A Neonatal Rodent Model of Retroorbital Vein Injection
Summary
This protocol aims to demonstrate a reproducible venous administration route that can be used in rats and mice throughout the neonatal period. This procedure is important for preclinical rodent studies that wish to mirror drug administration in neonatal care units primarily using intravenous administration.
Abstract
Intravenous (iv) injection is the most used route of drug administration in neonates in the clinical setting. Therefore, retroorbital vein injection is an important method for compound administration in research, where successful proof-of-concept studies can progress into much-needed neonatal clinical trials. Most intravenous studies in neonatal rodents use the superficial temporal/facial vein. However, retroorbital injection becomes unreliable in neonatal rodents older than 2 days after the skin darkens and the vein is no longer visible. In the present protocol, we describe the retroorbital injection of the venous sinus in both the neonatal mouse and rat at ages when the superficial temporal vein is no longer visible, but the eyes have not opened yet. Eye-opening facilitates retro-orbital injection by enabling the researcher to clearly see that they are not perforating the eye when inserting the needle. We demonstrate that this technique can be performed in a reliable and reproducible manner without adverse effects. Additionally, we show that it can be used in many studies, such as administering compounds to study neonatal brain injury.
Introduction
Animal research is an essential step leading to clinical trials, and as such, it is important that animal studies closely mimic procedures and treatments performed in the clinical setting. However, there are several challenges in translating clinical practices to neonatal rodent studies. These include the small size of the neonatal rodent and the gap in neonatal research and knowledge compared to adult research, among others1,2.
Administration of different substances like drugs or cells can be performed via multiple routes, including intraperitoneal (ip), subcutaneous (sc), and intravenous (iv) injections. Injection by iv is the preferential route of administration of compounds in human neonates. In neonates, the iv route of administration is advantageous compared to other routes because it maximizes systemic distribution of drugs and has high bioavailability3,4. A well-maintained iv lines may be used for repeated drug administration. In rodent studies, iv injections must be performed in the tail, facial/temporal veins, or in the retroorbital sinus5. Tail vein injection is routinely used in adult rodents, as it provides two lateral caudal parallel veins to choose from5. However, these veins have a small diameter, which excludes their use in neonates. Most neonatal iv injections have been performed in the superficial facial/temporal vein, as it is visible from postnatal day 0 (P0)-P2 and allows for a relatively large volume administration5. However, this route becomes unreliable around P36 once the animal gains skin coloration, thereby making the superficial facial/temporal vein difficult to see with the unassisted eye. Administration iv via the neonatal transverse sinus has been described in one study7; however, this requires opening the skin above the transverse sinus and injecting AAV9 at P0-P1 with assistance from a microscope.
When investigating a potential treatment or establishing a relevant neonatal injury model, it is important to consider that neonatal rodents can have different organ development timing compared to humans. Our protocol is based on the differences in neonatal central nervous system development between humans and rodents. As an example, the term newborn human brain approximately corresponds to a P7 rat and a P10 mouse brain8. As the distribution of substances injected retroorbitally is similar to that of the other iv sites, with high blood levels rapidly being achieved, we consider it a suitable route. This technique has been well-described by Yardeni and colleagues, who injected compounds into the ophthalmic venous sinus in P1-P2 mice9. In the current protocol, we show a simple and feasible method of performing retroorbital injections in older neonatal rodents which have yet to open their eyes.
Protocol
All procedures listed in this protocol conformed to the Swedish Board of Agriculture and were approved by the Gothenburg Animal Ethics Committee (825-2017 and 2195-19). C57BL/6 mice and Wistar rats were bred in-house with a 12 h light/dark cycle and free access to food and water. All experimental procedures followed the ARRIVE guidelines10.
1. Workspace setup
- For the duration of this procedure, collect the experimental animals from the dam cage and place them in a separate cage on a heated pad (35-37 °C).
NOTE: If using a light source (albino animals), a non-heat light source that can be positioned underneath the animal's head should be used.
2. Needle and solution
- Use a needle with 29-31 G (around 0.30 mm).
- For accurate volumes, draw up the solution to be injected from a pipetted volume.
NOTE: A maximum of 5 μL/ g body weight should be injected in each retroorbital sinus.
3. Setup
- Place the animals on a flat surface (Figure 1A) in lateral recumbency (Figure 1C).
- Induce full-body isoflurane anesthesia (5% induction, 3% maintenance).
NOTE: The animals should be placed under anesthesia using a mouthpiece. The eyelid and tear duct area should not be covered (Figure 1D). - Check the depth of anesthesia using the paw withdrawal reflex method.
NOTE: No pre-operative analgesia is required as this is not considered an invasive procedure11.
4. Injection procedure
NOTE: If possible, use a light source under the animal's head (Figure 1C), to facilitate view of the venous sinus (Figure 1E). No sterilization of the area being injected is required, as the eye lid is still closed.
- With its head facing to the right, administer the injection into the right retroorbital sinus (right-handed operator example).
- Insert the needle, bevel down, at the front of the eye socket – the equivalent of the medial canthus, at an angle of approximately 40°. This angle allows the needle to be directed to the back of the eye orbit.
- Advance 1/3 of the needle (around 2 mm) into the area of the retroorbital sinus located behind the eye orbit.
- Inject in a gentle, smooth, and fluid motion.
- Wait for a moment, before withdrawing the needle slowly to avoid backflow.
CAUTION: Do not aspirate. - Use a new sterile syringe for each animal to avoid contamination.
NOTE: When injecting a clear solution, the vein should turn momentarily clear.
5. Post-injection care
- Place the pup in the recovery box, rested on a protected warming device (35-37 °C).
- Wait for the recovery and check for any signs of distress before returning the pup to the dam.
NOTE: Dye-injected practice animals should be immediately euthanized as per the IACUC approved protocols.
CAUTION: If the eye swells during the injection, it means that the needle is not inserted into the venous plex and is instead in the eye orbit. The neonatal skull is very soft, if the needle perforates it, then the injection will go into the meninges or even the brain parenchyma.
Representative Results
The present technique was performed on a flat surface, with a mouthpiece for global anesthesia (Figure 1A). The mouthpiece should not block access to the medial canthus (Figure 1B). In albino animals, a fiber optic light source was placed below the animal, to assist with the visualization of the veins (Figure 1B). The needle was placed at an angle of approximately 40°, and advanced around 2 mm into the medial canthus (Figure 1C). Injection of trypan blue dye in a P5 albino rat allowed clear visualization of the dye in the retroorbital sinus (Figure 1C).
The retroorbital injection technique described in this protocol was successfully used to administer the tracer biotin-dextran (BDA, 10,000 Da)12. The use of visible tracers in vascular research can, for example, provide an alternative to using radioactive sucrose extravasation from the blood vessels, enabling the use of the same brains for other histological measurements12.
Recently, we have established a neonatal rat model of germinal matrix hemorrhage (GMH)13. In brief, P5 Wistar rats received a single intracranial injection of 0.3 U of collagenase VII into the medial striatum. GMH results in rupture of the vessels in the germinal matrix and is one of the prevalent causes of preterm brain injury and mortality14. To further characterize the GMH model, we used a retroorbital injection of the BDA tracer (Figure 2) to investigate the effects of GMH in blood-brain barrier function and integrity14.
When compared to saline-injected controls (Figure 2A), successful retroorbital injection of the BDA tracer14 allowed assessment of the tracer presence in the brain vasculature 10 min after BDA injection (Figure 2B). This technique was then used to detect penumbra vascular leakage of BDA at the individual blood vessel level in GMH-injured animals (Figure 2C, red arrows) which can then be quantified10.
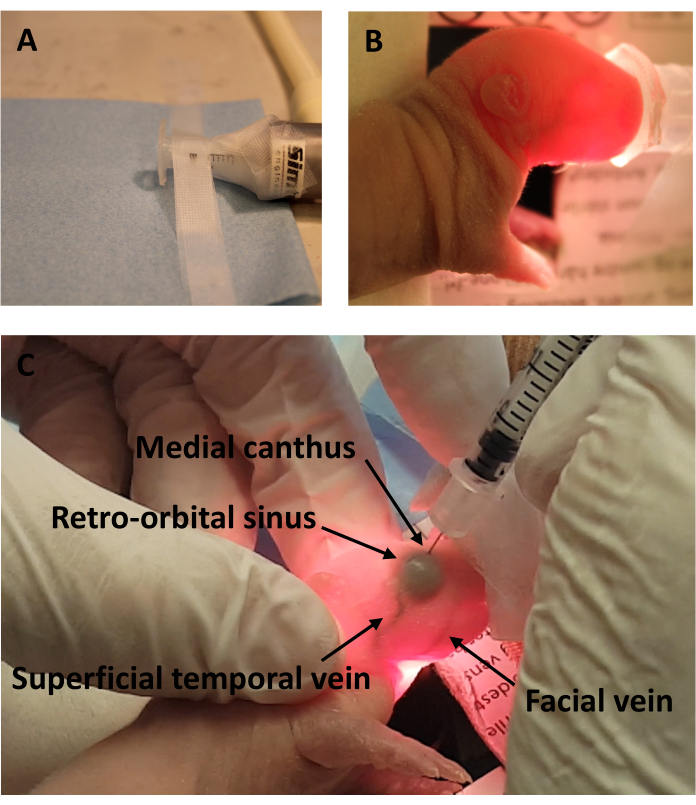
Figure 1: Experimental setup with diagram view of the blood vessels after trypan blue dye administration. (A) Anesthesia setup (B) without and (C) with fiber optic light source. (D) Retroorbital injection of trypan blue dye in the P10 C5BL/6 mouse. (E) Diagram view of the blood vessels in the P5 Wistar rat following trypan blue dye injection. Please click here to view a larger version of this figure.
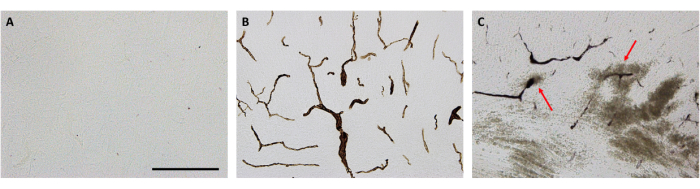
Figure 2: Representative brain micrographs showing the distribution of the BDA tracer. (A) No positive stain in saline-injected control animals. (B) BDA tracer dissolved in saline, at a dose concentration of 2.0 – 2.5 mg per animal was visible within blood vessels of the brain (cortex). (C) BDA tracer leaking into the brain parenchyma following GMH (red arrows). Scale bar = 200 μm. Adapted from Andersson et al., 202114. Please click here to view a larger version of this figure.
Discussion
This protocol provides a clear and precise method for the injection of substances into the retroorbital sinus of neonatal mice and rats. This is important because it shows that retroorbital injections can be performed reliably and reproducibly in rodents older than P2, where the superficial temporal/facial vein is no longer discernible, and in animals younger than P12, where the eye lids have not yet opened, and the eyeball is not exposed. Furthermore, the neonatal retroorbital injection is well tolerated by both the pups and the dams, with minimal risks of side effects once the technique has been mastered.
Injections via iv have an advantage over other routes of administration as they allow for injection of high concentration, as well as both low and high pH, providing that the rate of injection is kept constant and low to avoid rupture of the vessel. Furthermore, iv injections allow for faster distribution of compounds as they enter directly into the systemic circulation thus bypassing potential delays from poor absorption observed in other routes of administration. This allows for immediate access and nearly 100% bioavailability of compounds.
Clinically iv is the preferred route of administration in neonates (< 28 days of age). This is especially true in neonatal intensive care settings as iv cannulation allows for easy access to provide drugs/fluids. Injections via the sc route have been somewhat used in neonates, particularly for the administration of erythropoietin15. However, concerns have been raised, with a study suggesting iv infusion as a superior alternative16. Oral administration is not often a practical option when neonates are in a hospital intensive unit setting. Additionally, compared to adults, neonates have differences in their gastrointestinal tract, including delayed gastric emptying and decreased intestinal motility which can affect drug absorption. Intramuscular injections are difficult to administer as a result of the small muscular mass of neonates3,4.
In rodent research, one of the most widely used methods of iv injections is the tail vein injection. However, this method is inviable when working with neonates. Other iv sites such as the superficial temporal/facial vein6 become invisible at P3. Neonatal transverse sinus has been described in one study and was performed at P0-P1 and, with assistance of a microscope, opening the skin and advancing a capillary needle though the skull into the transverse sinus, allowing 2-4 μl volume injections7. Few studies have documented the use of the external jugular vein at P7 in rats17. However, this is an invasive technique that requires surgical opening of the skin and exposure of the external jugular vein18. In studies in adult rodents, retroorbital administration has been shown to be as effective as tail vein injection5 thus reinforcing the viability and relevancy of the retroorbital route. The retroorbital injection causes minimal distress and once mastered can be performed by a single person with minimal equipment and allows multiple injections (ensuring that eyes are alternated). Previous studies have shown that retroorbital injection has been used to administer adeno-associated virus 9 in mice at P0-P1 or at P14-P2111 or FITC-dextran at P1719 indicating an increasing acceptance of this method.
There are some limitations associated with retroorbital injection in neonates. As with all iv injections, injected volume is limited, and we recommend 5 μL/g for this procedure. Additionally, the retroorbital injection requires full body anesthesia. To minimize complications, it is suggested the use of inhaled anesthesia agents such as isoflurane, as these are quicker in anesthesia induction, have fast metabolism and have a rapid recovery rate. Training is required, preferably using colored dye in terminally anesthetized animals, to avoid potential swelling around the injection site or eye trauma due to incorrect placement of the needle bevel. Due to the small size of these animals, finer needles are required, with small needle gauge. Injection of cells must be performed in single-cell suspension, to avoid blockage of vessels, and to ensure cell viability. Encouragingly, a study by Amer and colleagues has shown that injection of mammalian cells using 30 G syringes still provides reliable cell viability even at high cell density ejection20.
In summary, establishment of a reliable iv route in neonates is of clinical significance, as this is the preferred route of administration in humans. The retroorbital injection can be easily mastered, is reproducible and provides a relevant alternative to other iv injection sites, such as tail and temporal/facial vein which cannot be reliable used throughout the rodent neonatal period. Thus, the neonatal retroorbital injection allows for the delivery of drugs, cells and other compounds at appropriate neonatal ages.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The work performed in this protocol was funded by Hasselblad Foundation (2020-2021, ERF), Åke Wibergs Foundation (M19-0660, ERF), Swedish Research Council (2019-01320, HH; 2021-01872, CM), Public Health Service at the Sahlgrenska University Hospital (ALFGBG-965174, HH ; ALFGBG-966107, CM), The Swedish Brain Foundation (FO2022-0110, CM), Åhlen Foundation (223005, CM) and Horizon 2020 Framework Program of European Union (grant agreement no. 87472/PREMSTEM, HH).
Materials
| BD Micro-Fine Demi 0,3 ml 30G (0,30mm) | BD | 256370 | 1 per animal per injection |
| Biotin-dextran (BDA) tracer | ThermoFischer | D1956 | 2.0-2.5 mg per animal |
| Fiber optic light source | Euromex | ||
| HP 062 Heating Plate | Labotect | ||
| Isoflurane | Vetmedic | Vnr 17 05 79 | |
| Tryptan blue solution (0.4%) | Sigma | T8154 | 5 μl/ g body weight |
References
- Laughon, M. M., et al. Drug labeling and exposure in neonates. JAMA Pediatr. 168 (2), 130-136 (2014).
- Das, A., et al. Methodological issues in the design and analyses of neonatal research studies: Experience of the nichd neonatal research network. Semin Perinatol. 40 (6), 374-384 (2016).
- Ku, L. C., Smith, P. B. Dosing in neonates: Special considerations in physiology and trial design. Pediatr Res. 77 (1-1), 2-9 (2015).
- Linakis, M. W., et al. Challenges associated with route of administration in neonatal drug delivery. Clin Pharmacokinet. 55 (2), 185-196 (2016).
- Steel, C. D., Stephens, A. L., Hahto, S. M., Singletary, S. J., Ciavarra, R. P. Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Anim (NY). 37 (1), 26-32 (2008).
- Gombash Lampe, S. E., Kaspar, B. K., Foust, K. D. Intravenous injections in neonatal mice). J Vis Exp. , e52037 (2014).
- Hamodi, A. S., Martinez Sabino, A., Fitzgerald, N. D., Moschou, D., Crair, M. C. Transverse sinus injections drive robust whole-brain expression of transgenes. Elife. 9, 53639 (2020).
- Semple, B. D., Blomgren, K., Gimlin, K., Ferriero, D. M., Noble-Haeusslein, L. J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 106-107, 1-16 (2013).
- Yardeni, T., Eckhaus, M., Morris, H. D., Huizing, M., Hoogstraten-Miller, S. Retro-orbital injections in mice. Lab Anim (NY). 40 (5), 155-160 (2011).
- Kilkenny, C., et al. Animal research: Reporting in vivo experiments: The arrive guidelines). Br J Pharmacol. 160 (7), 1577-1579 (2010).
- Prabhakar, S., Lule, S., Da Hora, C. C., Breakefield, X. O., Cheah, P. S. Aav9 transduction mediated by systemic delivery of vector via retro-orbital injection in newborn, neonatal and juvenile mice. Exp Anim. 70 (4), 450-458 (2021).
- Ek, C. J., Habgood, M. D., Dziegielewska, K. M., Potter, A., Saunders, N. R. Permeability and route of entry for lipid-insoluble molecules across brain barriers in developing monodelphis domestica. J Physiol. 536, 841-853 (2001).
- Jinnai, M., et al. A model of germinal matrix hemorrhage in preterm rat pups). Front Cell Neurosci. 14, 535320 (2020).
- Andersson, E. A., Rocha-Ferreira, E., Hagberg, H., Mallard, C., Ek, C. J. Function and biomarkers of the blood-brain barrier in a neonatal germinal matrix haemorrhage model. Cells. 10 (7), (2021).
- Ohls, R. K., et al. Effects of early erythropoietin therapy on the transfusion requirements of preterm infants below 1250 grams birth weight: A multicenter, randomized, controlled trial. Pediatrics. 108 (4), 934-942 (2001).
- Costa, S., et al. How to administrate erythropoietin, intravenous or subcutaneous. Acta Paediatr. 102 (6), 579-583 (2013).
- Fernandez-Lopez, D., et al. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J Neurosci. 32 (28), 9588-9600 (2012).
- Sugiyama, Y., et al. Intravenous administration of bone marrow-derived mesenchymal stem cell, but not adipose tissue-derived stem cell, ameliorated the neonatal hypoxic-ischemic brain injury by changing cerebral inflammatory state in rat. Front Neurol. 9, 757 (2018).
- Li, S., et al. Retro-orbital injection of fitc-dextran is an effective and economical method for observing mouse retinal vessels. Mol Vis. 17, 3566-3573 (2011).
- Amer, M. H., White, L. J., Shakesheff, K. M. The effect of injection using narrow-bore needles on mammalian cells: Administration and formulation considerations for cell therapies. J Pharm Pharmacol. 67 (5), 640-650 (2015).

