High-Efficiency Gene Disruption in Primary Bone Marrow-Derived Macrophages Using Electroporated Cas9-sgRNA Complexes
Summary
This protocol describes the procedure for genome editing in mouse bone marrow-derived macrophages using Cas9-sgRNA ribonucleoprotein complexes assembled in vitro and delivered by electroporation.
Abstract
Bone marrow-derived macrophages (BMDMs) from mice are a key tool for studying the complex biology of tissue macrophages. As primary cells, they model the physiology of macrophages in vivo more closely than immortalized macrophage cell lines and can be derived from mice already carrying defined genetic changes. However, disrupting gene function in BMDMs remains technically challenging. Here, we provide a protocol for efficient CRISPR/Cas9 genome editing in BMDMs, which allows for the introduction of small insertions and deletions (indels) that result in frameshift mutations that disrupt gene function. The protocol describes how to synthesize single-guide RNAs (sgRNA-Cas9) and form purified sgRNA-Cas9 ribonucleoprotein complexes (RNPs) that can be delivered by electroporation. It also provides an efficient method for monitoring editing efficiency using routine Sanger sequencing and a freely available online analysis program. The protocol can be performed within 1 week and does not require plasmid construction; it typically results in 85% to 95% editing efficiency.
Introduction
Macrophages are innate immune cells that play critical roles in tissue repair and immunity1,2. Immortalized macrophage cell lines, such as mouse RAW 264.7 cells or human THP-1 cells, have several beneficial characteristics, including robust growth and ease of gene disruption by delivering vectors for RNA interference or CRISPR/Cas93,4. However, oncogenic transformation dramatically alters their physiology, which results in the aberrant activation of some pathways and muted responses of others5,6. Primary bone marrow-derived macrophages (BMDMs) more closely recapitulate in vivo macrophage physiology, but remain challenging to genetically manipulate due to the low efficiency of both plasmid transfection and viral transduction in these primary immune cells7,8. Thus, more efficient methods for disrupting gene function are needed.
CRISPR/Cas9 genome editing is a powerful tool for genetic manipulation across a range of biological systems, including mammalian cells9,10,11,12. The Streptococcus pyogenes Cas9 protein efficiently and specifically cleaves double-stranded DNA when complexed with a sequence-specific guide RNA. DNA repair through the non-homologous end joining (NHEJ) of the cleaved DNA results in small insertions or deletions (indels) that create frameshift mutations. In early studies, Cas9 and sgRNAs were delivered through plasmid or lentiviral vectors, which are effective delivery methods for many cell lines9,10. However, primary cells and, in particular, primary immune cells are often refractory to these methods due to the low efficiency of vector delivery by transfection or transduction. Subsequently, methods have been developed to generate sgRNA-Cas9 complexes in vitro and to deliver them via electroporation, and these methods have achieved high efficiency in a variety of cell types13,14. The results have suggested the possibility of using this approach to carry out genome editing in primary macrophages.
Here, we provide a protocol for using sgRNA-Cas9 ribonucleoprotein complexes (RNPs) to carry out genome editing in primary BMDMs. It contains steps to mitigate the activation of the immune sensors present in primary immune cells and results in up to 95% editing at targeted loci with minimal toxicity. This protocol also includes workflows to evaluate editing efficiency using routine polymerase chain reaction (PCR) and Sanger sequencing, followed by in silico analysis by Tracking of Indels by Decomposition (TIDE)15, a well-validated online software tool.
Protocol
1. sgRNA design
NOTE: This step describes selection of the target sequences and design of the sgRNAs. It is helpful to design guides that are in the first large coding exon, so that any translated protein is disrupted early in the open reading frame. It is also helpful to select target sequences that lie within the same exon, as this will streamline the analysis of the editing efficiency (step 6). The examples of genome editing provided with this protocol used sgRNAs targeting the first exon of the Src gene and the Cblb gene, as well as in the non-coding Rosa26 locus of the mouse genome.
- Identify 20 nucleotide genomic sequences to target, using one of several free online designing tools (Table 1). Select four to five guides that are non-overlapping within each gene, as Cas9 activity varies by the specific guide chosen, and a priori prediction of a highly active guide is not possible.
NOTE: It is critical to ensure the proper orientation of the guide relative to the sequence of the targeted locus. Design tools typically provide the guide sequence in a 5' to 3' orientation, irrespective of which chromosomal strand is targeted. To verify the strand orientation, check that there is a protospacer adjacent motif (PAM) sequence for S. pyogenes Cas9 (nGG) immediately 3' of the targeted genomic sequence. The sgRNA sequence does not include the PAM.
2. sgRNA synthesis
NOTE: This step describes how to synthesize sgRNAs using PCR to generate a template for in vitro transcription (IVT), and then purify the sgRNA using spin columns (Figure 1A). Custom synthetic sgRNAs are commercially available through several vendors as an alternative to PCR/IVT.
- Perform PCR to generate the IVT template for T7 RNA polymerase. The PCR reaction uses universal primers that contain a T7 RNA polymerase promoter and trans-activating CRISPR RNA (tracrRNA) sequence (Table 2), in addition to short individual primers with the gene-specific guide sequence.
- This is the non-amplifying overlap extension step. Dilute the PCR buffer to 1x and add an additional 1 mM MgCl2. Then, add 0.25 µL of high-fidelity DNA polymerase, 0.5 mM deoxynucleotide triphosphate (dNTP), 0.02 mM guide-specific primer, and 0.02 mM T7 reverse long universal primer (Table 2) to a total volume of 46 µL. Then, place the tube in the thermocycler and run two cycles of: 95 °C for 15 s, 37 °C for 15 s, and 72 °C for 15 s. Cool the reactions on ice.
- This is the PCR amplification step. To each reaction, add 2 µL of 25 mM forward amplification primer and 2 µL of 25 mM reverse amplification primer. The final reaction volume is 50 µL. Denature for 30 s at 95 °C. Then, run 35 cycles of: 95 °C for 15 s, 65 °C for 20 s, and 72 °C for 15 s. Perform an additional extension at 72 °C for 2 min.
- Check the PCR reaction product on a 2% agarose gel. Overlap-extension PCR results in a 127 bp dsDNA molecule with the T7 promoter upstream of the gene-specific 20 nucleotide guide and the tracrRNA immediately downstream (Figure 1B).
- IVT template purification: There are numerous protocols and kits that work well for this. This protocol uses solid-phase reversible immobilization (SPRI) beads. Mix 50 µL of the PCR product with 90 µL of beads and follow the manufacturer's instructions. Elute the DNA by adding 40 µL of buffer (5 mM Tris, 0.1 mM ethylenediaminetetraacetic acid [EDTA]) and heat at 65 °C for 5 min.
- Measure the DNA concentration of the IVT template using absorption at a wavelength of 260 nm. A DNA concentration of at least 50 ng/µL is needed for IVT. The IVT template can be stored at -20 °C.
- IVT reaction
- Use a commercial kit designed to produce high yields of IVT (see Table of Materials). Follow the manufacturer's recommendations to perform the IVT reaction. Using 250 ng of IVT template in a 20 µL reaction, incubate at 37 °C for 4-16 h.
- Check the IVT sgRNA product by running 1 µL of the reaction on a 2% agarose gel. A bright RNA band should be observed, running similarly to 70 bp dsDNA (Figure 1C). Though not mandatory, to obtain sharp and more resolved bands, use an RNA sample buffer with urea, and denature the sample at 65 °C for 5 min prior to loading.
NOTE: Faint higher-molecular weight bands and slight migration differences may be seen with some guide RNAs due to differences in the RNA secondary structure.
- Dephosphorylate the sgRNA IVT product to minimize the activation of RIG-I (a foreign RNA sensor) and subsequent cell death following electroporation. For each 20 µL IVT reaction, add 69 µL of molecular-grade water and 15 U of calf intestinal phosphatase (CIP) in 1x CIP buffer. Incubate for 2 h at 37 °C.
- Degrade the IVT template to minimize the activation of cellular DNA sensors, which trigger cell death following electroporation. To each IVT reaction, add 2 U of RNase-free DNase. Incubate for 15 min at 37 °C. The IVT product can be stored at -20 °C for 1 day or -80 °C for several months.
- Purify the IVT product using spin columns. For this step, SPRI bead purification kits are inferior.
- Bind the RNA to the column by adding 220 µL of binding buffer to the IVT product, followed by 1 mL of 100% molecular biology-grade ethanol. Mix well and load the column. Thereafter, follow the manufacturer's instructions. Perform the final wash in a laminar flow biosafety cabinet to avoid contamination of the sgRNA.
- Perform the elution in the laminar flow hood to avoid contamination. Elute the RNA using 30 µL of sterile 10 mM Tris buffer (pH 8.0).
- Measure the concentration of sgRNA by measuring the absorbance at a wavelength of 260 nm.
NOTE: Typical sgRNA yields are at least 100 µg. - Store the sgRNA at -80 °C. Make aliquots to avoid more than three freeze-thaw cycles.
3. Preparation for electroporation
NOTE: All steps should be performed in a laminar flow hood to avoid contamination. This protocol uses a commercially available electroporation system (see Table of Materials) with 10 µL tips.
- Thaw the BMDMs 48-72 h before use and expand them on non- tissue culture (TC) treated plates.
NOTE: Per reaction, 4 x 105 cells are needed. - Sterilize PCR tubes for reaction assembly. Prepare one PCR tube per reaction, and heat them in a thermocycler for 10 min at 98 °C. Prepare the tubes in batches and store them closed. Place the sterilized PCR tubes on ice when ready to use.
- Clean the pipette station with 70% ethanol and place it in the laminar flow hood. Set the parameters to 1,900 V, 20 ms, and one pulse.
- Remove a transfection tube from the package with care to keep it sterile and fill it with 3 mL of "E" buffer. Ensure that the E buffer is at room temperature prior to electroporation. Insert the tube in the station and push it down until it clicks.
- For each reaction, aliquot 2.5 µL of sgRNA at a concentration of 1,100 ng/µL. Dilute the sgRNA in cold phosphate-buffered saline (PBS) with 0.9 mM CaCl2 and 0.5 mM MgCl2 (PBS + Ca/Mg). Leave it on ice.
- Prepare two plates: a 12-well uncoated plate for the cells and an additional 24-well plate to hold the culture media. Aliquot 1.5 mL of antibiotic-free medium per reaction into the wells of the 24-well plate.
- Prepare Cas9 protein. There are several commercial and academic suppliers of sterile-purified Cas9 protein. Dilute Cas9 to a final concentration of 20 µM with cold PBS + Ca/Mg. For each electroporation reaction, aliquot 1 µL of 20 µM Cas9 into sterile PCR tubes. Leave on ice.
- Prepare cells for electroporation.
- Remove the growth medium. Wash the cells using PBS without CaCl2 and MgCl2 (PBS-Ca/Mg) to remove the serum and divalent cations that promote adhesion. Then, add PBS + 1 mM EDTA and incubate at room temperature for about 5 min until the cells begin to detach.
- Pipette gently up and down to detach the cells. Transfer the cells to a low protein-binding 15 mL tube. Pellet the cells at 500 x g for 5 min.
NOTE: Macrophages are adherent to plasticware. The use of low protein-binding centrifuge tubes significantly improves the cell yield. - Work quickly to avoid prolonged incubation of cells in PBS + EDTA. Remove most of the supernatant, leaving about 1 mL of supernatant in the tube. Resuspend the cells in the remaining supernatant, and then transfer to a low protein-binding 1.5 ml microfuge tube.
- Remove a small aliquot of cells to count. Pellet the remaining cells by centrifuging at 500 x g at room temperature for 5 min.
- During centrifugation, count the cells and warm the Cas9 and sgRNA tubes to room temperature.
- Remove all the supernatant from the cell pellet. Resuspend the cells in PBS + Ca/Mg at a concentration of 4 x 107 cells/mL (4 x 105 cells per 10 µL reaction).
NOTE: Each kit is supplied with a limited amount of electroporation buffer (Buffer R, Buffer T). However, we find that the electroporation efficiency using PBS + Ca/Mg is equivalent to the proprietary buffers. - When electroporating sgRNAs for different genes, aliquot the cells into different low protein-binding tubes for each gene to avoid cross-contamination between the sgRNAs. Keep the cells at room temperature until ready for electroporation.
4. RNP assembly
- Keep the sgRNA and Cas9 at room temperature in order to avoid precipitation. Add 2.5 µL of sgRNA to 1 µL of the diluted Cas9 in sterilized PCR tubes. Dispense the sgRNA slowly over 15 s with mixing to prevent precipitation.
- Incubate for 5 min at room temperature to allow RNP complex formation.
5. RNP delivery by electroporation
- Prepare the electroporation tip (cuvette). Depress the plunger to extend the stem. Insert the stem into the tip.
NOTE: Ensure that the tip is secure and that the plunger slides smoothly and extends beyond the tip sheath. If the tip is not properly positioned, air bubbles may be introduced into the tip, causing tip failure. - Resuspend the cells by pipetting up and down, and then load the electroporation tip with the cells. Withdraw the full 10 µL volume capacity of the tip, avoiding bubbles.
- Starting with the negative control sgRNA, transfer 10 µL of cells in the electroporation tip to the sample tube containing 3.5 µL of RNP from step 4. Pipette up and down three times to mix well. Draw 10 µL of the mixture back into the tip, ensuring no bubbles are present.
- Place the tip into the electroporation station, lowering it into the buffer. Avoid contacting plastic surfaces with tip to maintain sterility. Push Start on the touchscreen display.
- After completion, the display will indicate a successful pulse. Remove the pipette from the device and place the cells in a dry well of the labeled, uncoated 12-well plate.
- Rinse the tip by pipetting up and down twice in 15 mL of PBS + Ca/Mg. Set aside the pipette with the tip still attached. Ensure that the tip does not touch anything and remains sterile.
NOTE: In many experiments, it is possible to reuse the tip for multiple samples, such as after the inactive negative control sgRNA sample, or during the initial evaluations of the guide activity when four to five sgRNAs are tested per gene and the sole read-out is the editing efficiency. However, during functional analysis on edited cells, a new tip is recommended for each gene, as small amounts of sgRNA or cell carryover could impact the experimental results. - Immediately add 1 mL of antibiotic-free medium (aliquoted in step 3.6) to the cells and gently shake the plate to mix.
- Repeat steps 5.2-5.6 for the remaining reactions.
- Return the cells to the incubator.
- Check the viability of the cells 1-2 h post-electroporation. The cells should be >90% adherent. Optional: Add gentamicin (5 µg/mL) to the cells 1-2 h post-electroporation to help prevent contamination if this will not interfere with the downstream experiments.
6. Assessing the editing efficiency
NOTE: Most editing is complete after 48 h.
- Check the cell monolayer 48 h after electroporation. If the cells are over 50% confluent, then proceed further. If the cells are <50% confluent, wait an additional 1-2 days. The genomic DNA analysis to determine the editing efficiency requires 1 x 105 cells, in addition to the cells needed for the downstream experiment.
- Prepare genomic DNA (gDNA).
- Wash the cells with PBS(-Ca/Mg). Add 1 mL of PBS + 1 mM EDTA. Incubate at room temperature for about 5 min until the cells begin to detach from the plate.
- Detach the cells by pipetting. Transfer to a low protein-binding microfuge tube.
- Count the cells and remove an aliquot of 1 x 105 cells. The remaining cells can be re-plated for use in further experiments. Generally, the experiments are conducted 5 days after electroporation in order to allow decay of the protein.
- Pellet the cells for gDNA analysis for 15 s at maximum speed in a microfuge tube.
- Resuspend the pellet in 50 µL of lysis buffer. Vortex to detach any cells stuck to the walls of the tube, and heat at 98 °C for 10 min to lyse the cells and inactivate endogenous DNase.
- Place the tubes on ice.
- Add 1 µL proteinase K (20 mg/mL). Incubate for at least 1 h at 37 °C; it can be left overnight for convenience.
- Heat to 98 °C for 10 min to inactivate the proteinase K.
- Centrifuge at room temperature for 5 min at maximum speed. Remove the supernatant, which contains the DNA. This crude preparation without further purification works well for most PCR reactions.
- Evaluate the editing efficiency with Sanger sequencing.
- Design PCR primers 200-300 bp upstream of the most 5' guide sites and 200-300 bp downstream of the most 3' guide sites. The typical amplicon size is ~500 bp. Design a nested sequencing primer at least 125 bp away from the nearest guide site (Figure 2A).
- Perform PCR using 2 µL of gDNA.
- Prepare the PCR product for sequencing. This protocol uses an exonuclease I/shrimp alkaline phosphatase (ExoI/SAP) treatment. Commercial DNA purification kits also work but require more hands-on time.
- Prepare 15 µL of the PCR product. Add 7.5 µL of water, 1 µL of 10x ExoI buffer, 10 U of ExoI, and 1 U of SAP to degrade the dNTPs and primers that interfere with Sanger sequencing.
- Incubate at 37 °C for 30 min, followed by 85 °C for 20 min to deactivate the enzymes.
- Carry out Sanger sequencing on the Exo/SAP-treated PCR product; use a known amount of DNA size marker to estimate the size of the PCR product present in the sample. The sequencing reactions may require optimization, as the TIDE software needs high-quality sequence chromatograms to accurately estimate the editing efficiency. The sequence chromatogram for the un-edited locus should provide clear peaks with minimal background (Figure 2B, C).
- To estimate the editing efficiency, use TIDE to analyze the Sanger sequencing chromatogram files using the edited gDNA and the unedited control gDNA. (Table 1, Figure 2). Upload the Sanger sequencing files and run the software with the default settings.
Representative Results
The IVT template is a 127 bp PCR product (Figure 1B). The full-length IVT product is a 98 nt RNA, which migrates similarly to a 70 bp double-stranded DNA fragment (Figure 1C).
After electroporation, the cells should be >90% viable, with a total cell count of >70% of the starting cell number. The resulting pool of mutant cells should have a diverse set of indels, starting near the Cas9 cleavage site. The analysis of the targeted genes by PCR and Sanger sequencing should show multiple nucleotides at each position downstream of the Cas9 cleavage site (Figure 2).
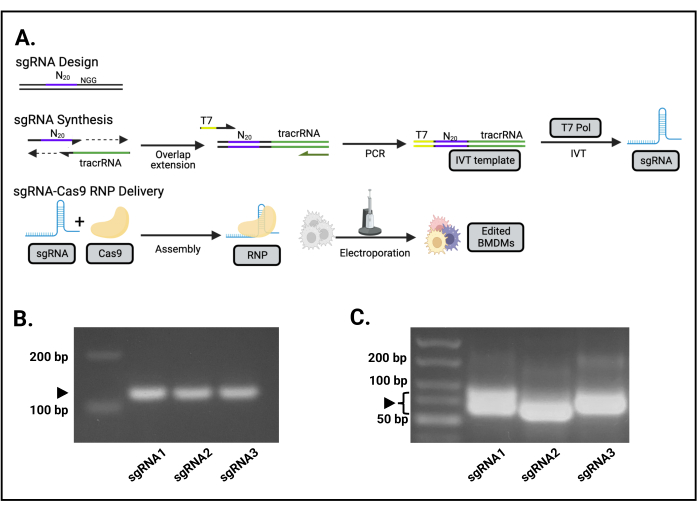
Figure 1: Overview of the sgRNA-Cas9 editing process. (A) Schematic for the guide design, sgRNA generation, and Cas9-sgRNA delivery. (B) The PCR product from IVT for Src sgRNAs 1, 2, and 3 resolved on a 2% agarose gel. The arrow indicates the correct 127 bp PCR products. (C) The RNA products following IVT for Src sgRNAs 1, 2, and 3 resolved on a 2% agarose gel. The brackets indicate the correct sgRNA products. The variable migration of the sgRNA is due to RNA secondary structure. This figure is reprinted from the first author's master's thesis16. Please click here to view a larger version of this figure.
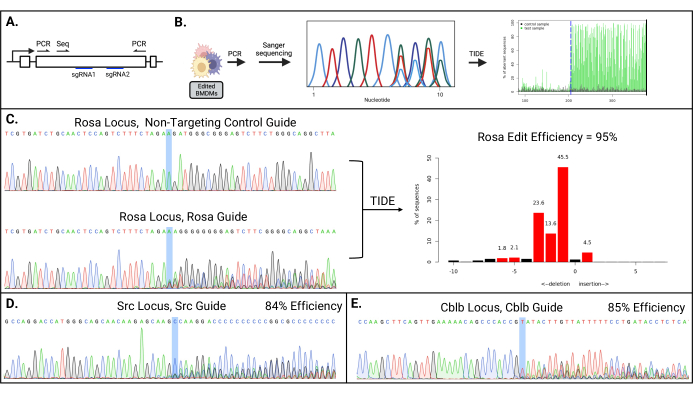
Figure 2: High editing efficiency achieved for multiple targeted genes. (A) Schematic of PCR and Sanger sequencing primers. (B) Schematic of the workflow for TIDE to assess the editing efficiency. (C) Representative Sanger sequencing chromatogram of the ROSA26 locus from BMDMs electroporated with a control non-targeting sgRNA-Cas9 RNP (top) and ROSA26-specific sgRNA (bottom); the Cas9 cleavage site is highlighted. The TIDE output (right) with the calculated editing efficiency and the percentage of sequences harboring the indicated number of indels. (D,E) Sanger sequencing chromatograms of the (D) Src and Cblb genes in the edited BMDMs. This figure is reprinted from the first author's master's thesis16. Please click here to view a larger version of this figure.
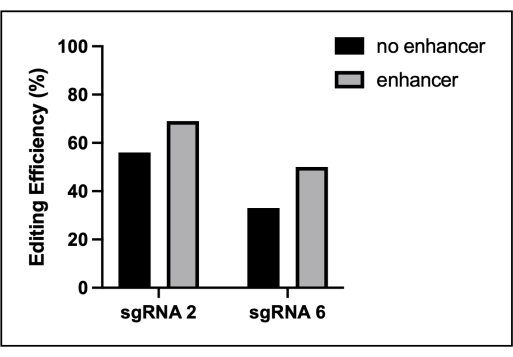
Figure 3: Moderate increase in editing efficiency due to the commercial electroporation enhancer. The BMDMs were electroporated with low-efficiency guides to evaluate the effect of a commercial editing enhancer. Prior to electroporation, 1 µL of the enhancer was added to the assembled RNPs to a final concentration of 4 µM. The editing efficiency of the indicated Src sgRNA was evaluated using TIDE. This figure is reprinted from the first author's master's thesis16. Please click here to view a larger version of this figure.
| Tool Name | URL | ||
| Synthego – CRISPR Design Tool | https://www.synthego.com/products/bioinformatics/crispr-design-tool | ||
| The Broad Institute – CRISPick | https://portals.broadinstitute.org/gppx/crispick/public | ||
| Tracking of Indels by Decomposition (TIDE) | http://shinyapps.datacurators.nl/tide/ | ||
Table 1: URLs of online tools.
| Primer Name | Sequence | ||
| Guide-Specific Primer | ggatcctaatacgactcactatag[N20]gttttagagctagaa | ||
| Guide-Specific Primer – Cbl-b Guide 3 | ggatcctaatacgactcactatagAAAATATCAAGTATATACGGgttttagagctagaa | ||
| Guide-Specific Primer – Cbl-b Guide 4 | ggatcctaatacgactcactatagGGTAAAATATCAAGTATATAgttttagagctagaa | ||
| Guide-Specific Primer – Rosa | ggatcctaatacgactcactatagCTCCAGTCTTTCTAGAAGATgttttagagctagaa | ||
| Guide-Specific Primer – Scramble | ggatcctaatacgactcactatagGCACTACCAGAGCTAACTCAgttttagagctagaa | ||
| Guide-Specific Primer – Src Guide 2 | ggatcctaatacgactcactatagTCACTAGACGGGAATCAGAGgttttagagctagaa | ||
| Guide-Specific Primer – Src Guide 5 | ggatcctaatacgactcactatagCAGCAACAAGAGCAAGCCCAgttttagagctagaa | ||
| Guide-Specific Primer – Src Guide 6 | ggatcctaatacgactcactatagAGCCCAAGGACGCCAGCCAGgttttagagctagaa | ||
| T7 Reverse Long Universal Primer | aaaaaagcaccgactcggtgccactttttcaagttgataacggactagccttattttaacttgctatttctagctctaaaac | ||
| Universal Forward Amplification Primer | ggatcctaatacgactcactatag | ||
| Universal Reverse Amplification Primer | aaaaaagcaccgactcgg | ||
Table 2: Oligonucleotides used in the PCR to generate the template for the IVT of the sgRNA. The 20 nucleotide target sequences for gene-specific primers are capitalized.
| BMDM Growth media. Store at 4 C. | |||
| DMEM | |||
| Fetal bovine serum | 0.1 | ||
| L-glutamine | 0.2 M | ||
| MCSF supernatant from 3T3-MCSF Cells** | 0.1 | ||
| Sodium pyruvate | 11 mg/mL | ||
| Lysis Buffer. Store at 4 C. | |||
| 2-mercaptoethanol (add immediately prior to use) | 0.01 | ||
| MgCl2 | 5 mM | ||
| Tris | 20 mM | ||
| Triton-X 100 | 0.005 | ||
| **3T3-MCSF cells are grown in DMEM+10% FCS. Supernatent with MCSF is harvested on the 5th day after reaching 100% confluence. As an alternative 10ng/ml recombinant MCSF can be used in lieu of conditioned media | |||
Table 3: Compositions of the media and buffers.
Supplementary File 1: Raw sequencing file for ROSA_ Mock Please click here to download this File.
Supplementary File 2: Raw sequencing file for ROSA_TargettingGuide Please click here to download this File.
Supplementary File 3: Raw sequencing file for ScrambleGuide Please click here to download this File.
Supplementary File 4: Raw sequencing file for SrcG5+SrcG6 Please click here to download this File.
Supplementary File 5: Raw sequencing file for CBLB_Mock Please click here to download this File.
Supplementary File 6: Raw sequencing file for CBLB_TargetingGuide Please click here to download this File.
Supplementary File 7: Raw sequencing file for Mock_Guide2Locus Please click here to download this File.
Supplementary File 8: Raw sequencing file for Mock_Guide6Locus Please click here to download this File.
Supplementary File 9: Raw sequencing file for SrcGuide2_Enhancer Please click here to download this File.
Supplementary File 10: Raw sequencing file for SrcGuide2_NoEnhance Please click here to download this File.
Supplementary File 11: Raw sequencing file for SrcGuide6_Enhancer Please click here to download this File.
Supplementary File 12: Raw sequencing file for SrcGuide6_NoEnhancer Please click here to download this File.
Discussion
Genome editing using electroporated Cas9-sgRNA complexes allows effective disruption of gene function in BMDMs. The editing efficiency varies by the target sequence and gene. Typically, four to five sgRNAs are generally screened to identify one that is highly active. Some loci have lower editing efficiencies, most likely due the chromatin structure. In these cases, several modifications can be made to increase the editing efficiency. Co-delivery of two active sgRNAs to the same exon results in improved editing for some genes. However, when two guides are co-transfected, we have observed that TIDE may lose accuracy. Therefore, alternative techniques to assess editing, such as Western blotting, may be required. In addition, the inclusion of a commercially available NHEJ enhancer often increases the editing efficiency by ~20% (Figure 3).
This approach has several advantages. The direct delivery of sgRNA-Cas9 to cells does not require the time-consuming steps of plasmid construction or lentiviral vector production to transduce BMDMs. The process of synthesizing sgRNA, electroporation, and generating mutant cells can be completed in 1 week. This technique can also be used with BMDMs derived from genetically modified mice to create double-mutant cells. Although chemical methods exist for transfecting primary immune cells with siRNA or mRNA17, these chemical methods are significantly less effective than electroporation at delivering Cas9-sgRNA complexes to immune cells18. There are several types of electroporation devices available commercially. These devices are anticipated to work similarly for the delivery of Cas9-sgRNA complexes, although the voltage parameters likely need to be optimized for each individual device. While this protocol has been used primarily on mouse BMDMs, in limited experiments, similar results have been obtained with rat BMDMs (unpublished data). It is possible that other types of primary macrophages, such as mouse peritoneal macrophages or alveolar macrophages, might also be amenable to this approach, though this has not yet been tested.
This method has some limitations. The number of cells produced by this protocol is somewhat limited; our standard conditions generate 4 x 105 cells per electroporation. However, it may be possible to scale up the yield significantly, as the efficiency is undiminished in a 10 µL reaction with up to 2.4 x 106 cells using the same amount of RNP (unpublished data). In addition, 100 µL electroporation tips are available. Another drawback of this method is the expense; the costs of both the electroporator and the electroporation consumables are substantial. These expenses can be significantly mitigated by reusing the tips when using different sgRNAs targeting the same gene and by using PBS instead of the proprietary buffers. While the compositions of the proprietary buffers are not known, presumably the electrolyte composition of PBS with 0.9 mM CaCl2 and 0.5 mM MgCl2 approximates the electrical conductance of the proprietary buffer. These changes reduce the cost of consumables by roughly 80% in this protocol.
There are several critical steps in the protocol, deviations from which could dramatically affect the efficiency of the gene editing. One of the potential pitfalls is that standard IVT kits, which are not designed for high yields, often produce insufficient IVT product. In addition, the use of standard polypropylene microfuge tubes instead of low-binding tubes can cause significant cell loss by adhesion. The incomplete dephosphorylation of the IVT product and the presence of residual DNA in the sgRNA preparation may result in the activation of macrophage immune sensors and subsequent toxicity. Higher or longer voltage pulses may also result in increased cell death.
In summary, this genome editing protocol, which uses electroporation to deliver Cas9-sgRNA RNPs, is an efficient method to disrupt genes in mouse BMDMs. This allows users to rapidly screen for phenotypes in primary cells that more closely recapitulate the complex biology of macrophages in vivo.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was funded by the NIH grant 5R01AI144149. The schematic figures were created with BioRender.
Materials
| 3T3-MCSF Cell Line | Gift from Russell Vance | not applicable | |
| Alt-R Cas9 Electroporation Enhancer | IDT | 1075915 | |
| Ampure XP Reagent Beads | Beckman Coulter | A63880 | |
| Calf intestinal alkaline phosphatase | NEB | M0525S | |
| DNase | NEB | M0303S | |
| DPBS +Ca/Mg (0.9mM CaCl2 and 0.5mM MgCl2) | Thermo Fisher | 14040-133 | |
| DPBS -Ca/Mg | Thermo Fisher | 14190-144 | |
| ExoI | NEB | M0293S | |
| Fetal Calf Serum (FCS) | Corning | 35-015-CV | |
| Herculase DNA polymerase & buffer | Agilent | 600677 | |
| HiScribe T7 High Yield RNA Synthesis Kit | NEB | E2040S | |
| LoBind conical tubes 15 mL | Eppendorf | 30122216 | |
| LoBind Eppendorf tubes 2 mL | Eppendorf | 22431102 | |
| NEBuffer r2.1 | NEB | B6002S | |
| Neon Transfection System | Thermo Fisher | MPK5000, MPP100, MPS100 | |
| Neon Transfection System 10 uL Tips | Thermo Fisher | MPK1025 or MPK1096 | |
| PBS + 1mM EDTA | Lonza | BE02017F | |
| Proteinase K | Thermo Fisher | EO0491 | |
| rCutSmart Buffer for ExoI | NEB | B6004S | |
| Ribolock | Thermo Fisher | EO0384 | |
| RNA loading dye | NEB | B0363S | |
| RNeasy Mini Kit | Qiagen | 74104 | |
| S. pyogenes Cas9-NLS | University of California Macro Lab | not applicable | Available to non-UC investigators through https://qb3.berkeley.edu |
| S. pyogenes Cas9-NLS, modified 3rd Generation | IDT | 1081059 | |
| SAP | NEB | M0371S |
References
- Murray, P. J., Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nature Reviews Immunology. 11 (11), 723-737 (2011).
- Wynn, T. A., Chawla, A., Pollard, J. W. Macrophage biology in development, homeostasis, and disease. Nature. 496 (7446), 445-455 (2013).
- Paddison, P. J., Caudy, A. A., Hannon, G. J. Stable suppression of gene expression by RNAi in mammalian cells. Proceedings of the National Academy of Sciences. 99 (3), 1443-1448 (2002).
- Laugel, B., et al. Engineering of isogenic cells deficient for MR1 with a CRISPR/Cas9 lentiviral system: Tools to study microbial antigen processing and presentation to human MR1-restricted T cells. The Journal of Immunology. 197 (3), 971-982 (2016).
- Andreu, N., et al. Primary macrophages and J774 cells respond differently to infection with Mycobacterium tuberculosis. Scientific Reports. 7, 42225 (2017).
- Roberts, A. W., et al. Cas9+ conditionally-immortalized macrophages as a tool for bacterial pathogenesis and beyond. eLife. 8, e45957 (2019).
- Oberdoerffer, P., et al. Efficiency of RNA interference in the mouse hematopoietic system varies between cell types and developmental stages. Molecular and Cellular Biology. 25 (10), 3896-3905 (2005).
- Ma, S., et al. YTHDF2 orchestrates tumor-associated macrophage reprogramming and controls antitumor immunity through CD8+ T cells. Nature Immunology. 24 (2), 255-266 (2023).
- Cong, L., et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 339 (6121), 819-823 (2013).
- Mali, P., et al. RNA-guided human genome engineering via Cas9. Science. 339 (6121), 823-826 (2013).
- Cho, S. W., Kim, S., Kim, J. M., Kim, J. S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nature Biotechnology. 31 (3), 230-232 (2013).
- Jinek, M., et al. RNA-programmed genome editing in human cells. eLife. 2, e00471 (2013).
- Kim, S., Kim, D., Cho, S. W., Kim, J., Kim, J. S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Research. 24 (6), 1012-1019 (2014).
- Lin, S., Staahl, B. T., Alla, R. K., Doudna, J. A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife. 3, 04766 (2014).
- Brinkman, E. K., Chen, T., Amendola, M., van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Research. 42 (22), e168 (2014).
- Craft, J. CRISPR-Cas9-mediated genome editing in primary murine bone marrow-derived macrophages. University of California, Davis. , (2022).
- Herb, M., Farid, A., Gluschko, A., Krönke, M., Schramm, M. Highly efficient transfection of primary macrophages with in vitro transcribed mRNA. Journal of Visualized Experiments. (153), e60143 (2019).
- Yu, X., et al. Improved delivery of Cas9 protein/gRNA complexes using lipofectamine CRISPRMAX. Biotechnology Letters. 38 (6), 919-929 (2016).

