Elucidating the Metabolism of 2,4-Dibromophenol in Plants
Summary
The present protocol describes a simple and efficient method for the identification of 2,4-dibromophenol metabolites in plants.
Abstract
Crops can be extensively exposed to organic pollutants, since soil is a major sink for pollutants discarded into the environment. This creates potential human exposure through the consumption of pollutant-accumulated foods. Elucidating the uptake and metabolism of xenobiotics in crops is essential for the assessment of dietary exposure risk in humans. However, for such experiments, the use of intact plants requires long-term experiments and complex sample preparation protocols that can be affected by various factors. Plant callus cultures combined with high-resolution mass spectrometry (HRMS) may provide a solution for the accurate and time-saving identification of metabolites of xenobiotics in plants, as it can avoid interference from the microbial or fungal microenvironment, shorten the treatment duration, and simplify the matrix effect of intact plants. 2,4-dibromophenol, a typical flame retardant and endocrine disrupter, was chosen as the model substance due to its widespread occurrence in soil and its uptake potential by plants. Herein, plant callus was generated from asepsis seeds and exposed to sterile 2,4-dibromophenol-containing culture medium. The results showed that eight metabolites of 2,4-dibromophenol were identified in the plant callus tissues after 120 h of incubation. This indicates that 2,4-dibromophenol was rapidly metabolized in the plant callus tissues. Thus, the plant callus culture platform is an effective method to evaluate the uptake and metabolism of xenobiotics in plants.
Introduction
An increasing number of organic pollutants have been discarded into the environment due to anthropogenic activities1,2, and soil is considered a major sink for these contaminants3,4. The contaminants in the soil can be taken up by plants and potentially transferred to higher trophic-level organisms along food chains, by directly entering the human body through crop consumption, consequently leading to unintended exposure5,6. Plants utilize different pathways to metabolize xenobiotics for detoxification7; elucidating the metabolism of xenobiotics is important, as it controls the actual fate of contaminants in plants. As the metabolites can be excreted by leaves (to the atmosphere) or the roots, determining the metabolites in the very early phases of exposure hence provides the possibility to test an extended number of metabolites8. However, studies using intact plants require long-term experiments and complex sample preparation protocols that can be affected by various factors.
Plant callus cultures, therefore, are a good alternative for studying the metabolism of xenobiotics in planta, as they can greatly shorten the treatment time. These cultures exclude microbial interference and photochemical degradation, simplify the matrix effect of intact plants, standardize the cultivation conditions, and require less experimental effort. Plant callus cultures have been successfully applied as an alternative approach in metabolic studies of triclosan9, nonylphenol10, and tebuconazole8. These studies showed that the metabolic patterns in callus cultures were similar to those in intact plants. This study proposes a method for the efficient and accurate identification of metabolites of xenobiotics in plants without complex and time-consuming protocols. Here, we use plant callus cultures in combination with high-resolution mass spectrometry for the analysis of metabolites with low-intensity signals11,12.
To this end, carrot (Daucus carota var. sativus) callus suspensions were exposed to 100 µg/L of 2,4-dibromophenol for 120 h in a shaker at 130 rpm and 26 °C. 2,4-dibromophenol was chosen due to its disruptive endocrine activity13 and widespread occurrence in soil14. The metabolites were extracted and analyzed by high-resolution mass spectrometry. The protocol proposed here can investigate the in planta metabolism of other types of organic compounds that can be ionized.
Protocol
1. Differentiation of carrot callus
NOTE: Autoclave all equipment used here and perform all operations in a UV-sterilized ultra-clean workbench.
- Vernalize the seeds by immersing the uniform carrot seeds (Daucus carota var. sativus) into deionized water at 4 °C for 16 h.
- Surface-sterilize the vernalized seeds with 75% ethanol for 20 min, and then rinse three times with sterile deionized water under aseptic conditions.
- Further sterilize the seeds with 20% H2O2 for 20 min, and wash them with sterilized deionized water six times under aseptic conditions.
- Aseptically germinate the seeds by sowing them on hormone-free MS medium (pH 5.8, autoclaved at 121 °C for 20 min) containing 1% agar-gel, and incubating at 26 °C with a 16 h photoperiod (350 µmol/m2s) for 15 days.
- Obtain the explants by cutting the hypocotyl and cotyledon of the seedlings into small pieces (0.5 cm).
- Transform the explants to Petri dishes (two to four explants per dish) containing 15-20 mL of aseptic MS medium supplemented with auximone (2,4-dichlorophenoxyacetic acid; 1 mg/L) and phytokinin (6-benzylaminopurine; 0.5 mg/L) under aseptic conditions.
- Incubate the explants in the dark at 26 °C for 3-4 weeks to induce the callus.
- Separate the callus tissues (around 1 cm diameter) formed from the initial explants using a sterile scalpel and forceps.
NOTE: The newly formed callus tissues are white to creamy yellow in color and loosely attach to the initial explants.
2. 2,4-dibromophenol treatment
- Dissolve 1 µg of 2,4-dibromophenol in 10 mL of aseptic liquid MS medium (the final concentration of 2,4-dibromophenol is 100 ppb, pH 5.6-7.0).
- Add 3 g of the fresh carrot callus (step 1.8) into glass flasks containing the prepared 2,4-dibromophenol solution (from step 2.1) under aseptic conditions. Consider this as the 2,4-dibromophenol treatment.
NOTE: The glass flasks were autoclaved and sealed using paraffin film. - Include a medium control containing the 2,4-dibromophenol solution only (prepared in step 2.1) to evaluate the abiotic degradation of 2,4-dibromophenol.
- Include a blank control containing the carrot callus only (no 2,4-dibromophenol solution) to check for any potential contamination.
- Prepare the blank control containing carrot by adding 3 g of fresh collected carrot callus only into 10 mL of sterile MS medium.
- Incubate the 2,4-dibromophenol treatment and the medium and blank controls at 130 rpm and 26 °C in the dark in an incubator for 120 h.
- Remove the glass flasks from the incubator to collect the samples from the 2,4-dibromophenol treatment and controls after 120 h of incubation.
NOTE: All samples were prepared in triplicate.
3. Sample preparation
- Separate the callus carefully from the MS medium by filtration with glass fiber filters (0.45 µm) for the 2,4-dibromophenol treatment and the controls. Collect the callus after washing with ultrapure water three times.
- Freeze-dry the collected callus with liquid nitrogen, and subsequently homogenize the collected callus (0.2 g) with a high-throughput tissue grinder at 70 Hz for 3 min.
- Spike the homogenized callus by adding 50 µL of 25 mg/L surrogate 4-n-NP-d4 with a glass microsyringe and subsequently vortexing for 1 min.
- Sonicate the samples with 5 mL of methanol/water (1:1, v/v) in an ultrasonicator (150 W, 40 kHz) filled with ice water for 30 min, to extract 2,4-dibromophenol and the metabolites.
- Centrifuge the suspensions at 8,000 x g at 4 °C for 10 min, and collect the supernatants by pipetting.
- Repeat the extraction processes for the callus sample three times and combine the extracts.
- Pass the extracts through hydrophilic lipophilic balanced solid phase extraction (HLB SPE) cartridges with a flow rate of 1 mL/min.
NOTE: The HLB SPE cartridges were pretreated sequentially with 6 mL of methanol and 6 mL of water to remove any interferences. - Elute the analytes by passing 6 mL of methanol through the HLB SPE cartridges. Then, concentrate the obtained eluents to 1 mL under a gentle stream of nitrogen gas for instrumental analysis.
- Inject 10 µL of resultant eluents into the UPLC-Q-TOF-MS for 2,4-dibromophenol and their metabolites analysis15.
- Filter all the samples with a 0.22 µm nylon membrane before instrumental analysis.
4. Instrumental analysis
NOTE: 2,4-dibromophenol and their metabolites' analyses were performed on an ultra-performance liquid chromatograph (UPLC) in combination with a micrOTOF-QII mass spectrometer equipped with an electrospray ionization (ESI), operating in the positive and negative ion mode.
- Open the column heater door and install the UPLC column by connecting the column inlet to the injection valve and column outlet to the mass spectrometer's inlet.
NOTE: A C18 column (50 mm x 2.1 mm; 1.7 µm particle size) was used for the separation of analytes at 40 °C. - Connect mobile phase A (ultrapure water) and mobile phase B (chromatography-grade methanol) to the instrument by inserting the end of the solvent tubes A and B into the corresponding solvent bottles, respectively.
- Filter all mobile phases (500 mL for each) through a 0.22 µm filter, and sonicate for more than 30 min.
- In the software window, click on Instrument | Inlet Method to edit the conditions for the liquid chromatogram.
- Set the gradient conditions of mobile phase B as follows: a flow rate of 1.0 mL/min; 0-0.5 min, 5%; 0.5-3.5 min, 5% to 50%; 3.5-6.5 min, 50% to 100%; 6.5-7 min, 100%; 7-10 min, 100% to 5%.
- Set the injection rate of the samples into the UPLC-Q-TOF-MS as 0.2 mL/min.
NOTE: The injection of the sample is programmed using a fully automatic sampler.
- In the software window, select MS Method and then set up the parameters of Q-TOF-MS: a drying gas (N2) flow rate of 8 L/min, a temperature of 300-350 °C; a capillary voltage of 4,500 V; a collision energy of 5-45 V; and a full scan range of 40-800 Da.
- Place the sample vials in the corresponding locations of the sample trays by serial number, and re-insert the sample trays into the sample chamber.
NOTE: Keep the sample trays flat and make sure that the door of the sample chamber is closed. - In the software window, select File | New to create a database. Name the database.
- Load the sample program created above by selecting MS File | Inlet file | Inject Volume.
- Save the database in the sample folder of the project by clicking File | Save.
- Select Run | Start in the software main window, and then select Acquire Sample Data and click OK in the window of the start sample list run to collect data.
NOTE: The real-time chromatogram can be viewed by clicking Chromatogram | Real-time Update during the data acquisition process. - Process the data in the software by selecting the target data row and clicking the Chromatogram window to view the MS scan chromatogram.
- In the Chromatogram window, click Display | TIC | ScanWaveDS | Add trace | OK to get the daughter scan mass spectra.
- Identify the metabolites by comparing the chromatograms of the 2,4-dibromophenol treatment and the controls.
- Elucidate the metabolite candidates by the retention time, mass, and fragmentation patterns16,17.
NOTE: The error of mass accuracy between the experimental m/z values of the parent ions of the metabolite candidates to their corresponding theoretical m/z should be less than 10 ppm.
Representative Results
The steps of the protocol are depicted in Figure 1. Following the protocol, we compared the chromatogram of the carrot callus extract from the 2,4-dibromophenol treatment to the controls, and found eight distinct peaks that are present in the 2,4-dibromophenol treatment but absent in the controls (Figure 2). This indicates that a total of eight metabolites of 2,4-dibromophenol (M562, M545, M661, M413, M339, M380, M424, and M187) were successfully detected in the 2,4-dibromophenol-treated carrot callus. Additionally, the peak of the parent 2,4-dibromophenol (retention time = 0.85 min) was not found in the chromatogram of the 2,4-dibromophenol treatment (Figure 2), indicating that 2,4-dibromophenol was rapidly metabolized in the carrot callus under the experimental conditions.
The chromatographic and mass information used to identify the metabolites of 2,4-dibromophenol in the carrot callus are summarized in Table 1. 2,4-dibromophenol incubated in carrot callus led to the formation of metabolites by direct conjugation with glucose (M562, M545, M661, and M413) and amino acids (M339, M380, and M424). For instance, M413 produced fragments at m/z 250.8954 and 163.1485, which correspond to dibutyl phthalate (DBP) and glucose (C6H11O5). M413 was further metabolized to form disaccharide conjugation metabolites M661, M545, and M562, by adding pentose or hexose. The M339, M380, and M424 were speculated to be 2,4-dibromophenol alanine, 2,4-dibromophenol acetylalanine, and 2,4-dibromophenol acetylaspartic acid, as they have the characteristic neutral loss of amino acid (C3H6NO2), acetylalanine (C5H8NO3), and acetylaspartic acid (C6H8NO5), producing the corresponding fragments at m/z 89.0932, 129.1140, and 173.1235, respectively15. The presented results suggest that plant callus cultures can be used as an efficient and reliable tool to elucidate the metabolism of xenobiotics in crops.
Figure 1: Method schematic. Please click here to view a larger version of this figure.
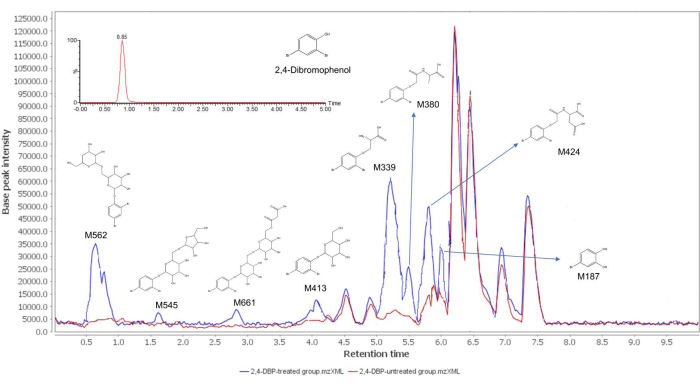
Figure 2: The chromatograms for 2,4-dibromophenol (theinserted picture) and the metabolites of 2,4-dibromophenol. This figure has been adapted with permission from Sun et al.15. Copyright (2018) American Chemical Society. Please click here to view a larger version of this figure.
| Metabolite | RT (min) | ESI Mode | Observed m/z |
Calculated m/z |
Predicated formula | Fragments (m/z) | Confidence level | |||
| M562 | 0.7 | -H | 562.201 | 562.201 | C18H26Br2O10 | 250.8954(-DBP) | Level 2b | |||
| 170.9914(-Br) | MS, MS2 | |||||||||
| M545 | 1.6 | -H | 545.151 | 545.1506 | C17H22Br2O10 | 250.8954(-DBP) | Level 2b | |||
| 170.9914(-Br) | ||||||||||
| 528.1433(-OH) | MS, MS2 | |||||||||
| M661 | 2.9 | -H | 661.222 | 661.2228 | C21H26Br2O14 | 250.8954(-DBP) | Level 2b | |||
| 410.3274(-C15H23O13) | MS, MS2 | |||||||||
| M413 | 4.1 | -H | 413.036 | 413.036 | C12H14Br2O6 | 250.8954(-DBP) | Level 1 | |||
| 163.1485(-C6H11O5) | ||||||||||
| 207.8938(250-CO2) | Synthetic Standard, RT, MS, MS2 | |||||||||
| M339 | 5.2 | +H | 339.994 | 339.9886 | C9H9Br2NO3 | 250.8954(-DBP) | Level 2b | |||
| 87.0773(-C3H6NO2) | MS, MS2 | |||||||||
| M380 | 5.5 | -H | 380.01 | 380.0094 | C11H11Br2NO4 | 250.8954(-DBP) | Level 2b | |||
| 129.1140(-C5H8NO3) | MS, MS2 | |||||||||
| M424 | 5.8 | -H | 424.012 | 424.0189 | C12H11Br2NO6 | 250.8954(-DBP) | Level 2b | |||
| 173.1235(-C6H8NO5) | MS, MS2 | |||||||||
| M187 | 6.1 | -H | 187.995 | 187.9988 | C6H5BrO2 | 109.1027(-Br) | Level 1 | |||
| 170.9914(-OH) | Authentic Standard, RT, MS, MS2 | |||||||||
Table 1: Summary of 2,4-dibromophenol and its metabolites discovered in carrot callus extracts. This table has been adapted with permission from Sun et al.15. Copyright (2018) American Chemical Society.
Discussion
This protocol was developed to efficiently identify the biotransformation of xenobiotics in plants. The critical step of this protocol is the culture of the plant callus. The most difficult part is the differentiation and maintenance of the plant callus, because the plant callus is easily infected and developed to plant tissues. Therefore, it is important to make sure that all equipment used is autoclaved, and all operations are performed under aseptic conditions. The differentiation and maintenance of the plant callus should be done in the dark to avoid autotrophic growth and overdevelopment. Additionally, the dose and types of phytohormones supplemented into the MS medium is critical for the differential of the plant callus, which should take the plant species into consideration. An overdose of phytohormones causes the callus to develop a vascular system, but insufficient phytohormones limit the differentiation of the plant callus18. The MS medium and phytohormone stocks should be prepared fresh. It is highly recommended to autoclave the MS medium before the introduction of the phytohormones and the aseptic hypocotyl.
Pigments such as chlorophyll in intact plants are a general problem in LC-HRMS measurements19,20. The plant callus is derived from aseptic hypocotyl and is transparent without chlorophyll. This means that plant callus culture can optimize the matrix effect of the intact plant and offer an easy but efficient sample preparation technique without pigment removal steps. The analysis of the metabolites of xenobiotics in plant callus was achieved with LC-HRMS in a non-targeted way15. The plant callus culture combined with non-targeted analysis allows for the effective identification of a large-scale profiling of metabolic compounds. These advantages make the method ideal for the mechanistic understanding of the metabolism of xenobiotics in plants. However, there are still several limitations for the protocol. For instance, the protocol can only be applied as a reference for the actual situation that occurs in plants in the field, due to the complex environmental conditions. Additionally, since different plant calluses exhibit different metabolic abilities, the identified metabolites of xenobiotics may vary with the type of callus.
Knowing the metabolic transformation of chemicals in plants is important for their safe development and application. The method proposed here is efficient and reliable for the screening of the generated metabolites in plants, and can support the evaluation of the associated risk to ecosystems and human health through the food chain transfer or direct dietary intake of crops. This protocol can investigate the persistence of xenobiotics in plants and help screen emerging contaminants. Considering its full metabolic capability, with fewer costs and decreased time and effort, the plant callus culture is a good tool to compare the metabolic behaviors of many compounds and build up a database for the prediction of possible metabolites of xenobiotics.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (21976160) and Zhejiang Province Public Welfare Technology Application Research Project (LGF21B070006).
Materials
| 2,4-dichlorophenoxyacetic acid | WAKO | 1 mg/L | |
| 20% H2O2 | Sinopharm Chemical Reagent Co., Ltd. | 10011218-500ML | |
| 4-n-NP, >99% | Dr. Ehrenstorfer GmbH | ||
| 4-n-NP-d4 | Pointe-Claire | ||
| 6-benzylaminopurine | WAKO | 0.5 mg/L | |
| 75% ethanol | Sinopharm Chemical Reagent Co., Ltd. | 1269101-500ML | |
| 7890A-5975 gas chromatography | Agilent | ||
| ACQULTY ultra-performance liquid chromatography | Waters | ||
| Amber glass vials | Waters | ||
| Artificial climate incubator | Ningbo DongNan Lab Equipment Co.,LTD | RDN-1000A-4 | |
| Autoclaves | STIK | MJ-Series | |
| C18 column | ACQUITY UPLC BEH | ||
| Centrifuge | Thermo Fisher | ||
| DB-5MS capillary column | Agilent | ||
| Dichloromethane | Sigma-Aldrich | 40071190-4L | |
| Freeze dryer | SCIENTZ | ||
| High-throughput tissue grinder | SCIENTZ | ||
| Methanol | Sigma-Aldrich | ||
| MicrOTOF-QII mass spectrometer | Bruker Daltonics | ||
| Milli-Q system | Millipore | MS1922801-4L | |
| Murashige & Skoog medium | HOPEBIO | HB8469-7 | |
| N-hexane | Sigma-Aldrich | H109658-4L | |
| Nitrogen blowing instrument | AOSHENG | MD200-2 | |
| NP isomers, >99% | Dr. Ehrenstorfer GmbH | ||
| Oasis HLB cartridges | Waters | 60 mg/3 mL | |
| Research plus | Eppendorf | 100-1000 µL | |
| Seeds of Little Finger carrot (Daucus carota var. sativus) | Shouguang Seed Industry Co., Ltd | ||
| Shaking Incubators | Shanghai bluepard instruments Co.,ltd. | THZ-98AB | |
| Solid phase extractor | AUTO SCIENCE | ||
| Ultrasound machine | ZKI | UC-6 | |
| UV-sterilized ultra-clean workbench | AIRTECH |
References
- Chakraborty, P., et al. Baseline investigation on plasticizers, bisphenol A, polycyclic aromatic hydrocarbons and heavy metals in the surface soil of the informal electronic waste recycling workshops and nearby open dumpsites in Indian metropolitan cities. Environmental Pollution. 248, 1036-1045 (2019).
- Abril, C., Santos, J. L., Martin, J., Aparicio, I., Alonso, E. Occurrence, fate and environmental risk of anionic surfactants, bisphenol A, perfluorinated compounds and personal care products in sludge stabilization treatments. Science of the Total Environment. 711, 135048 (2020).
- Xu, Y. W., et al. Determination and occurrence of bisphenol A and thirteen structural analogs in soil. Chemosphere. 277, 130232 (2021).
- Cai, Q. Y., et al. Occurrence of nonylphenol and nonylphenol monoethoxylate in soil and vegetables from vegetable farms in the Pearl River Delta, South China. Archives of Environmental Contamination and Toxicology. 63 (1), 22-28 (2012).
- Wang, S. Y., et al. et al Migration and health risks of nonylphenol and bisphenol a in soil-winter wheat systems with long-term reclaimed water irrigation. Ecotoxicology and Environmental Safety. 158, 28-36 (2018).
- Gunther, K., Racker, T., Bohme, R. An isomer-specific approach to endocrine-disrupting nonylphenol in infant food. Journal of Agricultural and Food Chemistry. 65 (6), 1247-1254 (2017).
- Van Eerd, L. L., Hoagland, R. E., Zablotowicz, R. M., Hall, J. C. Pesticide metabolism in plants and microorganisms. Weed Science. 51 (4), 472-495 (2003).
- Hillebrands, L., Lamshoeft, M., Lagojda, A., Stork, A., Kayser, O. Evaluation of callus cultures to elucidate the metabolism of tebuconazole, flurtamone, fenhexamid, and metalaxyl-M in Brassica napus L., Glycine max (L.) Merr., Zea mays L., and Triticum aestivum L. Journal of Agricultural and Food Chemistry. 68 (48), 14123-14134 (2020).
- Macherius, A., et al. Metabolization of the bacteriostatic agent triclosan in edible plants and its consequences for plant uptake assessment. Environmental Science & Technology. 46 (19), 10797-10804 (2012).
- Sun, J. Q., et al. Uptake and metabolism of nonylphenol in plants: Isomer selectivity involved with direct conjugation. Environmental Pollution. 270, 116064 (2021).
- Schymanski, E. L., et al. Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environmental Science & Technology. 48 (4), 2097-2098 (2014).
- Moschet, C., Anumol, T., Lew, B. M., Bennett, D. H., Young, T. M. Household dust as a repository of chemical accumulation: new insights from a comprehensive high-resolution mass spectrometric study. Environmental Science & Technology. 52 (5), 2878-2887 (2018).
- Ren, Z., et al. Hydroxylated PBDEs and brominated phenolic compounds in particulate matters emitted during recycling of waste printed circuit boards in a typical e-waste workshop of South China. Environmental Pollution. 177, 71-77 (2013).
- de Wit, C. A. An overview of brominated flame retardants in the environment. Chemosphere. 46 (5), 583-624 (2002).
- Sun, J. Q., Chen, Q., Qian, Z. X., Zheng, Y., Yu, S. A., Zhang, A. P. Plant Uptake and Metabolism of e,4-Dibromophenol in Carrot: In Vitro Enzymatic Direct Conjugation. Journal of Agricultural and Food Chemistry. 66 (17), 4328-4335 (2018).
- Chibwe, L., Titaley, I. A., Hoh, E., Simonich, S. L. M. Integrated framework for identifying toxic transformation products in complex environmental mixtures. Environmental Science & Technology Letters. 4 (2), 32-43 (2017).
- Hollender, J., Schymanski, E. L., Singer, H. P., Ferguson, P. L. Nontarget screening with high resolution mass spectrometry in the environment: ready to go. Environmental Science & Technology. 51 (20), 11505-11512 (2017).
- Nafisi, M., Fimognari, L., Sakuragi, Y. Interplays between the cell wall and phytohormones in interaction between plants and necrotrophic pathogens. Phytochemistry. 112, 63-71 (2015).
- Zhang, Q., et al. Multiple metabolic pathways of 2,4,6-tribromophenol in rice plants. Environmental Science & Technology. 53 (13), 7473-7482 (2019).
- Hou, X., et al. Glycosylation of tetrabromobisphenol A in pumpkin. Environmental Science & Technology. 53 (15), 8805-8812 (2019).

