decreases the separation of the disc cell from the remaining fragile head structure of the glandular trichome, enabling the retention of the headspace content.
This protocol is presented in a detailed step-by-step fashion designed to assist the technical practice of isolating C. sativa glandular capitate trichomes. The protocol provides a manageable workflow that results in isolated trichomes with a high concentration and purity that are appropriate for downstream molecular analysis.
Protocol
NOTE: The plant material used in this study consisted of four C. sativa ARO-Volcani strains (CS-11, CS-12, CS-13, and CS-14) that were grown in the Volcani Center, Israel, as described elsewhere11. Glandular capitate stalked trichomes were isolated from mature flowering inflorescences, and glandular capitate sessile trichomes were isolated from large fan leaves from mature non-flowering mother plants. All plant material was freshly picked and immediately stored at −80 °C.
CAUTION: Dry ice and LN are used throughout the protocol. These substances are extremely hazardous. The isolated trichomes can contain dry ice particles that can create dangerous gas pressure when inserted into sealed tubes; therefore, all caps should be needle-punctured. The use of protective goggles, appropriate lab wear, and gloves for handling at extremely low temperatures is highly advised.
1. Setup for the initial separation of trichomes from the plant material
- Crush a dry ice block into small fine flakes using a hammer and a hard flat object in a 5 L plastic container.
- Sieve the fine dry ice flakes (smaller than 5 mm) from the non-crushed dry ice with a large strainer (via pores smaller than 5 mm) into another 5 L plastic container. Pour approximately 200 cm3 (200 mL mark) of the dry ice flakes into an upright 1 L glass beaker.
- Add up to 10 g of frozen C. sativa inflorescences (from now on referred to as plant material) onto the first layer of crushed dry ice, and cover with an additional layer of 200 cm3 of finely crushed dry ice.
- Cover the opening of the 1 L glass beaker with two to three layers of 1 mm screen door mosquito net, and secure it to the outer sides of the glass cup with rubber bands (Figure 1).
- Pour LN into a large round-bottom stainless steel container (see Table of Materials), where the isolated trichomes will be collected.
- Insert a 350 µm mesh in a flour sifter/sieve strainer to cover the flour sifter mesh from below (Figure 2). If a flour sifter with a detachable plastic ring is available, insert the 350 µm mesh so that it is secured under the flour sifter's mesh. If not, secure the 350 µm mesh to the circumference of the flour sifter with rubber bands.
- Place the flour sifter above the large round-bottom stainless steel container filled with LN (Figure 3). Ensure that the width of the container exceeds that of the flour sifter to minimize the loss of the sifted mass outside the round-bottom stainless steel container.
2. Separation of trichomes from the plant material
- Shake the 1 L glass with its opening pointing downward toward the flour sifter as if using a large salt shaker (Figure 4).
- Set aside the 1 L glass beaker every 2-3 min to allow the sifting of the crushed dry ice and plant material accumulated on the flour sieve.
- Sift the flour sieve horizontally to facilitate the passage of the plant material into the LN in the round-bottom stainless steel container below.
- Add LN to the stainless steel container and crushed dry ice to the 1 L glass beaker when their levels run low. Replenish the used plant material in the glass beaker with an additional 10 g of fresh plant material when the plant material on the flour sieve is depleted. The replenishment usually does not have to be repeated more than twice.
- Repeat steps 2.1-2.4 until a sufficient amount of enriched trichomes has been collected in the round-bottom stainless steel container. Generally, 20 g of fresh plant material is sufficient for RNA extraction and transcriptome analysis.
- Verify that there is a white powder-like substance (consisting of plant debris, glandular trichomes, and crushed dry ice) at the bottom of the stainless steel container submerged in the LN. A microscopic observation will help to determine if a sufficient amount of trichomes has been collected; however, this step might obstruct the protocol's flow. Usually, 10-20 g of initial plant material suffices for most isolations; however, there may be differences in the trichome density of the plants.
NOTE: From this point, the experiment can be paused and restarted later. If paused for a short time, adequate amounts of LN should be added so that the trichomes remain submerged. Alternatively, the trichomes can be collected and stored at −80 °C for up to 3 months for later use, as described in step 5.
3. Separation of glandular capitate trichomes (stalked and sessile) from other trichome types, such as bulbous and cystolithic trichomes, and debris
- Add a small amount of LN to a clean small round-bottom stainless steel container.
- Fold a 40 cm x 40 cm micro-sieve with a 150 µm mesh size twice to obtain a 20 cm x 20 cm fold, and open it. Ensure it resembles a cone-like shape (Figure 5).
- Secure the mesh cone to the edge of the round-bottom stainless steel container with one or two clothespins so that the opened part of the cone is upright and its pointed part is partly submerged in the LN.
- Gently pour the LN and the white powder-like substance from step 2.6 into the micro-sieve cone.
- Gently apply a wide brush to gather and transfer any remaining plant material from the first container into the 150 µm mesh cone.
- Add more LN to the first container, and repeat the brushing motion until all the plant material is transferred to the micro-sieve cone.
- Carefully detach the clothespin from the lid of the container, and open the micro-sieve cone so that the trichomes are located in the middle of the opened micro-sieve. Hold all four corners of the micro-sieve together so that the middle part containing the trichomes remains submerged in the LN (Figure 6).
- While holding all four corners of the micro-sieve, gently dip and shake the micro-sieve in the LN in the steel container as if infusing a tea bag. Continue this dipping/shaking (vertical and horizontal) motion for 1 min. There is a tradeoff between the isolation quality and quantity. Longer dipping motions may result in larger amounts of trichomes, but their isolation grade may be poorer. Overall, 1 min is recommended as adequate for both quality and quantity.
NOTE: This sieving motion is the most critical part of the protocol and should be executed accordingly. - Optional: Scoop the non-sifted plant debris and larger trichomes (still submerged in the LN) that are wrapped in the center of the micro-sieve into a 13 mL or 50 mL test tube, and store at −80 °C for future use.
CAUTION: To avoid the buildup of pressured gas (from the dry ice and LN residues), puncture a hole in the cap of the test tube with a sterilized needle. The cap can be replaced once the pressure has reduced, which is usually after 24 h.
4. Passing trichomes through micro-sieves of decreasing pore size
- Transfer the sifted trichomes submerged in the LN at the bottom of the small round-bottom stainless steel container sequentially through micro-sieves with decreasing pore sizes (105 µm, 80 µm, 65 µm, and 50 µm), in the same manner as presented in step 3.
5. Collection of the desired trichomes
- Remove the desired trichomes submerged in the LN and wrapped in the 50 µm micro-sieve using a pre-chilled (in LN) spoon. Place them on a clean plate.
NOTE: In this final isolation stage, the desired trichomes are collected from the sieve. - Quickly transfer the powder-like trichomes into a labeled pre-chilled 1.5 mL tube via a pre-chilled spatula or a scoopula inserted into the tube (Figure 7). Store the tubes immediately at −80 °C for further studies.
NOTE: A schematic workflow chart depicting the whole isolation protocol is given in Figure 8.
6. Observation and analysis of the purified trichomes
- Place a small amount (10 mg) of isolated trichomes on a microscope slide using a pre-cooled (via LN) spatula. Add a drop of water, and directly observe on a light microscope without staining. Evaluate the overall isolation and contamination degree using low (10x) and high (40x) magnifications. Enrichment is visually estimated by the relative amount of trichomes with respect to non-trichome tissue, as shown in Figure 9.
- Perform RNA extraction and quality analysis from 50 mg of isolated C. sativa glandular capitate stalked and sessile trichomes.
NOTE: A commercial extraction kit (see Table of Materials) employing a poly-A-based strategy of mRNA purification was used for the RNA extraction.- Resuspend 50 mg of the isolated trichomes in the lysis solution, vortex for 30 s, sonicate in an ice-cold ultrasonic cleaning unit at 35 kHz for 5 min, and extract the mRNA from the samples according to the manufacturer's protocol (see Table of Materials).
- To estimate the integrity of the RNA extracted from the trichomes, determine the RNA concentration, the total amount, and the RNA integrity number (RIN) values using a lab-on-a-chip system (see Table of Materials).
- Perform RNAseq analysis of the trichome fractions (optional). RNAseq analysis and bioinformatics analyses of sequenced reads can be performed by standard outsourcing services
Representative Results
The main modification included in this protocol compared to conventional trichome isolation protocols is substituting the standard isolation medium with LN. Using LN as an isolation medium allows a relaxed workflow, because as long as the samples are submerged in LN, metabolic degradation is not likely to occur. Furthermore, as the protocol avoids the hazardous components (i.e., aurintricarboxylic acid and β-mercaptoethanol) used in traditional trichome isolation medium, the work is not restricted to a chemical hood. Nevertheless, it is important to take the necessary precautions while handling dry ice and LN.
To assess the advantages of the present protocol, the trichome isolation results from the present protocol were compared with those obtained using a conventional trichome isolation procedure (which used an aqueous buffer, glass beads, and a fine sieve for trichome isolation)12, initially using a light microscope. A comparison of the components from different isolation phases via light microscope inspection illustrates the increasing trichome isolation degree as the isolation process progresses. In the initial stage of the isolation process (105-150 µm micro-sieve), pieces of leaf tissue were predominant in the isolated portion (Figure 9A), in contrast to the final isolation product, which was composed almost entirely of isolated trichomes (Figure 9C). Care should be taken to validate that the isolated samples are contamination-free (Figure 9B).
Although the trichome density was not quantified in this study, the quality of the isolated glandular capitate stalked and sessile trichomes in the final isolation step can be observed in Figure 10. The purity can be compared to that obtained with trichome isolation using a conventional protocol12 (Figure 10C). In the present protocol, the delicate stalked trichome head structure is retained, and the whole heads of the isolated stalked trichome are visible (Figure 10D), whereas using the conventional protocol, the complete trichome head structure is missing, and only the disc cells are present (Figure 10E). The yield can also be greatly increased since the use of dry ice and LN obviates the limit of plant material that can be extracted. An additional advantage of our non-aqueous protocol is that it uses only dry ice and LN, and, therefore, no residual components of the isolation medium are left once they evaporate. Using LN prevents the release of sticky secondary metabolites that can lead to the aggregation of the trichomes13. In the present protocol, the isolated trichomes in the final isolation step were recovered as a dry fine powder.
The extremely low temperatures maintained throughout the present protocol are likely to suffice in meeting the objectives of conventional buffer media-preventing RNA degradation (as long as the samples are kept submerged) and facilitating downstream molecular applications. In order to validate this assumption, we extracted RNA from the isolated trichomes and estimated the RNA integrity using commercially available kits. The obtained high RIN values (9.4 to 10) and distinct gel chromatography (Figure 11) clearly indicate a high RNA integrity. The RNA samples were further analyzed by RNAseq, and >20 M high-quality reads were obtained from all libraries.
In order to validate that, indeed, the mRNA from the isolated trichomes fraction is enriched in trichome-expressed genes, we compared our gene expression results for the inflorescence and corresponding isolated stalked trichomes to previous results presented by Livingston et al.14, which characterized the gene expression of purified trichomes of Cannabis (adapted from their Supplemental Table 1). Their protocol consisted of blending in aqueous buffer, filtering through sieves and, finally, trichome purification using a Percoll gradient. Trichome-expressed genes were characterized as those most highly correlated (p > 0.95) with the gene expression of cannabidiolic acid synthase (CBDAS), a trichome-specific gene marker14. The comparison of gene expression between the results obtained with the present protocol and those presented in Livingston et al.14 show that the 12 most highly enriched genes in our trichome fraction were also enriched in the Livingston et al. study14, including the trichome marker gene CBDAS (Table 1). Notably, a significantly higher enrichment ratio was obtained from the present protocol. These results confirm the validity of the present protocol for the study of trichome-enriched gene expression.
In addition, we compared the expression data of five actin genes of Cannabis, since actin is frequently used as a reference gene for transcriptome studies (Table 2). Our results for both the isolated stalked and sessile trichomes were comparable to those reported by the Livingston et al. study14.
Finally, we calculated the expression and trichome enrichment data for the chlorophyll a-b binding protein family genes, which are presumably not trichome enriched (Table 3). Indeed, the 12 members of this gene family showed a trichome enrichment factor of <1, serving as a negative control for trichome enrichment. These combined results indicate the unique expression patterns of the inflorescence and isolated trichome fractions and support the trichome integrity and quality of the isolated trichome fraction.
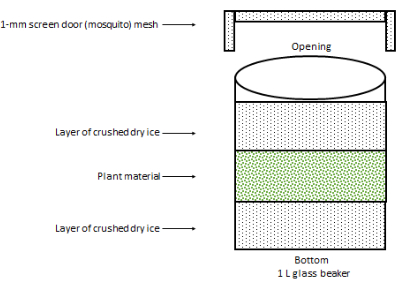
Figure 1: Setup for loading the 1 L glass beaker. The 1 mm screen door mesh is secured to the sides of the glass beaker via a few rubber bands (not shown). The 1 L glass beaker should be loaded in an upright position. Please click here to view a larger version of this figure.
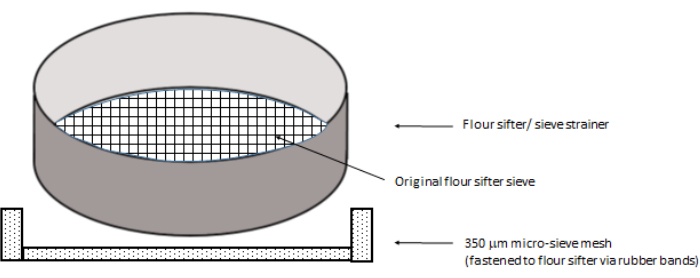
Figure 2: Setup of the flour sifter. The flour sifter is fitted with a 350 µm micro-sieve via rubber bands at the bottom (not shown). Please click here to view a larger version of this figure.
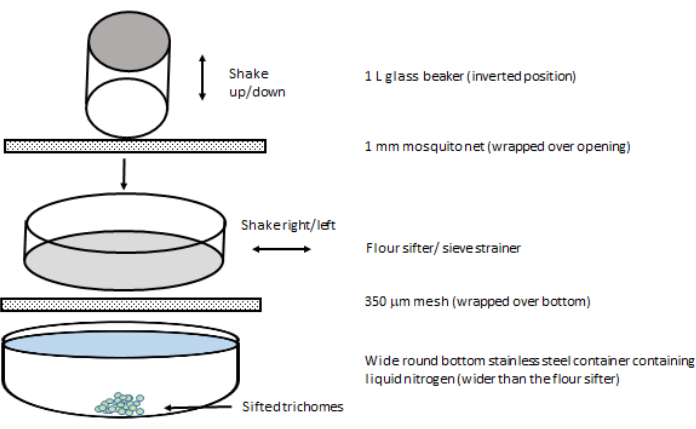
Figure 3: Setup of the first step of trichome isolation. Please click here to view a larger version of this figure.
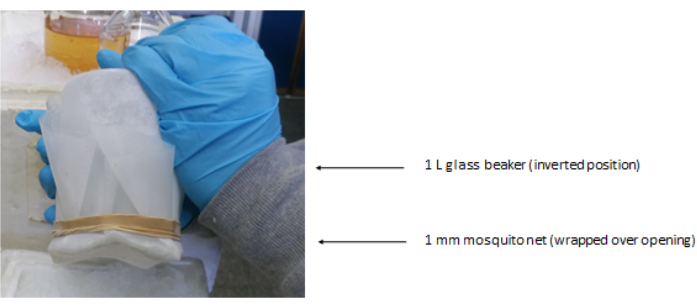
Figure 4: Setup of the glass beaker. The glass beaker fitted with three layers of 1 mm screen-door (mosquito) mesh secured via rubber bands at the opening. The opening of the beaker is pointing downward. Please click here to view a larger version of this figure.
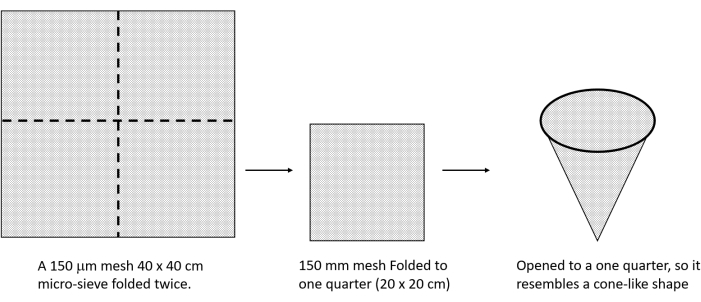
Figure 5: Setup of the micro-sieve cone structure used for trichome isolation. Please click here to view a larger version of this figure.
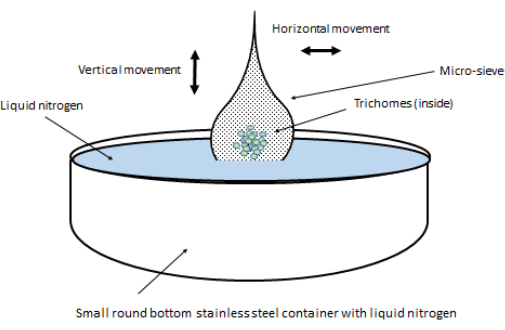
Figure 6: Setup for the dipping and shaking motion of the micro-sieve containing isolated trichomes. The horizontal/vertical movement should resemble a tea bag infusion. The setup is identical for each mesh size used. Please click here to view a larger version of this figure.
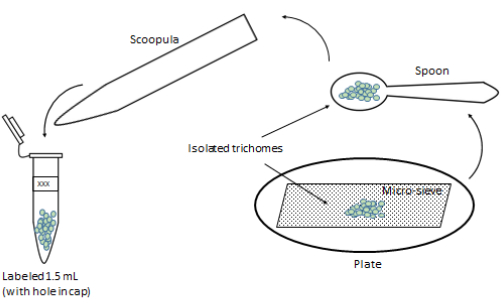
Figure 7: Setup for transferring the final isolated trichomes. The final isolated trichomes are transferred to a labeled 1.5 mL tube. All the utensils should be pre-chilled with LN. Please click here to view a larger version of this figure.
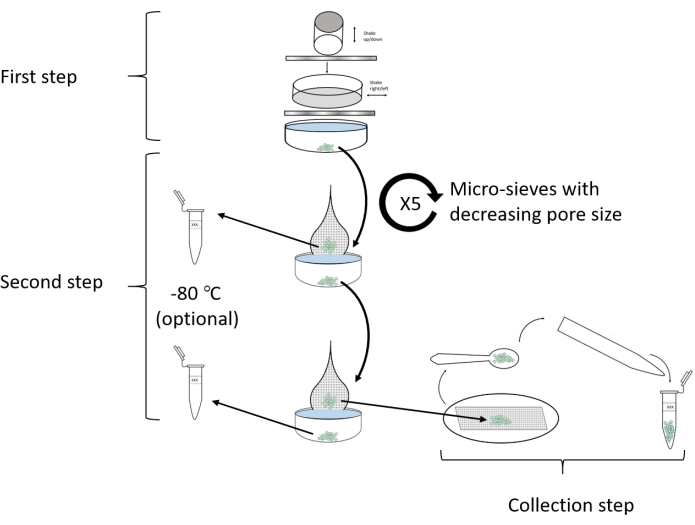
Figure 8: Flowchart outlining the protocol. The isolation of glandular capitate trichomes from C. sativa involves three steps: (1) the initial detachment of the trichomes from the plant source and passage via two sieves (1 mm and 350 µm), (2) passing the plant material via five micro-sieves of decreasing pore sizes (150 µm, 105 µm, 80 µm, 65 µm, and 50 µm), and (3) the collection of the isolated trichomes into a pre-chilled 1.5 mL tube. Retained tissue from any of the micro-sieve stages may similarly be collected. Please click here to view a larger version of this figure.
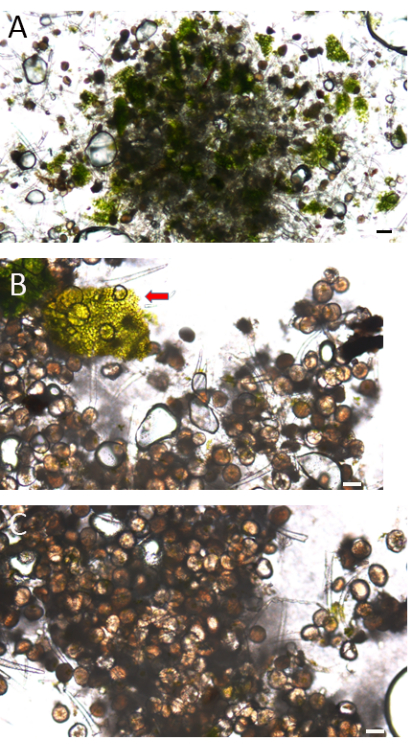
Figure 9: Microscopy images of the isolated trichomes using the current protocol. (A,B,C) Glandular capitate sessile trichomes from C. sativa fan leaves. (A) Mid-isolation, from micro-sieves with pore sizes ranging from 105 µm to 150 µm. Note the retention of large amounts of green, non-trichome material. (B) End of isolation, from micro-sieves with pore sizes ranging from 50 µm to 65 µm, contaminated with plant source (red arrow), and (C) free of contamination. Scale bars = (A) 100 µm, (B,C) 50 µm. Please click here to view a larger version of this figure.
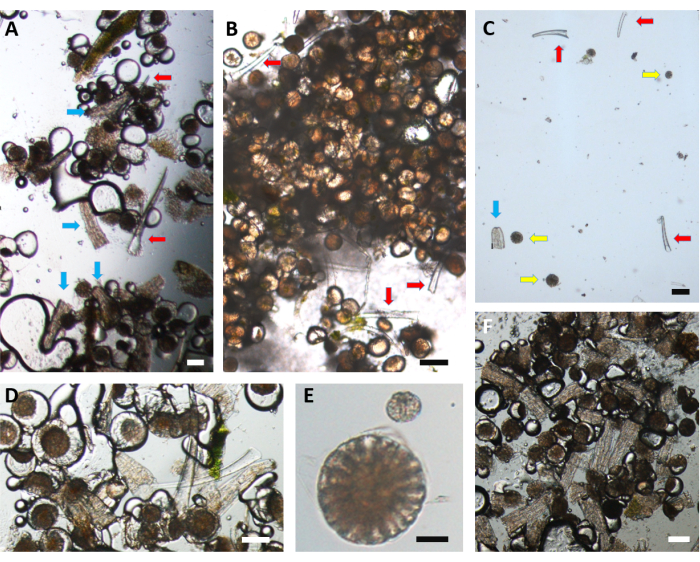
Figure 10: Microscopy images of the isolated trichomes. (A,B) Glandular capitate trichomes from C. sativa: (A) stalked type, isolated using the current protocol, and (B) sessile type, isolated using the current protocol. (C) Stalked trichomes isolated using a conventional protocol. The low yield and lack of uncompromised heads of the trichomes are apparent. (D,E) Stalked glandular capitate trichomes isolated using (D) the present protocol and (E) the conventional protocol. (F) Glandular stalked trichomes isolated from 65 µm to 105 µm micro-sieves (close-up). Note the high isolation of stalked trichomes, with detached heads and stalk parts. The red arrows indicate cystolithic trichomes, the blue arrows indicate a few stalk parts from the glandular capitate stalked trichomes, and the yellow arrows indicate separated disc cells. Scale bars = (A,C,D,E,F) 100 µm, (B) 50 µm. Please click here to view a larger version of this figure.
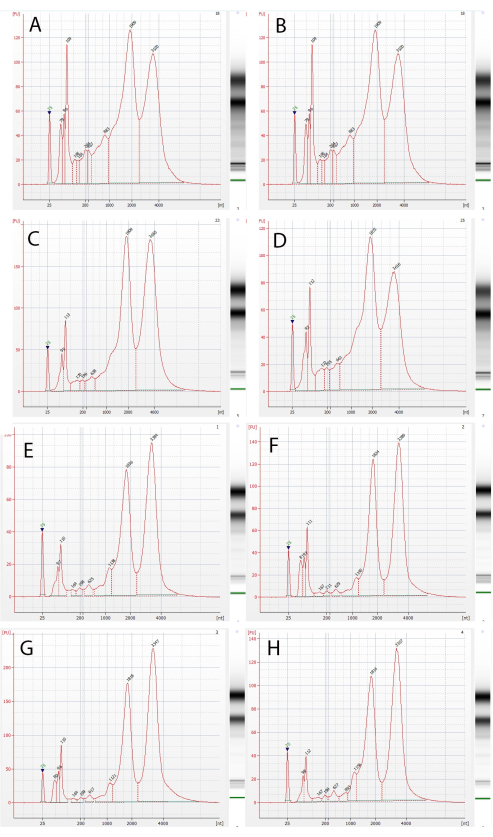
Figure 11: Analysis of the integrity of RNA extracted from the trichomes. Chromatographs and gels of extracted RNA samples from glandular capitate stalked and sessile trichomes isolated using the present protocol. Results for the RNA from (A–D) the stalked trichomes of the four cultivar lines used in this study, CS-11, CS-12, CS-13, and CS-14, respectively, and (E–H) the sessile trichomes, CS-11, CS-12, CS-13, and CS-14. Please click here to view a larger version of this figure.
Table 1: Comparison of the trichome-enriched gene expression obtained with RNAseq using the present protocol to the results obtained by Livingston et al.14. The 12 genes displaying the highest correlation to cannabidiolic acid synthase (CBDAS) expression by Livingston et al.14 (from their Supplemental Table Data S4, adapted with permission from Wiley publishers), considered a trichome-specific gene marker, were chosen for comparison. Please click here to download this Table.
Table 2: Comparison of the gene expression (FPKM) of five Cannabis actin genes of isolated stalked and sessile trichomes from the present protocol (our gene expression data from Cannabis variety CS-1111 (Var CS-11)) with previously published results adapted from Livingston et al.14. The results of Livingston et al.14 were presented based on the Finola FN reference genome, and their data were translated to the CS10.2 reference genome. Please click here to download this Table.
Table 3: Gene expression (FPKM) of Cannabis chlorophyll a-b binding genes of whole inflorescences and isolated stalked trichomes from the present protocol (our gene expression data from Var CS-11). Please click here to download this Table.
Discussion
Compared to the currently available trichome isolation protocols, two main modifications are described in the present protocol. These include the detachment of the trichomes from the plant material using dry ice in the initial step and substituting LN for the commonly used liquid buffer medium. The first modification for C. sativa trichome purification is based on an earlier protocol that introduced the use of crushed dry ice to detach the trichomes from geranium pedicels5. While traditional trichome isolation protocols generally use a small (50 mL) test tube, a 1 L glass beaker was used in this study with a generous amount of crushed dry ice, allowing a larger volume of initial plant material (up to 10 g) to be processed. Furthermore, in the present protocol, two additional passages of the trichomes through micro-sieves were introduced in the first isolation step. The first step employs a vertical up-and-down vigorous saltshaker-like motion, and the second employs a horizontal sifting motion through the micro sieve in the flour sifter. The combined horizontal and vertical sieving motions are suggested to promote an enhanced separation based on the trichome size and shape.
The second, most significant modification addresses the isolation medium in which the passage through the micro-sieves is conducted. In the present protocol, the conventional aqueous isolation medium is completely replaced by LN. A previous protocol8 also used LN in the first step for trichome isolation, separating trichomes from the plant material (by grating the flower material against a mesh sieve), but continued with a liquid buffer extraction and Percoll density separation. Substituting LN in place of the conventional isolation medium eliminates the need for special procedures associated with the toxic components of the conventional isolation medium, allowing a relaxed workflow and high throughput.
A key feature of the present protocol relies on the propensity of dry ice and LN to sublimate quickly at room temperature. This feature enables their generous application throughout the isolation process. In contrast, using a large amount of the conventional isolation buffer would lead to technical complications concerning handling its large volumes.
While the isolation process in the present protocol promotes the retention of the fragile head structure of the glandular capitate stalked trichome, the conventional protocols promote the separation of the disc cells from the remaining gland material, which is easily washed away, leaving only the disc cells. This phenomenon can clearly be seen in our results comparing a conventional isolation protocol to our new protocol (Figure 10D,E). Besides the microscopic observation, the quality analysis of the RNA extracted from the trichomes (isolated using this protocol) clearly indicates that the RNA integrity is retained during the isolation process and that it is suitable for transcriptomic analysis.
Furthermore, the gene expression results from our isolated trichome fraction indicate that the fraction is highly enriched in established trichome-expressed genes (Table 1) and, reciprocally, that non-trichome-expressed genes are unrepresented (Table 3).
The main limitation of the present protocol is its exclusive suitability for isolating trichomes according to their propensity to pass through micro-sieves. While the protocol cannot be directly applied to trichome isolation using a density gradient, it is suitable for isolating the trichomes prior to the density gradient step, if necessary. In this study, the trichomes isolated using this protocol did not undergo a proteomic characterization, and their suitability for such an application needs to be verified. However, as the RNA samples extracted from both stalked and sessile trichomes exhibited no degradation, as indicated by the chomatographs and high RIN values, it can be assumed that the trichomes isolated with the present protocol are likely to meet the requirements needed for proteomic analysis.
The isolation degree (from the initial plant source and other types of trichomes) of the glandular capitate sessile trichomes from the fan leaves is very high, as the fan leaves lack the glandular capitate stalked trichome type15,16, and the cystolithic and bulbous trichomes are easily isolated from the sessile trichomes due to their structural and size differences. However, with regard to the isolation of glandular capitate stalked type trichomes, it is best to address their isolation degree as enrichment, as the plant source, the mature female inflorescence, contains all four trichome types, in addition to the pre-stalk type14. It stands to reason that most of the isolated glandular capitate trichomes are of the stalked type, as their elevated position (in relation to the epidermal-bound sessile trichomes) enhances the likelihood of them colliding with a dry ice particle and becoming detached from the plant. Furthermore, the juncture connecting the head and stalk parts of the stalked trichome is considered a point of weakness associated with the abscission of the gland head17. Thus, it would be accurate to refer to the stalked trichome portion as a highly enriched fraction with potentially low contamination of the sessile type. However, further analysis was not conducted to validate this assumption.
The suitability of the present protocol to extract glandular trichomes from other plant species is yet to be determined. The high trichome density in C. sativa presumably contributes to the success of the present protocol. However, it seems unlikely that this is the sole reason, as a relatively large plant material source (up to 10 g per isolation cycle) can be effectively processed. Other studies have presented detailed protocols for isolating different trichome types from other plants, for example, rosette leaf trichomes from Arabidopsis thaliana18,19. While the applicability of the present protocol for isolating other trichome types was not studied in this work, elements presented in this work, especially the substitution of LN for the isolation buffer, are likely to improve the trichome isolation process.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge financial support from CannabiVar Ltd. All plant material was generously provided by Professor Hinanit Koltai from the Volcani Center, Israel.
Materials
| Bioanalyzer RNA Pico 6000 chip | Agilent, Germany | Reorder number 5067-1513 | Lab-on-a-chip system |
| Transsonic-310 | Elma, Germany | D-78224 | Ultrasonic cleaning unit |
| TruSeq RNA Sample Prep Kit v2 | Illumina, USA | RS-122-2001 | Sample preperation for RNA sequencing library |
| Spectrum Plant Total RNA Kit | SIGMA-ALDRICH, USA | STRN50-1KT | Plant Total RNA Kit |
| Nylon micro-sieve with a mesh size of 350 µm (40 x 40 cm or larger than the circumference of the flour sifter) | Sinun Tech, Israel | r0350n350210 | Nylon screen aperture |
| Nylon micro-sieve with mesh size of 150 µm (size of 30 x 30 cm) | Sinun Tech, Israel | r0150n360465 | Nylon screen aperture |
| Nylon micro-sieve with mesh size o 105 µm (size of 30 x 30 cm) | Sinun Tech, Israel | r0105n320718 | Nylon screen aperture |
| Nylon micro-sieve with mesh size o 80 µm (size of 30 x 30 cm) | Sinun Tech, Israel | r0080n370465 | Nylon screen aperture |
| Nylon micro-sieve with mesh size o 65 µm (size of 30 x 30 cm) | Sinun Tech, Israel | r0065n340715 | Nylon screen aperture |
| Nylon micro-sieve with mesh size o 50 µm (size of 30 x 30 cm) | Sinun Tech, Israel | r0080n370465 | Nylon screen aperture |
| Up to 10 g of frozen plant material (stored in -80 oC or liquid nitrogen) | |||
| Suitable gloves for handling low temperatures | |||
| Safety goggles | |||
| 1 mm screen door (mosquito) mesh (strip of 30 x 100 cm) | |||
| Large strainer (colander) with holes approximately 5 mm | |||
| 1 L glass beaker | |||
| 1 block of dry ice (0.5-1 kg) | |||
| Hammer and hard flat object | |||
| Two 5 L plastic containers | |||
| Rubber bands | |||
| Large flour sifter or sieve strainer- preferably one with a detachable plastic ring on the circumference | |||
| Several large and small round bottom stainless steel containers. One of them should be larger than the flour sifter's circumference (approximately 40 cm in diameter), to minimize the loss of the sifted mass outside the round bottome stainless steel container | |||
| Pre-chilled (via liquid nitrogen) stainless steel spoon, spatula, and scoopula | |||
| Clean plate | |||
| Several clothespins | |||
| Pre-chilled (via liquid nitrogen) labeled 1.5 mL tubes with holes poked on the lid with a sterile needle | |||
| Two containers of liquid nitrogen | |||
| 1 cm wide painting brush |
References
- Schilmiller, A. L., Last, R. L., Pichersky, E. Harnessing plant trichome biochemistry for the production of useful compounds. Plant Journal for Cell & Molecular Biology. 54 (4), 702-709 (2008).
- Liu, Y., Jing, S. X., Luo, S. H., Li, S. H. Non-volatile natural products in plant glandular trichomes: chemistry, biological activities and biosynthesis. Natural Product Reports. 36 (4), 626-665 (2019).
- Fairbairn, J. W. The trichomes and glands of Cannabis sativa L. UN Bulletin on Narcotics. 23, 29-33 (1972).
- Booth, J. K., Page, J. E., Bohlmann, J. Terpene synthases from Cannabis sativa. PLoS One. 12 (3), 0173911 (2017).
- Yerger, E. H., et al. A rapid method for isolating glandular trichomes. Plant Physiology. 99 (1), 1-7 (1992).
- Gershenzon, J., Maffei, M. M., Croteau, R. Biochemical and histochemical localization of monoterpene biosynthesis in the glandular trichomes of spearmint (Mentha spicata). Plant Physiology. 89 (4), 1351-1357 (1989).
- Gershenzon, J., et al. Isolation of secretory cells from plant glandular trichomes and their use in biosynthetic studies of monoterpenes and other gland products. Analytical Biochemistry. 200 (1), 130-138 (1992).
- Conneely, L. J., Mauleon, R., Mieog, J., Barkla, B. J., Kretzschmar, T. Characterization of the Cannabis sativa glandular trichome proteome. PLoS One. 16 (4), 0242633 (2021).
- Liu, Y., Zhu, P., Cai, S., Haughn, G., Page, J. E. Three novel transcription factors involved in cannabinoid biosynthesis in Cannabis sativa L. Plant Molecular Biology. 106 (1-2), 49-65 (2021).
- Slone, J. H., Kelsey, R. G. Isolation and purification of glandular secretory cells from Artemisia tridentata (ssp. vaseyana) by Percoll density gradient centrifugation. American Journal of Botany. 72 (9), 1445-1451 (1985).
- Namdar, D., et al. Terpenoids and Phytocannabinoids Co-Produced in Cannabis Sativa Strains Show Specific Interaction for Cell Cytotoxic Activity. Molecules. 24 (17), 3031 (2019).
- McDowell, E. T., et al. Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiology. 155 (1), 524-539 (2011).
- Bergau, N., Santos, A. N., Henning, A., Balcke, G. U., Tissier, A. Autofluorescence as a signal to sort developing glandular trichomes by flow cytometry. Frontiers in Plant Science. 7, 949 (2016).
- Livingston, S. J., et al. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. The Plant Journal. 101 (1), 37-56 (2019).
- Turner, J. C., Hemphill, J. K., Mahlberg, P. G. Gland distribution and cannabinoid content in clones of Cannabis sativa L. American Journal of Botany. 64 (6), 687-693 (1977).
- Turner, J. C., Hemphill, J. K., Mahlberg, P. G. Quantitative determination of cannabinoids in individual glandular trichomes of Cannabis sativa L. (Cannabaceae). American Journal of Botany. 65 (10), 1103-1106 (1978).
- Hammond, C. T., Mahlberg, P. G. Morphology of glandular hairs of Cannabis sativa from scanning electron microscopy. American Journal of Botany. 60 (6), 524-528 (1973).
- Marks, M. D., et al. A new method for isolating large quantities of Arabidopsis trichomes for transcriptome, cell wall, and other types of analyses. The Plant Journal. 56 (3), 483-492 (2008).
- Huebbers, J. W., et al. An advanced method for the release, enrichment and purification of high-quality Arabidopsis thaliana rosette leaf trichomes enables profound insights into the trichome proteome. Plant Methods. 18 (1), 12 (2022).

