Motor Imagery Brain-Computer Interface in Rehabilitation of Upper Limb Motor Dysfunction After Stroke
Summary
The purpose of this study is to provide an important reference for the standard clinical operation of motor imagery brain-computer interface (MI-BCI) for upper limb motor dysfunction after stroke.
Abstract
The rehabilitation effect of patients with moderate or severe upper limb motor dysfunction after stroke is poor, which has been the focus of research owing to the difficulties encountered. Brain-computer interface (BCI) represents a hot frontier technology in brain neuroscience research. It refers to the direct conversion of the sensory perception, imagery, cognition, and thinking of users or subjects into actions, without reliance on peripheral nerves or muscles, to establish direct communication and control channels between the brain and external devices. Motor imagery brain-computer interface (MI-BCI) is the most common clinical application of rehabilitation as a non-invasive means of rehabilitation. Previous clinical studies have confirmed that MI-BCI positively improves motor dysfunction in patients after stroke. However, there is a lack of clinical operation demonstration. To that end, this study describes in detail the treatment of MI-BCI for patients with moderate and severe upper limb dysfunction after stroke and shows the intervention effect of MI-BCI through clinical function evaluation and brain function evaluation results, thereby providing ideas and references for clinical rehabilitation application and mechanism research.
Introduction
Nearly 85% of stroke patients have motor dysfunction1, especially due to the limited rehabilitation effect of patients with moderate and severe upper limb motor dysfunction, which seriously affects patients’ ability to live independent daily lives and has been the focus and difficulty of research. Non-invasive brain-computer interface (BCI) is known as an emerging treatment for rehabilitation of motor dysfunction after stroke2. BCI is the direct conversion of the sensory perception, imagery, cognition, and thinking of users or subjects into actions, without reliance on peripheral nerves or muscles, to establish direct communication and control channels between the brain and external devices3. At present, the paradigms of BCI for clinical rehabilitation include motor imagery (MI), steady-state visual evoked potentials (SSVEP), and auditory evoked potentials (AEP) P3004, of which the most commonly used and convenient is motor imagery brain-computer interface (MI-BCI). MI is an intervention that uses visual/kinesthetic motor imagery to visualize the execution of motor tasks (such as hand, arm, or foot movements). On the one hand, previous studies have demonstrated that activation of the associated motor cortex during MI is similar to actual motor execution5. On the other hand, unlike other paradigms, MI can activate a specific area of activity through motor memory without any external stimulus to improve motor function; this is conducive to implementation in stroke patients, especially when combined with hearing dysfunction6.
Moreover, MI-BCI has been shown to have a positive effect on improving motor dysfunction in stroke patients. Cheng et al. reported that compared with simple soft robotic glove intervention, the soft robotic glove based on MI-BCI combined with tasks oriented to daily life activities showed more obvious functional improvement and more lasting kinesthetic experience in chronic stroke patients after 6 weeks of intervention. Furthermore, it was also able to elicit the perception of motor movements7. Additionally, Ang et al. included 21 chronic stroke patients with moderate to severe upper limb dysfunction for randomized intervention. The clinical function was evaluated before and after intervention by the Fugl-Meyer assessment of upper extremity (FMA-UE). The results showed that, compared with simple haptic knob (HK) robot intervention (HK group) and standard arm therapy intervention (SAT group), the motion gain effect of HK based on MI-BCI intervention (BCI-HK group) was significantly better than that of the other two groups8. However, the specific operation of MI-BCI still requires normative standards, and the mechanism of neural remodeling must be fully understood, which limits the clinical application and promotion of MI-BCI. Therefore, by showing the intervention process of MI-BCI in a 36-year-old male stroke patient with upper limb motor dysfunction, this study will summarize the functional outcome changes and brain function remodeling before and after the intervention to demonstrate the complete operation process of MI-BCI and provide ideas and references for clinical rehabilitation application and mechanism research.
Protocol
This project was approved by the Medical Ethics Association of the Fifth Affiliated Hospital of Guangzhou Medical University (approval No. KY01-2021-05-01) on August 19, 2021. The trial was registered in the Chinese Clinical Trials Registry (registration number: NO. ChiCTR2100050162) on August 19, 2021. All patients signed the informed consent form.
1. Recruitment
- Inclusion criteria
- Recruit patients who meet the diagnostic criteria of stroke formulated by the fourth National Conference on Cerebrovascular Disease; have a confirmed cerebral apoplexy by brain computed tomography or magnetic resonance imaging with the disease course ≥ 6 months; had a stroke leading to one hand dysfunction at Brunnstrom stage 1-3; have a Montreal cognitive assessment (MoCA) ≥ 26; aged from 50 to 75 years; right-handedness prevails; have voluntarily signed the informed consent.
- Exclusion criteria
- Exclude patients with previous history of upper limb fracture, deformity of upper limb, etc.; their condition is unstable or changes occur during the experiment, which affects the experimental results; unable to complete the experiment due to serious heart, lung, liver and kidney and other important organ diseases; severe cognitive dysfunction or aphasia; suffering from serious mental disorders; taking medications that reduce the threshold of seizures; have any implanted device or metal that may be affected by the magnetic field generated by transcranial magnetic stimulation (TMS).
2. MI-BCI training
NOTE: The rehabilitation robot for upper limb hand function based on motor imagery is selected in this study. The device comprises an electroencephalogram (EEG) cap (Figure 1A), a computer terminal (i.e., control interface; Figure 1B), an external manipulator (Figure 1E), and a 23 inch computer display screen (Figure 1C).
- Preparation of subjects
- Explain the training purpose and method and note these for the subjects.
- Put the MI-BCI EEG cap on the subject to ensure that the Cz point of the subject's head coincides with the Cz point of the cap9,10. The subject's Cz point is the vertex of the head center. To confirm this, check the intersection point of the ear bead line and the human median line passing through the nose and eyebrow center. Determine the Cz point of the EEG cap according to the international 10-20 EEG positioning system11.
- Keep the ears exposed from the ear seam of the head cap, and adjust the chin strap to fix the head cap.
- Insert 24 electrodes dipped in normal saline into the groove of the EEG cap and clip 2 reference electrodes into the two ear lobes.
NOTE: The electrode should be kept in a wet state without dripping. If the electrode is too dry, the EEG signal acquisition process will be affected. If the electrode is too wet, the dripping saline might affect the functional performance of the subject. - As the operator, pay attention to EEG acquisition during the training. If there is a small range of EEG disturbance, first consider whether the corresponding electrode shows dryness, water shortage, or other conditions with the training. At this time, stop the training and EEG acquisition immediately and properly wet the electrode before the training continues.
- If the EEG signal has an extensive range of disturbance, first consider whether the reference electrode has fallen off or not. At this time, stop the training immediately and clamp the reference electrode at the earlobe again.
- Put the manipulator on the patient and adjust it to a comfortable training position to prevent upper limb forearm pain caused by over-tightening or slipping.
- Software operation
- Open the training software MI-BCI upper limb hand function rehabilitation robot, click the User List, and enter the patient information including name, disease name, date of birth, date of illness, and location of the affected side.
- Adjust the stability of the EEG signal to no obvious clutter.
- Click the Resting EEG button to let the patient complete the resting EEG acquisition process according to the voice and text prompts: Please close your eyes to relax and please open your eyes. This lasts for 60 s.
- Click the Task Setting button to set the task difficulty and training duration. Set the initial training difficulty upward or downward from level 9 according to the actual situation of the patients, and set the training duration to 30 min.
- During the training, the MI-BCI system will automatically adjust the task difficulty according to the patient's performance. Check the difficulty of training as reflected by the size of the objects grasped; the higher the difficulty of the task, the finer the objects grasped.
- Click the Task EEG button to start the formal training. The screen on the patient side displays: Please close your eyes and relax for 5s.
- After 5 s, the screen on the patient side displays: Please open your eyes and imagine your hand open/make a fist, which lasts for 3 s. Ask the patient to follow the on-screen commands.
- The screen on the patient side displays the gripping video/opening video, to assist the patient to simulate the actions demonstrated by the video in imagination. Ask the patient to perform this motion imagination task that lasts for 5 s.
- The system extracts the EEG signal of the patient's motor imagination, uses the algorithm to analyze the score of the motor intention, and displays it on the screen of the patient's side. Check the robot feedback, that will be displayed in 4 s.
NOTE: The model adopted by the MI-BCI system is FBCNet (multi-band filtering + channel separation convolution + variance layer +FC) to extract features and score EEG signals12. The threshold value of the MI-BCI system is determined based on a large number of clinical trial data and core EEG algorithm. The threshold value is 60 points. - Check the motor intention displayed. If the patient's motor intention is ≥ 60 points (threshold value), the system recognizes that the specified action has been achieved. If the patient's intention to move < 60 points, and the system determines that the patient cannot perform the specified movement, ensure that the audio prompts the patient to keep going and not get discouraged and the patient complies to this.
- During the training process, observe the EEG waveform in real time. If there is any abnormal situation, suspend the training in time, and restart the training after the signal is adjusted to be stable. If the patient has pain or discomfort during the training, stop the training and record the reason for termination.
3. Clinical evaluation
- Information collection
- Collect and record basic personal information about the subjects, such as name, sex, date of birth, education level, etc.
- Complete the collection of the subject's medical history, such as clinical history, medication history, personal history, etc.
- Motor function evaluation
- Evaluate the Fugl-Meyer assessment of upper extremity (FMA-UE)13 on stroke subjects.
The total score is 66. The higher the score, the better the patient's functional performance. - Evaluate the Wolf motor function test (WMFT)14 on stroke subjects. The total score is 85. The higher the score, the better the patient's functional performance.
- Evaluate the Fugl-Meyer assessment of upper extremity (FMA-UE)13 on stroke subjects.
- Cognitive function assessment
- Evaluate the mini-mental state examination (MMSE)15 on stroke subjects. According to the educational level, divide the score into illiterate ≤17, education level of primary school ≤20, and education level of junior high school and above ≤24.
- Emotional function assessment
- Evaluate the Hamilton anxiety scale (HAMA)16 on stroke subjects. The score ranges are: ≥21, there is evident anxiety; ≥14 points, definitely have anxiety; and more than 7 points, might have anxiety. A score of less than 7 indicates no symptoms of anxiety.
- Evaluate the Hamilton depression scale (HAMD)17 on stroke subjects. A score of < 7 is average, a score of 7-17 indicates that the individual might have depression, a score of 17-24 indicates that the individual can be diagnosed with depression, and a score of >24 indicates that the individual has severe depression.
4. fNIRS brain function assessment
For this study, place 10 sources and 12 detectors on the fNIRS test head caps to correspond to the eight regions of interest (ROIs) in this study, including the bilateral dorsolateral prefrontal cortex (DLPFC), the dorsolateral promoter cortex (PMC), the dorsolateral primary motor cortex (M1), and the dorsolateral primary sensory cortex (S1; Figure 2).
- Preparation of subject
- Explain the training purpose and method and note them for the subjects. Ensure that the subjects understand the experiment and are familiar with the relevant procedures; this helps improve patient compliance.
- Put the fNIRS test head cap on the subject, determine the Cz point of the subject's head, and ensure that it coincides with the Cz point of the head cap as described in step 2.1.2.
- Adjust the head cap for the subject to ensure that both ears are exposed from the ear seam of the cap, the bilateral ear edges of the cap are naturally fitted to the bilateral mastoid process, the leading edge of the cap is naturally fitted to the forehead, and the trailing edge is naturally fitted to the posterior occipital part. Adjust the chin band to fix the cap.
- Adjust the tightness of the jaw band appropriately. If it is too loose, it will lead to the displacement of the head cap during the experiment, which will result in inaccurate positioning and affect signal acquisition. If it is too tight, it will cause discomfort to the subject, which will reduce compliance and affect the functional performance of the subject.
- Pre-collection and collection of data
- Enter the fNIRS control software, select the Experimental Subject, and establish patient treatment files according to the patient information.
- Click the Pre-collection button to calibrate the signals. According to the signal intensity of the site displayed by the near-infrared functional brain imaging system, improve the signal intensity and stability of the site by adjusting the light source or receiver of the head cap so that it is closer to the subject's scalp.
- When the system displays all sites as green, the signal strength is stable. Stop the pre-collection and click the Automatic gain button to make the final adjustment to the signal.
NOTE: In the pre-collection process, the near-infrared brain functional imaging system presents different colors to represent the signal strengths of sites. Gray represents low signal strength, yellow represents good signal strength, green represents excellent signal strength, and red represents over-strong signal strength. - Click the Start button to collect the signals. Observe and record various conditions in the experiment, such as signal fluctuations and poor electrode contact.
- fNIRS-motor task assessment
- Select the Motor Task paradigm in the fNIRS system.
- Place the patient's upper limb on the test table and ask the patient to rest for 10 s before the experiment.
- Ask the patient to follow the exercise rhythm to grasp the affected hand in three blocks during the experiment, each block includes 30 s task and 30 s rest. Each task consists of 15 trials, each trial includes 1 s gripping and 1 s opening of the grasp.
- During each rest, ask the patients to close their eyes and rest. If the subject is unable to grasp the affected hand, ask the subject to perform an exercise of motor imagery. The test lasts for 190 s (Figure 3). After all three blocks are complete, end the task, save the data, and import it to the self-built database.
- fNIRS-cognitive task assessment (Stroop task)
- Run behavioral research software and choose the Cognitive Task paradigm. Select the patient treatment files and then select the Congruence Test.
- Ask the subject to place the healthy hand on the button of the keyboard, then ask the subject to rest for 10 s before the trial starts. Perform three blocks of the congruence test, each block includes 60 s task and 30 s rest. Each task consists of 10 trials, each consisting of 2000 ms fixation and 4000 ms stimulus-response with a total duration of 280 s (Figure 4).
- When left symbol is displayed on the left of the field font, press the ← button on the keyboard as soon as possible according to the character meaning (that is, left).
- When right symbol is displayed on the left of the field grid, click the → button on the keyboard as soon as possible according to the meaning of the character (i.e., right).
- Select the Incongruence Test; the procedure is the same as that of the congruence test.
- When right symbol is displayed on the left of the field font, ignore the character meaning and press the ← button on the keyboard as soon as possible according to the position where the text appears (i.e., left).
- When left symbol is displayed on the right side of the field font, ignore the character meaning and click the → button on the keyboard as soon as possible according to the position of the text (i.e., right).
- Complete the task, save the data, and export it to the self-created database.
5. Post-treatment
- Perform the evaluation as done for the clinical function evaluation and fNIRS brain function evaluation in the pre-treatment. Perform all the evaluations after the end of the 10th training session (see steps 3.1 to 4.4 for details).
6. Data processing and analysis
- Summarize and analyze patients' personal information and clinical assessment scale data.
- Use a commercial software to preprocess near-infrared data. Perform elimination of time interval and motion artifacts of interference tests, selection of a bandpass filter (0.01-0.2 Hz) to remove noise, calculation of the relative changes of oxygenated hemoglobin (HbO) and deoxygenated hemoglobin (HbR) according to the modified Beer-Lambert law and convert the optical density signal into a blood oxygen concentration signal.
NOTE: HbO is more sensitive to changes between different conditions than HbR, so only HbO data is used in this study scheme for subsequent analysis. - Select HbO in blood oxygen type as analysis data. Set up the GLM design matrix and select the task phase in the task. Click the Estimate button to fit the established design matrix with the collected data.
- Use the established linear correlation model to calculate the beta value in each ROI. Export the parameters, accuracy rate (ACC) and response time (RT), in the Stroop task through the behavioral research software.
Representative Results
The study presents the clinical function and remodeling of brain function before and after MI-BCI intervention in a 36-year-old male stroke patient. More than 4 months after cerebral hemorrhage, the imaging results showed chronic bleeding focus in the right frontal lobe and the right basal ganglia region-radiative crown region. The patient was diagnosed with left limb motor dysfunction during convalescence from a cerebral hemorrhage. Simple outpatient treatment of MI-BCI was performed in the hospital for 10 days (30 min/session/day) and brain function assessment and clinical function assessment were completed before and 10 days after treatment (Figure 5). The results of clinical evaluation before and after treatment were FMA-UE = 12/14 (before/after); WMFT (scores) = 33/34; WMFT (s) = 127.43/90.91; MMSE = 30/30; HAMA = 6/0; HAMD = 4/0 (Table 1).
The Stroop cognitive paradigm assessment results showed that the ACC of the congruence test and the incongruence test was the same in the pre-treatment while the RT of the incongruence test was longer than that of the congruence test (ACCCongruence = ACCIncongruence = 100%, RTCongruence = 856.6 ms, RTIncongruence = 880.7 ms). The ACC of the congruence test and the incongruence test was the same in the post-treatment and the RT of the incongruence test was also longer than that of the congruence test (ACCCongruence = ACCIncongruence = 100%, RTCongruence = 803.2 ms, RTIncongruence = 870.1 ms). It could be seen from the comparison before and after treatment that for the congruence test, the ACC remained unchanged and the RT became shorter after treatment (ACCPre = ACCPost = 100%, RTPre = 856.6 ms, RTPost = 803.2 ms). For the incongruence test, the ACC remained unchanged, and the RT was shorter after treatment (ACCPre = ACCPost = 100%, RTPre = 880.7 ms, RTPost = 870.1 ms; Table 2).
The results of the fNIRS evaluation showed that the beta value of R-M1 in the post-treatment was higher than that in the motor task paradigm of the pre-treatment (R-M1Pre = 0.016, R-M1Post = 0.044). The beta value of bilateral DLPFC in the post-treatment was higher than that in the congruence test of the pre-treatment (R-DLPFCPre = 0.009, R-DLPFCPost = 0.010; L-DLPFCPre = -0.062, L-DLPFCPost = 0.003). The beta value of all ROIs in the post-treatment was higher than that in the incongruence test of the pre-treatment (R-DLPFCPre = -0.032, R-DLPFCPost = 0.030; L-DLPFCPre = -0.007, L-DLPFCPost = 0.026; R-PMCPre = 0.014, R-PMCPost = 0.087; L-PMCPre = 0.030, L-PMCPost = 0.111; R-S1Pre = -0.035, R-S1Post = 0.081; L-S1Pre = 0.024, L-S1Post = 0.081; R-M1Pre = 0.000, R-M1Post = 0.025; L-M1Pre = 0.031, L-M1Post = 0.093). The rest of the brain regions and their data are shown in Figure 6 and Table 3, respectively.
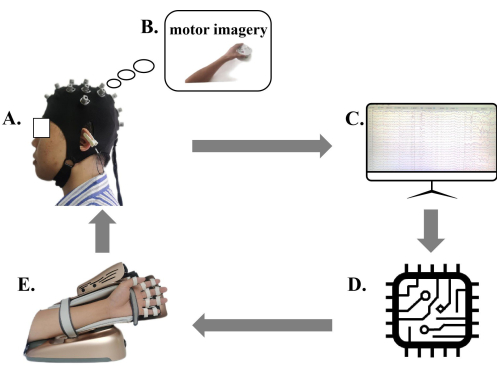
Figure 1. Diagram of the motor imagery brain computer interface (MI-BCI) upper limb rehabilitation training system. (A) Patients wear MI-BCI EEG caps. (B) The patient side display provides motor imagery. (C) EEG display screen. (D) The MI-BCI system analyzes and interprets the EEG signal. (E) The robotic arm for motion performing and feedback. Patients wearing MI-BCI EEG caps perform motor imagery according to the motion images provided by the patient side display, and the MI-BCI system based on EEG analyzes and interprets the patient's EEG signal. The robotic arm provides corresponding feedback according to the patient's motion intention score. Please click here to view a larger version of this figure.

Figure 2. Regions of interest and the channel setting. In the protocol, eight regions of interest (ROIs) were selected, including the bilateral dorsolateral prefrontal cortex (DLPFC), the dorsolateral promoter cortex (PMC), the dorsolateral primary motor cortex (M1), and the dorsolateral primary sensory cortex (S1). Abbreviations: DLPFC = prefrontal cortex; PMC = premotor cortex; M1 = primary motor cortex; S1 = primary sensory cortex. Please click here to view a larger version of this figure.
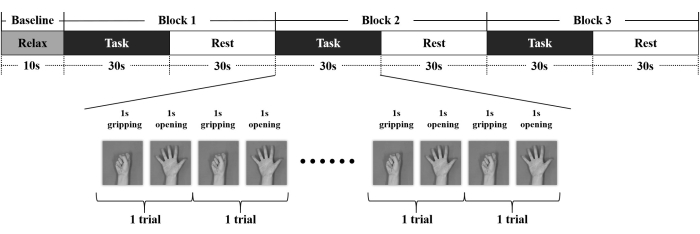
Figure 3. The Motor task paradigm and the fNIRS design. The motor task paradigm lasted 190 s and included a resting state rest of 10 s and three blocks, each block includes 30 s task and 30 s rest. Each task consists of 15 trials, each trial includes 1 s gripping and 1 s opening of the grasp. Please click here to view a larger version of this figure.
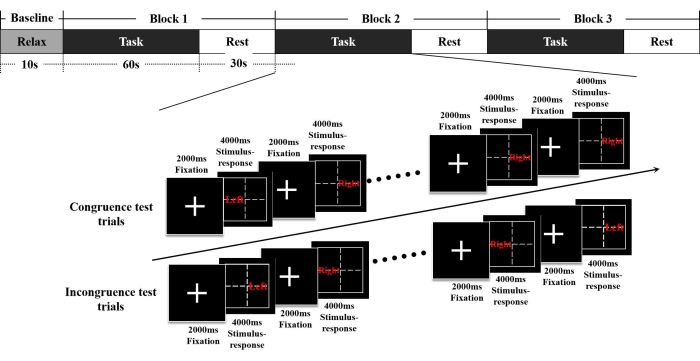
Figure 4. The Stroop paradigm and the fNIRS design. The congruence test lasted 280 s and included a resting state rest of 10 s and three blocks, each block includes 60 s task and 30 s rest. Each task consists of 10 trials, each consisting of 2000 ms fixation and 4000 ms stimulus-response. The procedure of the incongruence test is the same as that of the congruence test. Abbreviations:  = left;
= left;  = right. Please click here to view a larger version of this figure.
= right. Please click here to view a larger version of this figure.
Figure 5. MI-BCI training timeline. The protocol included 10 days of outpatient treatment (30 min/session/day) and brain function assessment and clinical function assessment were completed before and 10 days after treatment. Please click here to view a larger version of this figure.
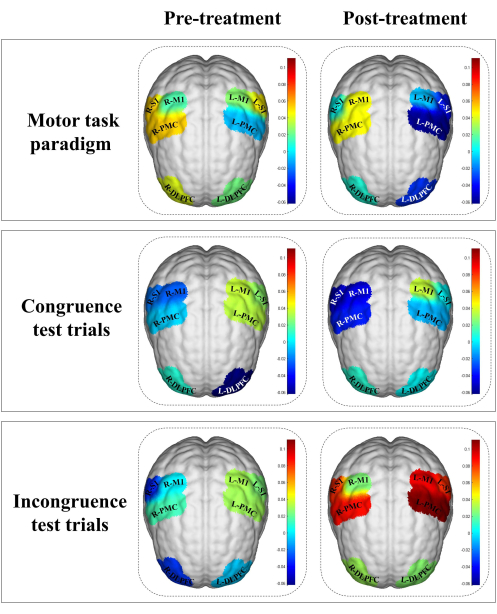
Figure 6. ROIs activation in the stroke patient before and after treatment. The beta values are indicated by color. The color bar represents intensity of activation of each region, in which blue means less activation whereas red means more activation. The results of the fNIRS evaluation showed that the beta value of R-M1 in the post-treatment was higher than that in the motor task paradigm of the pre-treatment. The beta value of bilateral DLPFC in the post-treatment was higher than that in the congruence test of the pre-treatment. The beta value of all ROIs in the post-treatment was higher than that in the incongruence test of the pre-treatment. Abbreviations:L-DLPFC = left dorsolateral prefrontal cortex; R-DLPFC = right dorsolateral prefrontal cortex; L-PMC = left promoter cortex; R-PMC = right promoter cortex; L-M1 = left primary motor cortex; R-M1 = right primary motor cortex; L-S1 = left primary sensory cortex; R-S1 = right primary sensory cortex; L = left; R= right. Please click here to view a larger version of this figure.
| Clinical function evaluation | Pre-treatment | Post-treatment |
| FMA-UE (scores) | 12 | 14 |
| WMFT (scores) | 33 | 34 |
| WMFT(s) | 127.43 | 90.91 |
| MMSE (scores) | 30 | 30 |
| HAMA (scores) | 6 | 0 |
| HAMD (scores) | 4 | 0 |
Table 1 Results of clinical function assessment before and after MI-BCI training. FMA-UE and WMFT were used to assess patients' motor function, MMSE was used to assess patients' cognitive function, and HAMA and HAMD were used to assess patients' emotional function. Abbreviations: FMA-UE = Fugl-Meyer assessment-upper extremity; WMFT = Wolf motor function test; MMSE = Mini-Mental State Examination; HAMA = Hamilton Anxiety Scale; HAMD = Hamilton Depression Scale.
| Pre-treatment | Post-treatment | ||
| Congruence test | ACC (%) | 100% | 100% |
| RT (ms) | 856.6 | 803.2 | |
| Incongruence test | ACC (%) | 100% | 100% |
| RT (ms) | 880.7 | 870.1 |
Table 2 Results of congruence test and incongruence test based on Stroop cognitive paradigm. The Stroop cognitive paradigm assessment results showed that the ACC of the congruence test and the incongruence test were the same in the pre-treatment while the RT of the incongruence test was longer than that of the congruence test. The ACC of the congruence test and the incongruence test was the same in the post-treatment and the RT of the incongruence test was also longer than that of the congruence test. It could be seen from the comparison before and after treatment that for the congruence test, the ACC remained unchanged, and the RT became shorter after treatment. For the incongruence test, the ACC remained unchanged, and the RT was shorter after treatment. Abbreviations: ACC = accuracy; RT = reaction time.
| Motor task paradigm | Congruence test trials | Incongruence test trials | ||||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| RDLPFC | 0.041 | 0.008 | 0.009 | 0.010 | -0.032 | 0.030 |
| LDPFC | 0.022 | -0.032 | -0.062 | 0.003 | -0.007 | 0.026 |
| RPMC | 0.054 | 0.045 | -0.006 | -0.036 | 0.014 | 0.087 |
| LPMC | -0.009 | -0.059 | 0.034 | -0.011 | 0.030 | 0.111 |
| RS1 | 0.053 | 0.004 | -0.021 | -0.051 | -0.035 | 0.081 |
| LS1 | 0.048 | -0.038 | 0.026 | 0.001 | 0.024 | 0.081 |
| RM1 | 0.016 | 0.044 | -0.025 | -0.047 | 0.000 | 0.025 |
| LM1 | 0.026 | -0.009 | 0.036 | 0.036 | 0.031 | 0.093 |
Table 3 Changes of ROIs beta values in the stroke patient before and after treatment. The results of the fNIRS evaluation showed that the beta value of R-M1 in the post-treatment was higher than that in the motor task paradigm of the pre-treatment. The beta value of bilateral DLPFC in the post-treatment was higher than that in the congruence test of the pre-treatment. The beta value of all ROIs in the post-treatment was higher than that in the incongruence test of the pre-treatment. Abbreviations: L-DLPFC = left dorsolateral prefrontal cortex; R-DLPFC = right dorsolateral prefrontal cortex; L-PMC = left promoter cortex; R-PMC = right promoter cortex; L-M1 = left primary motor cortex; R-M1 = right primary motor cortex; L-S1 = left primary sensory cortex; R-S1 = right primary sensory cortex; L = left; R = right.
Discussion
The rehabilitation period for moderate and severe upper limb motor dysfunction after stroke is long and the recovery is difficult, which has always been the focus of clinical rehabilitation research18. Traditional upper limb rehabilitation training is mostly simple peripheral intervention or central intervention19. Meanwhile, due to the lack of active participation of patients with moderate and severe limb dysfunction, passive treatment is mainly used, with poor rehabilitation effects20. Therefore, it is of great significance to continuously develop new stroke rehabilitation techniques/strategies21. MI-BCI has good clinical application potential as a non-invasive means of rehabilitation and has been gradually promoted22. In this study, MI-BCI was adopted as an intervention method to collect EEG signals generated by brain motor imagery, analyze and decode the patient's intention to move the affected limb, convert them (the patient's intention) into action instructions to control external devices, and feed them back to the brain through audio-visual and tactile feedback, thus forming a central-peripheral-central closed-loop system23. This method is more in line with the rehabilitation characteristics of central nervous system diseases and theoretically is more conducive to promoting the functional remodeling of injured cerebral nerves24.
The following points must be noted and explained in the MI-BCI training. First, contraindications must be clear before treatment. In addition to the contraindications mentioned in the exclusion criteria in recruitment, the operator should ensure that there are no fresh wounds on the subject's head prior to treatment. Since the process of wearing the EEG cap requires the sponge to be filled with saline and placed in the slot, there is a risk of wound infection if the subject has head trauma. Second, for patients with severe upper limb spasms, it is necessary to clarify the degree of spasm and pain and to determine whether wearing the manipulator will increase the degree of pain. If the patient cannot tolerate it, the training should be terminated. For patients with shoulder subluxation, pillows should be placed on the elbow to avoid aggravating the degree of subluxation. Third, in the preparation stage of MI-BCI training, the correct wearing of the EEG cap is very important for decoding EEG data during training. The wearing of the EEG cap should strictly follow the international 10-20 system to ensure that the Cz site on the EEG cap corresponds to the Cz site on the top of the subject's head9,10,11. Recalibration is required if the EEG cap shifts during training. Fourth, the MI-BCI operation process has strict requirements regarding the environment. MI-BCI requires subjects to concentrate intently to complete active motor imagery, and a noisy environment will affect patients' concentration. Fifth, attention should be paid to the fatigued condition of patients in the training process. MI-BCI training requires the patient to constantly perform motor imagery; for patients in high-intensity, long-time, focused training who are prone to fatigue, sleepiness, and sedentary discomfort, the operator must pay attention to the patient's physical condition. If it is necessary, the patient can take a break. If the above conditions cannot be improved, training can be terminated.
In this study protocol, FMA-UE and WMFT scores of patients increased before and after the treatment, suggesting an improvement trend in the motor function performance of patients. Accordingly, eight ROIs were selected for fNIRS brain function assessment in this study, including bilateral DLPFC, PMC, M1, and S1. The results showed that M1 of the affected side was activated after MI-BCI intervention in the motor task monitored by synchronous fNIRS. At the same time, bilateral DLPFC were activated in the Stroop congruence test and the incongruence test, and M1 and S1 were activated in the Stroop incongruence test, suggesting that MI-BCI intervention might have positive effects on the motor and cognitive function of stroke patients. The above results can provide a reference for subsequent MI-BCI-related cerebral nerve remodeling mechanisms and be of significance for further research on MI-BCI-related mechanisms.
This protocol has some limitations. First, this study presented a treatment plan for MI-BCI in patients with moderate and severe upper limb dysfunction after stroke and described the efficacy of MI-BCI, 10 days after intervention through clinical function evaluation and brain function evaluation results. However, this study protocol included only one stroke patient. Therefore, further verification should be conducted with a larger sample size in the future. Second, this study intervened for only 10 sessions; the long-term efficacy of MI-BCI requires further improvement in future studies. Moreover, this study used only the MI-BCI paradigm, which does not apply to stroke patients with cognitive dysfunction. BCI under other paradigms should be explored in future clinical practice to meet the rehabilitation needs of patients with cognitive dysfunction.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This study was supported by the National Science Foundation of Guangdong Province (No.2023A1515010586), Guangzhou clinical characteristic technology construction project (2023C-TS19), Education Science Planning Project of Guangdong Province (No.2022GXJK299), the General Guidance Program of Guangzhou Municipal Health and Family Planning (20221A011109, 20231A011111), 2022 Guangzhou Higher Education Teaching Quality and Teaching Reform Project of Higher Education Teaching reform General project (No.2022JXGG088/02-408-2306040XM), 2022 Guangzhou Medical University Student Innovation Ability Improvement Plan project (No.PX-66221494/02-408-2304-19062XM), 2021 school-level education science planning project (2021: NO.45), 2023 First-class Undergraduate Major Construction Fund of high-level University (2022JXA009, 2022JXD001, 2022JXD003)/(02-408-2304-06XM), Guangzhou Education Bureau university research project (No. 202235384), 2022 Undergraduate Teaching Quality and Teaching Reform Project of Guangzhou Medical University (2022 NO. 33), National Science Foundation of Guangdong Province (No. 2021A1515012197), and Guangzhou and University Foundation (No. 202102010100).
Materials
| MI-BCI | Rui Han, China | RuiHan Bangde | NA |
| E-Prime | version 3.0 | behavioral research software. | |
| fNIRS | Hui Chuang, China | NirSmart-500 | NA |
| NirSpark | preprocess near-infrared data |
References
- Dawson, J., et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet. 397 (10284), 1545-1553 (2021).
- Lin, Q., et al. The Frequency Effect of the Motor Imagery Brain Computer Interface Training on Cortical Response in Healthy Subjects: A Randomized Clinical Trial of Functional Near-Infrared Spectroscopy Study. Frontiers in Neuroscience. 16, 810553 (2022).
- Carino-Escobar, R. I., et al. Longitudinal Analysis of Stroke Patients’ Brain Rhythms during an Intervention with a Brain-Computer Interface. Neural Plasticity. 2019, 7084618 (2019).
- Mane, R., Chouhan, T., Guan, C. BCI for stroke rehabilitation: motor and beyond. Journal of Neural Engineering. 17 (4), 041001 (2020).
- Khan, M. A., Das, R., Iversen, H. K., Puthusserypady, S. Review on motor imagery based BCI systems for upper limb post-stroke neurorehabilitation: From designing to application. Computers In Biology And Medicine. 123, 103843 (2020).
- Hendricks, H. T., van Limbeek, J., Geurts, A. C., Zwarts, M. J. Motor recovery after stroke: a systematic review of the literature. Archives of Physical Medicine and Rehabilitation. 83 (11), 1629-1637 (2002).
- Cheng, N., et al. Brain-Computer Interface-Based Soft Robotic Glove Rehabilitation for Stroke. IEEE Transactions on Biomedical Engineering. 67 (12), 3339-3351 (2020).
- Ang, K. K., et al. Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: results of a three-armed randomized controlled trial for chronic stroke. Frontiers in Neuroengineering. 7, 30 (2014).
- Nuwer, M. R., et al. IFCN standards for digital recording of clinical EEG. The International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology. 52, 11-14 (1999).
- Klem, G. H., Lüders, H. O., Jasper, H. H., Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology. 52, 3-6 (1999).
- Uwe Herwig, ., Peyman Satrapi, ., Schönfeldt-Lecuona, C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topography. , (2003).
- Mane, R., Robinson, N., Vinod, A. P., Lee, S. W., Guan, C. A Multi-view CNN with Novel Variance Layer for Motor Imagery Brain Computer Interface. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2020, 2950-2953 (2020).
- Sanford, J., Moreland, J., Swanson, L. R., Stratford, P. W., Gowland, C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Physical Therapy. 73 (7), 447-454 (1993).
- Martinez, C., et al. A Reaching Performance Scale for 2 Wolf Motor Function Test Items. Archives of Physical Medicine and Rehabilitation. 101 (11), 2015-2026 (2020).
- Dufouil, C., et al. Population norms for the MMSE in the very old: estimates based on longitudinal data. Mini-Mental State Examination. Neurology. 55 (11), 1609-1613 (2000).
- Thompson, E. Hamilton Rating Scale for Anxiety (HAM-A). Occupational Medicine. 65 (7), 601 (2015).
- Zimmerman, M., Martinez, J. H., Young, D., Chelminski, I., Dalrymple, K. Severity classification on the Hamilton Depression Rating Scale. Journal of Affective Disorders. 150 (2), 384-388 (2013).
- Bai, X., et al. Different Therapeutic Effects of Transcranial Direct Current Stimulation on Upper and Lower Limb Recovery of Stroke Patients with Motor Dysfunction: A Meta-Analysis. Neural Plasticity. 2019, 1372138 (2019).
- Dimyan, M. A., Cohen, L. G. Neuroplasticity in the context of motor rehabilitation after stroke. Nature Reviews Neurology. 7 (2), 76-85 (2011).
- Bai, Z., Fong, K. N. K., Zhang, J. J., Chan, J., Ting, K. H. Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: a systematic review and meta-analysis. Journal of NeuroEngineering and Rehabilitation. 17 (1), 57 (2020).
- Yang, W., et al. The Effect of Brain-Computer Interface Training on Rehabilitation of Upper Limb Dysfunction After Stroke: A Meta-Analysis of Randomized Controlled Trials. Frontiers in Neuroscience. 15, 766879 (2021).
- Pandian, S., Arya, K. N. Stroke-related motor outcome measures: do they quantify the neurophysiological aspects of upper extremity recovery. Journal of Bodywork and Movement Therapies. 18 (3), 412-423 (2014).
- Potter, S. M., El Hady, A., Fetz, E. E. Closed-loop neuroscience and neuroengineering. Frontiers in Neural Circuits. 8, 115 (2014).
- Nowak, D. A., Grefkes, C., Ameli, M., Fink, G. R. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabilitation and Neural Repair. 23 (7), 641-656 (2009).

