Gene Expression Analyses in Human Follicles
Summary
Here, we describe a protocol outlining how to isolate human ovarian follicles from frozen-thawed cortical tissue to perform gene expression analyses.
Abstract
The ovary is a heterogeneous organ composed of different cell types. To study the molecular mechanisms occurring during folliculogenesis, the localization of proteins and gene expression can be performed on fixed tissue. However, to properly assess gene expression levels in a human follicle, this complex and delicate structure must be isolated. Hence, an adapted protocol previously described by Woodruff's laboratory has been developed to separate follicles (the oocyte and the granulosa cells) from their surrounding environment. The ovarian cortical tissue is first manually processed to obtain small fragments using two tools: a tissue slicer and a tissue chopper. The tissue is then enzymatically digested with 0.2% collagenase and 0.02% DNase for at least 40 min. This digestion step is performed at 37 °C and 5% CO2 and is accompanied by mechanical pipetting of the medium every 10 min. After incubation, the isolated follicles are collected manually using a calibrated microcapillary pipette under microscope magnification. If follicles are still present in the pieces of tissue, the procedure is completed with manual microdissection. The follicles are collected on ice in a culture medium and are rinsed twice in droplets of phosphate-buffered saline solution. This digestion procedure must be carefully controlled to avoid follicle deterioration. As soon as the structure of the follicles appears to be compromised or after a maximum of 90 min, the reaction is stopped with a 4 °C blocking solution containing 10% fetal bovine serum. A minimum of 20 isolated follicles (sized under 75 µm) should be collected to obtain an adequate amount of total RNA after RNA extraction for real-time quantitative polymerase chain reaction (RT-qPCR). After extraction, the quantification of total RNA from 20 follicles reaches a mean value of 5 ng/µL. The total RNA is then retrotranscribed into cDNA, and the genes of interest are further analyzed using RT-qPCR.
Introduction
The ovary is a complex organ composed of functional and structural units, including the follicles within the cortex and the stroma. Folliculogenesis, the process of follicle activation, growth, and maturation from a primordial quiescent state to a mature follicle able to be fertilized and to support early embryonic development, is widely studied in research1. Unraveling the mechanisms driving this phenomenon could improve fertility care for women2. Analyses on fixed human tissue allow the assessment of protein expression and gene localization within the functional units of the ovary3,4. However, specific techniques are needed to dissociate the follicles from the surrounding cortex to accurately assess gene expression levels within the ovarian follicles. Hence, in a previous study, a follicle isolation technique was developed to allow analyses of gene expression directly from the functional unit of the ovary5. Different approaches have been developed, such as enzymatic digestion and/or mechanical isolation, as well as laser capture microdissection, that allow follicle isolation within a piece of tissue6,7,8,9. Follicle isolation is widely used, either with human or animal ovarian tissue, to evaluate the gene expression profiles of follicles at all stages of development10,11,12. However, an optimal isolation procedure should take into account the fragile structure of the follicle within the dense cortex and, therefore, should be performed with care to avoid any damage7. This manuscript describes a procedure, adapted from a protocol described by Woodruff's laboratory, to isolate human follicles from frozen-thawed ovarian cortex in order to perform gene expression analyses13.
The first step of ovarian follicle isolation from frozen human tissue is the thawing procedure. This process is performed based on the clinical protocol used for the grafting of cryopreserved ovarian tissue, as previously described14,15. The process aims to remove the cryoprotectant agents by rinsing the ovarian cortex in decreasing concentrations of the medium. Then, the tissue is fragmented before enzymatic and mechanical isolation to retrieve the follicles. Follicles at different stages can be distinguished using a stereomicroscope with high magnification and good-quality optics in order to isolate the ones of interest. Each isolated follicle is measured using a ruler integrated into the microscope, and the follicles can be pooled according to their developmental stage: primordial follicles (30 µm), primary follicles (60 µm), secondary follicles (120-200 µm), and antral follicles (>200 µm)16. Further classification can be performed according to the morphology of the follicles: primordial follicles have one layer of flattened granulosa cells (GCs), primary follicles have one layer of cuboidal GCs, secondary follicles have at least two layers of cuboidal GCs, and the presence of a cavity among the GCs characterizes the antral stage. When follicles of interest are selected, RNA extraction is performed. The RNA quantity and quality are evaluated prior to real-time quantitative polymerase chain reaction (RT-qPCR) (Figure 1).
Protocol
This project was approved by the Erasme Hospital Ethical Committee (Brussels, Belgium). The patient included in this protocol underwent ovarian tissue cryopreservation (OTC) for fertility preservation before chemotherapy exposure in 2000. The patient signed informed written consent to donate her residual frozen tissue to research at the end of the storage period.
1. Thawing of cryopreserved ovarian tissue
- Prepare a 6-well plate containing five thawing solutions. The first well contains 5 mL of cryoprotectant solution composed of Leibovitz-15 medium, 0.1 mol/L sucrose, 1.5 mol/L dimethyl sulfoxide (DMSO), and 1% human serum albumin (HSA). The next wells contain 5 mL of Leibovitz-15 medium with decreasing concentrations of cryoprotectant: 1 mol/L, 0.5 mol/L, and 2 x 0 mol/L DMSO.
NOTE: The cryoprotectant solution can differ according to the protocol used in the fertility clinic. - Remove a vial containing an ovarian cortical fragment from liquid nitrogen in compliance with safety rules (cryogenic gloves, protective glasses, and closed shoes), and keep the tube at room temperature (RT) for 30 s.
- Soak the vial in double-distilled water for 2 min at RT with gentle agitation before opening the vial under a vertical hood and directly transferring it into the first well of the 6-well plate (on ice).
- Transfer the fragment successively into each well of the 6-well plate, with each containing decreasing concentrations of cryoprotectant in 5 mL of Leibovitz-15 medium. Gently agitate the tissue in each medium for 5 min (on ice).
2. Follicle isolation
NOTE: All experiments are conducted using RNase-free materials and under a vertical hood.
- Transfer the thawed tissue into a 2 mm gridded Petri dish filled with 10 mL of dissection medium (Leibovitz-15 medium, sodium pyruvate [2 mmol/L], L-glutamine [2 mmol/L], HSA [0.3%], penicillin G [30 µg/mL], and streptomycin [50 µg/mL]). Adjust the size of the tissue with a scalpel if necessary.
NOTE: Depending on the clinical protocol, the size of the frozen tissue can vary from 8 mm x 4 mm x 1 mm to 4 mm x 2 mm x 1 mm. For this protocol, two strips of 4 mm x 2 mm x 1 mm were used. - Pile up the three pieces of the tissue slicer, put the fragment between the two blocks, and cut the fragment in half using a blade, sliding through the blocks to obtain two fragments of 0.5 mm thickness.
- Use the tissue chopper to cut the fragment and obtain smaller pieces. If necessary, cut the remaining pieces manually with a scalpel until the tissue is totally shattered.
- Transfer the fragmented tissue into a gridded Petri dish filled with 7 mL of digestion medium (culture medium [McCoy's 5A medium, 3 mmol/L glutamine, 0.1% HSA, 30 µg/mL penicillin G, 50 µg/mL streptomycin, 2.5 µg/mL transferrin, 4 ng/mL selenium, and 50 µg/mL ascorbic acid] supplemented with 0.2 % collagenase and 0.02% DNase).
- Put the dish in the incubator at 5% CO2 and 37 °C. Every 10 min, take the Petri dish out of the incubator, and flush the tissue by pipetting up and down with a 1 mL pipette.
- After 45 min of incubation in the digestion medium, place the dish under a stereomicroscope with a magnification range of 5x-6.3x, and retrieve the follicles using a microcapillary pipette. Select the follicles of interest, and isolate them by sucking them up with a mouth pipette.
NOTE: Mouth pipetting should be performed carefully to avoid the loss of material into the pipe and contamination. - If the follicles remain stuck in a piece of cortex, isolate them mechanically with two 27 G syringes by ripping the cortex off with the tip of the syringes to release follicles from the stroma.
NOTE: Do not touch the follicles with the syringes to avoid damaging them. - Transfer the follicles with the microcapillary in a 4-well plate containing drops of 15 µL of the calibrated culture medium covered by 500 µL of oil culture (1 to 10 follicles per drop). This step maintains the follicle viability during the collection process. At the end of the procedure, rinse the follicles twice for 5 s each time into two drops of 15 µL of phosphate-buffered saline solution (PBS) covered by 500 µL of oil culture (on ice).
- Collect 20 follicles with a minimal quantity of PBS using the mouth pipette (maximum 10 µL) in an empty tube, and keep the tube on ice.
NOTE: For RNA stability, it is crucial to perform step 2.8 and step 2.9 on ice. Around 20 follicles (<75 µm) are needed to have enough RNA for standard RT-qPCR (SYBR Green). - After a maximum of 90 min of incubation in the digestion medium, stop the enzymatic reaction by adding into the Petri dish an excess of cold (4 °C) blocking solution (7.5 mL) composed of culture medium supplemented with 10% fetal bovine serum (FBS).
NOTE: The blocking solution can be added as soon as the experimenter observes follicles sticking on the plate or damaged follicles (asymmetric shape or darker-colored follicles). It is advised to perform follicle isolation within a maximum period of 2.5 h to avoid follicular damage and to perform RNA extraction immediately after isolation.
3. RNA extraction
NOTE: RNA extraction is performed following the instructions provided with an RNA extraction kit by adapting the elution volumes.
- Suspend the isolated follicles by adding 100 µL of the lysis solution provided with the RNA extraction kit to the tube containing the follicles under a chemical hood, and vortex at a high speed (2,500 rpm/min) to break the structure of the follicles. Add 50 µL of ethanol (100%) to the tube, and briefly vortex at a high speed.
NOTE: As the lysis buffer contains 2-mercaptoethanol and thiocyanic acid, this step must be performed with caution under a chemical hood. - Transfer the total volume of the tube (approximately 160 µL) to a microfilter cartridge assembly comprising a column and a collection tube, and centrifuge it for 10 s at 16,363 x g at 4 °C. Wash the column with 180 µL of wash solution 1, provided with the kit, and centrifuge the tube for 10 s at 16,363 x g at 4 °C.
- Add 180 µL of wash solution 2/3, provided with the kit, to the column, and centrifuge for 10 s at 16,363 x g at 4 °C. Perform this step twice. Centrifuge the tube one last time for 1 min to dry the filter, and place a new collection tube under the cartridge.
- Perform the elution of the nucleic acids in two steps:
- First, add 8 µL of warm elution solution (75 °C) to the filter, wait for 1 min at RT, and centrifuge for 30 s at 16,363 x g at 4 °C.
- Repeat this step with 7 µL of elution solution.
NOTE: The tube containing the eluted nucleic acids must be kept on ice. To avoid DNA contamination, a supplemental step of DNA degradation is highly recommended-step 3.5.
- Incubate the tube with 2 IU DNase and 1x DNase buffer for 20 min at 37 °C. Block the enzymatic activity with 1/10 DNase inactivation reagent for 2 min at RT.
- Centrifuge the tube for 1.5 min at 16,363 x g at 4 °C. Finally, collect the suspension containing the RNA, and transfer it to a new tube.
- Assess the quantity of RNA present in the sample by using a spectrophotometer (NanoDrop 2000 software > Nucleic Acid > RNA). Use 1 µL of elution solution as a blank, and measure the RNA quantity extracted from isolated follicles with 1 µL of solution.
- Check the 260/280 ratio for RNA purity (around 2.0). Store the sample at −80 °C, or directly retrotranscribe it to perform RT-qPCR.
NOTE: To assess the RNA integrity, the sample can be processed with a high-resolution automated electrophoresis system before or after freezing at −80 °C. In this work, following retrotranscription, RT-qPCR was performed with the following cycle:
Hold stage with 20 s at 50 °C and 10 min at 95 °C
40 PCR cycles with 15 s at 95 °C and 1 min at 60 °C
Melt curve stage with 15 s at 95 °C, 1 min at 60 °C, 30 s at 95 °C, and 15 s at 60 °C
Representative Results
Using this isolation procedure, the experimenter can retrieve follicles from the stromal environment to perform specific gene expression analyses. Based on the size and morphology of the follicles, it is possible to differentiate the different stages of folliculogenesis. The experimenter can select follicles of interest according to their size using an adapted microcapillary pipette. By using a microcapillary of maximum 75 µm, it is possible to discriminate primordial and primary follicles from secondary, antral, and mature follicles. Furthermore, the experimenter can confirm the follicle stage according to the follicle morphology. When studying follicle activation, quiescent and primary follicles are selected, and approximatiely 20 follicles are needed to reach an RNA concentration of around 5 ng/µL.
Two homogenized ovarian fragments (4 mm x 2 mm x 1 mm) from the same patient were processed to isolate follicles using this protocol (Figure 2). After a 1.5 h isolation procedure, two populations of follicles were retrieved according to the developmental stages to compare the RNA quantity and to highlight the relevance of follicle selection: 20 follicles of <75 µm (tube 1) and 15 follicles of <200 µm (tube 2). Then, RNA extraction was performed, and 4.8 ng/µL total RNA from tube 1 and 10.5 ng/µL total RNA from tube 2 were obtained using a spectrophotometer for quantification (Table 1). Using this microvolume spectrophotometer system, the 260/280 ratio was also measured to assess the RNA purity. This ratio reflects the potential contamination by protein or other reagents during extraction and must be close to 2.0 for RNA samples. The 260/280 ratios were 1.89 and 1.74 in tube 1 and tube 2, respectively, validating the purity of the samples (Table 1). The RNA quality was then verified by processing the samples with high-resolution automated electrophoresis. Following this procedure, the RNA integrity number (RIN) was estimated. The RIN value, from 1 (degraded) to 10 (intact), reflects the level of degradation of RNA in a sample. The RIN values were 7.1 and 7.9 in tube 1 and tube 2, respectively, validating the quality of the RNA from our samples (Table 1). To perform a RT-qPCR, the total volume of extracted RNA was first retrotranscribed into cDNA. Then, 1.25 ng of cDNA was added per well on a 96-well plate, with primers for housekeeping and target genes (Hypoxanthine-guanine phosphoribosyltransferase [HPRT], Kit Ligand [KL], and Growth Differentiation Factor 9 [GDF9]) and SYBR Green master mix17,18. After running a standard cycle on a real-time PCR system, the cycle thresholds (Ct) obtained were 30.87 (tube 1) and 29.56 (tube 2) for HPRT, 33.5 (tube 1) and 31.77 (tube 2) for KL, and 30.71 (tube 1) and 30.57 (tube 2) for GDF9 (Table 1).
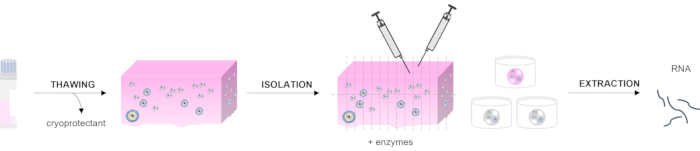
Figure 1: Schematic representation of the method for assessing RNA extraction from isolated human ovarian follicles. Three steps are described in this manuscript: tissue thawing, the isolation procedure, including mechanical and enzymatic processing, and RNA extraction from the follicles. Please click here to view a larger version of this figure.

Figure 2: Collected follicles following the isolation protocol. (A) Thawed cortical tissue. (B) The tissue slicer and (C) tissue chopper used to fragment the tissue. (D–E) Images of retrieved isolated follicles observed with a stereomicroscope with a 10x eyepiece and a wide zoom range (1x-6.3x); magnification settings of 50x-63x were used for the identification of primordial and primary follicles. Scalebars: 100 µm. Please click here to view a larger version of this figure.
| Tube | Number of follicles | Follicles size (µm) | RNA concentration (ng/µL) | 260/280 ratio | RNA Integrity Number (RIN) | HPRT Ct | KL Ct | GDF9 Ct | |
| 1 | 20 | <75 | 4.8 | 1.89 | 7.1 | 30.87 | 33.5 | 30.71 | |
| 2 | 15 | <200 | 10.5 | 1.74 | 7.9 | 29.56 | 31.77 | 30.57 | |
Table 1: Purity, RIN, and cycle thresholds of two homogenized ovarian fragments.
Discussion
The cryopreservation of ovarian tissue is a promising approach for preserving the fertility of cancer patients. In the clinic, thawed cortical tissue is grafted back into the patient after remission, allowing the resumption of ovarian function and fertility19,20. Besides clinical use, residual ovarian fragments may also be donated for research at the end of the storage period to study the mechanisms regulating folliculogenesis. Moreover, this tissue is particularly useful for developing alternatives to grafting when it is not feasible, such as in vitro culture systems or artificial ovaries. However, variations both within and between patients limit the feasibility and reproducibility of the experiments and the interpretation of the results. Indeed, follicular density dramatically decreases with age, and follicles are not equally distributed between fragments21. As the number of available fragments is limited, human tissue needs to be used with cutting-edge techniques. A wide range of techniques such as immunohistochemistry and immunofluorescence, as well as in situ hybridization, can be performed on fixed tissue to assess protein and gene localization3,18. To study the molecular mechanisms regulating folliculogenesis within the functional units of the ovary at different stages of development, the isolation of follicles is required.
Several studies have explored effective methods to separate follicles from the surrounding tissue in human and animal species22. The number of follicles retrieved and the quality of RNA after extraction may vary according to the techniques used. To study follicle activation (i.e., primordial and primary follicles), at least 20 pooled follicles are needed to perform standard RT-qPCR. By using alternative methods such as RNA or cDNA amplification or nested PCR, it is possible to work with fewer follicles23,24.
The dense nature of the ovarian cortex makes follicle isolation challenging, which has led to the use of methods involving a combination of enzymatic and mechanical isolation25. Different types of enzymes can be used, such as collagenase and DNase26. However, studies have reported follicle damage following enzymatic digestion, impacting further in vitro development27,28. To maintain follicle integrity, several teams are currently using adapted enzymatic digestion protocols or performing only mechanical isolation for subsequent follicular culture25,29,30,31. This manuscript reports a simple and efficient method to obtain isolated follicles for quantitative PCR analysis. Nevertheless, all experiments require a learning curve before reaching optimal results.
For fundamental research purposes, a revision of a previous protocol was developed to retrieve a large quantity of follicles without affecting their integrity13. The processing for gene expression analysis is directly performed following isolation to optimize RNA quality. It is also crucial to avoid prolonged digestion in order to limit the risk of selecting degenerated follicles. Thus, the digestion step remains crucial in this protocol. Three points require specific attention: (1) the flushing of the tissue during the enzymatic reaction every 10 min to provide homogeneous enzyme action; (2) the control of the follicle integrity by selecting healthy follicles during the procedure and stopping the reaction with blocking solution as soon as damage is observed; (3) the adaptation of the collagenase concentration, as tissue stiffness may vary between patients according to age. The concentration of collagenase can be decreased (to 0.12%) in some cases to prevent damage (i.e., in prepubertal patients)32.
In the last part of this protocol, RNA extraction from isolated follicles was performed following adapted instructions. This allows a wide range of experiments, such as RT-qPCR or RNA sequencing, providing fundamental information on various cellular processes specific to oocyte and granulosa cells. Numerous protocols and kits are available to extract RNA from tissue. We have reported here an efficient technique used in our laboratory that allows the extraction of a sufficient quantity of RNA to perform gene expression analyses by RT-qPCR. While direct RNA extraction is highly recommended following follicle isolation to maintain RNA integrity, it is also possible to freeze the isolated follicles at −80 °C if the extraction cannot be performed the same day. The RNA quantity may vary according to the size of the follicles, and growing follicles may interfere with the analysis if pooled with the quiescent follicles. Selection based on size is extremely relevant, depending on the objectives of the study, especially for the first step of folliculogenesis. However, the differentiation between primordial and primary follicles based only on size remains challenging in terms of properly assessing primordial follicle gene expression levels. The selection of primordial and primary follicles can be performed based on their morphology, but the discrimination between them with a stereomicroscope at a magnification of 63x is difficult.
In conclusion, the experimenter must be aware of the challenges of follicle isolation and take them into account when analyzing the results. Important parameters must be considered, such as (1) intra/inter-variations in follicle density between patients; (2) follicle integrity during follicle isolation; (3) differentiation between different follicles stages; and (4) RNA stability and integrity.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by an Excellence of Science (EOS) grant (ID: 30443682). I.D. is an associate researcher at Fonds National de la Recherche Scientifique de Belgique (FNRS).
Materials
| 2 mm gridded Petri dish | Corning | 430196 | |
| 2100 Bioanalyzer instrument | Agilent | G2939BA | |
| 2100 Expert software | Agilent | version B.02.08.SI648 | |
| 4-wells plate | Sigma Aldrich | D6789 | |
| 6-wells plate | Carl Roth | EKX5.1 | |
| Agilent total RNA 6000 pico kit | Agilent | 5067-1513 | |
| Ascorbic acid | Sigma Aldrich | A4403 | |
| Aspirator tube assemblies for microcapillary pipettes | Sigma Aldrich | A5177 | |
| Centrifuge | Eppendorf | 5424R | |
| Collagenase IV | LifeTechnologies | 17104-019 | |
| DMSO | Sigma Aldrich | D2650 | |
| DNase | Sigma Aldrich | D4527-10kU | |
| FBS | Gibco | 10270-106 | |
| GoScript reverse transcriptase | Promega | A5003 | |
| HSA | CAF DCF | LC4403-41-080 | |
| Leibovitz-15 | LifeTechnologies | 11415-049 | |
| L-Glutamine | Sigma Aldrich | G7513 | |
| McCoy’s 5A + bicarbonate + Hepes | LifeTechnologies | 12330-031 | |
| McIlwain tissue chopper | Stoelting | 51350 | |
| Microcapillary RI EZ-Tips 200 µm | CooperSurgical | 7-72-2200/1 | |
| Microcapillary RI EZ-Tips 75 µm | CooperSurgical | 7-72-2075/1 | |
| NanoDrop 2000/2000c operating software | ThermoFisher | version 1.6 | |
| NanoDrop spectrophotometer | ThermoFisher | 2000/2000c | |
| Penicillin G | Sigma Aldrich | P3032 | |
| PowerTrack SYBR green master mix | ThermoFisher | A46109 | |
| Primers: GDF9 | F: CCAGGTAACAGGAATCCTTC R: GGCTCCTTTATCATTAGATTG | ||
| Primers: HPRT | F: CCTGGCGTCGTGATTAGTGAT R: GAGCACACAGAGGGCTACAA | ||
| Primers: Kit Ligand | F: TGTTACTTTCGTACATTGGCTGG R: AGTCCTGCTCCATGCAAGTT | ||
| Real-Time qPCR Quantstudio 3 | ThermoFisher | A33779 | |
| RNAqueous-micro total RNA isolation kit | ThermoFisher | AM1931 | |
| Selenium | Sigma Aldrich | S9133 | |
| Sodium pyruvate | Sigma Aldrich | S8636 | |
| Stereomicroscope | Nikon | SMZ800 | |
| Streptomycine sulfate | Sigma Aldrich | S1277 | |
| Sucrose | Sigma Aldrich | S1888 | |
| Thermo Scientific Forma Series II water-jacketed CO2 incubators | ThermoFisher | 3110 | |
| Thomas Stadie-Riggs tissue slicer | Thomas Scientific | 6727C10 | |
| Transferrin | Roche | 10652202001 |
References
- Gougeon, A. Human ovarian follicular development: From activation of resting follicles to preovulatory maturation. Annales d’Endocrinologie. 71 (3), 132-143 (2010).
- Yang, D. Z., Yang, W., Li, Y., He, Z. Progress in understanding human ovarian folliculogenesis and its implications in assisted reproduction. Journal of Assisted Reproduction and Genetics. 30 (2), 213-219 (2013).
- Rosewell, K. L., Curry, T. E. Detection of ovarian matrix metalloproteinase mRNAs by in situ hybridization. Molecular Endocrinology. 590, 115-129 (2009).
- Tuck, A. R., Robker, R. L., Norman, R. J., Tilley, W. D., Hickey, T. E. Expression and localisation of c-kit and KITL in the adult human ovary. Journal of Ovarian Research. 8, 31 (2015).
- Oktay, K., et al. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertility and Sterility. 67 (3), 481-486 (1997).
- Bonnet, A., et al. Transcriptome profiling of sheep granulosa cells and oocytes during early follicular development obtained by laser capture microdissection. BMC Genomics. 12, 417 (2011).
- Chiti, M. C., et al. A modified and tailored human follicle isolation procedure improves follicle recovery and survival. Journal of Ovarian Research. 10 (1), 71 (2017).
- Kim, E. J., et al. Comparison of follicle isolation methods for mouse ovarian follicle culture in vitro. Reproductive Sciences. 25 (8), 1270-1278 (2018).
- Chen, J., et al. Optimization of follicle isolation for bioengineering of human artificial ovary. Biopreservation and Biobanking. 20 (6), 529-539 (2022).
- Babayev, E., Xu, M., Shea, L. D., Woodruff, T. K., Duncan, F. E. Follicle isolation methods reveal plasticity of granulosa cell steroidogenic capacity during mouse in vitro follicle growth. Molecular Human Reproduction. 28 (10), (2022).
- McDonnell, S. P., Candelaria, J. I., Morton, A. J., Denicol, A. C. Isolation of small preantral follicles from the bovine ovary using a combination of fragmentation, homogenization, and serial filtration. Journal of Visualized Experiments. (187), e64423 (2022).
- Schallmoser, A., Einenkel, R., Färber, C., Sänger, N. In vitro growth (IVG) of human ovarian follicles in frozen thawed ovarian cortex tissue culture supplemented with follicular fluid under hypoxic conditions. Archives of Gynecology and Obstetrics. 306 (4), 1299-1311 (2022).
- Xu, M., et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Human Reproduction. 24 (10), 2531-2540 (2009).
- Demeestere, I., Simon, P., Englert, Y., Delbaere, A. Preliminary experience of ovarian tissue cryopreservation procedure: Alternatives, perspectives and feasibility. Reproductive Biomedicine Online. 7 (5), 572-579 (2003).
- Demeestere, I., et al. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: Case report. Human Reproduction. 21 (8), 2010-2014 (2006).
- Gougeon, A. Dynamics of follicular growth in the human: A model from preliminary results. Human Reproduction. 1 (2), 81-87 (1986).
- Grosbois, J., Demeestere, I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Human Reproduction. 33 (9), 1705-1714 (2018).
- Grosbois, J., Vermeersch, M., Devos, M., Clarke, H. J., Demeestere, I. Ultrastructure and intercellular contact-mediated communication in cultured human early stage follicles exposed to mTORC1 inhibitor. Molecular Human Reproduction. 25 (11), 706-716 (2019).
- Oktay, K., et al. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. Journal of the American Medical Association. 286 (12), 1490-1493 (2001).
- Chung, E. H., Lim, S. L., Myers, E., Moss, H. A., Acharya, K. S. Oocyte cryopreservation versus ovarian tissue cryopreservation for adult female oncofertility patients: A cost-effectiveness study. Journal of Assisted Reproduction and Genetics. 38 (9), 2435-2443 (2021).
- Walker, C. A., Bjarkadottir, B. D., Fatum, M., Lane, S., Williams, S. A. Variation in follicle health and development in cultured cryopreserved ovarian cortical tissue: A study of ovarian tissue from patients undergoing fertility preservation. Human Fertility. 24 (3), 188-198 (2021).
- Simon, L. E., Kumar, T. R., Duncan, F. E. In vitro ovarian follicle growth: A comprehensive analysis of key protocol variables. Biology of Reproduction. 103 (3), 455-470 (2020).
- Rice, S., Ojha, K., Mason, H. Human ovarian biopsies as a viable source of pre-antral follicles. Human Reproduction. 23 (3), 600-605 (2008).
- Kristensen, S. G., Rasmussen, A., Byskov, A. G., Andersen, C. Y. Isolation of pre-antral follicles from human ovarian medulla tissue. Human Reproduction. 26 (1), 157-166 (2011).
- Dong, F. -. L., et al. An research on the isolation methods of frozen-thawed human ovarian preantral follicles. International Journal of Clinical and Experimental Medicine. 7 (8), 2298-2303 (2014).
- Lierman, S., et al. Follicles of various maturation stages react differently to enzymatic isolation: a comparison of different isolation protocols. Reproductive Biomedicine Online. 30 (2), 181-190 (2015).
- Abir, R., et al. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Human Reproduction. 14 (5), 1299-1301 (1999).
- Abir, R., et al. Morphological study of fully and partially isolated early human follicles. Fertility and Sterility. 75 (1), 141-146 (2001).
- McLaughlin, M., Albertini, D. F., Wallace, W. H. B., Anderson, R. A., Telfer, E. E. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Molecular Human Reproduction. 24 (3), 135-142 (2018).
- Abir, R., et al. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertility and Sterility. 68 (4), 682-688 (1997).
- Vanacker, J., et al. Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: Protocol for application in a clinical setting. Fertility and Sterility. 96 (2), 379-383 (2011).
- Amargant, F., et al. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell. 19 (11), 13259 (2020).

