Visualizing Oceanographic Data to Depict Long-term Changes in Phytoplankton
Summary
Here, we present a protocol for converting phytoplankton microscopic images into vector graphics and repetitive patterns to enable visualization of shifts in phytoplankton taxa and biomass over 60 years. This protocol represents an approach that can be utilized for other plankton time series and datasets globally.
Abstract
Oceanographic time series provide an important perspective on environmental processes in ecosystems. The Narragansett Bay Long-Term Plankton Time Series (NBPTS) in Narragansett Bay, Rhode Island, USA, represents one of the longest plankton time series (1959-present) of its kind in the world and presents a unique opportunity to visualize long-term change within an aquatic ecosystem. Phytoplankton represent the base of the food web in most marine systems, including Narragansett Bay. Therefore, communicating their importance to the 2.4 billion people who live within the coastal ocean is critical. We developed a protocol with the goal of visualizing the diversity and magnitude of phytoplankton by utilizing Adobe Illustrator to convert microscopic images of phytoplankton collected from the NBPTS into vector graphics that could be conformed into repetitive visual patterns through time. Numerically abundant taxa or those that posed economic and health threats, such as the harmful algal bloom taxa, Pseudo-nitzschia spp., were selected for image conversion. Patterns of various phytoplankton images were then created based on their relative abundance for select decades of data collected (1970s, 1990s, and 2010s). Decadal patterns of phytoplankton biomass informed the outline of each decade while a background color gradient from blue to red was used to reveal a long-term temperature increase observed in Narragansett Bay. Finally, large, 96-inch by 34-inch panels were printed with repeating phytoplankton patterns to illustrate potential changes in phytoplankton abundance over time. This project enables visualization of literal shifts in phytoplankton biomass, that are typically invisible to the naked eye while leveraging real-time series data (e.g., phytoplankton biomass and abundance) within the art piece itself. It represents an approach that can be utilized for many other plankton time series for data visualization, communication, education, and outreach efforts.
Introduction
Phytoplankton are primary producers representing the base of the food web across aquatic ecosystems1,2. While phytoplankton monitoring programs are key to identifying current and future changes in marine ecosystems, their support is declining over time 3. Due to their relatively short generation times and limited mobility, phytoplankton are particularly responsive to climate change, which makes them an important tool in time series monitoring. Phytoplankton time series are also important for informing ecosystem-based management of resource availability and providing context for episodic events, such as marine heatwaves4. Short-term time series, on the matter of years, give insights into phytoplankton community succession and seasonal dynamics (e.g., ref.5,6), whereas long-term time series, such as the Bermuda Atlantic Time Series (BATS) and Hawaii Ocean Times Series (HOTS) Programs, span more than two decades and enable the detection of long-term trends7,8. Such studies illustrate the benefit and importance of a highly resolved phytoplankton record for a complete understanding of long-term ecosystem change in dynamic marine environments. Further, visualizing and communicating these changes in phytoplankton, which cannot be seen by the naked eye, are more difficult to comprehend than for organisms that are large and readily visible, like fish and whales. Computer visualizations offer a technique to explore complex data sets9 and improved illustrative graphics are becoming readily available (e.g., Integration and Application Network, University of Maryland Center for Environmental Science). However, most studies in phytoplankton ecology, including many referenced here, still only present results as data graphs reducing their accessibility to general audiences. Given that phytoplankton represent the base of the food web in most marine systems, communicating their importance to the nearly 2.4 billion people who live within the coastal ocean is critical10. Here, we developed a protocol with the goal of visualizing the diversity and magnitude of phytoplankton, as collected by a phytoplankton monitoring program.
The Narragansett Bay Plankton Time Series (NBPTS) provides a long-term 60+ year (1959-Present) perspective on the effects of global change within a climate context on phytoplankton abundance, seasonality, and phenology (life history). Narragansett Bay (NBay) is a coastal estuary connected to the broader systems of the U.S. Northeast Shelf and Northwest Atlantic whose production has important implications for fisheries and human use along the coastal U.S.A.11. NBay is considered a highly seasonal system experiencing long-term (1950-2015) warming waters in the region as well as shifts in nutrients and an increase in water clarity12,13. In addition, a decline in phytoplankton biomass has occurred in upper NBay related to anthropogenic decreases in dissolved inorganic nitrogen, which is partially attributed to upgrades in wastewater treatment plants12. Shifts in phytoplankton taxa, particularly harmful algal blooms (HABs), are also occurring in NBay. Pseudo-nitzschia spp., which produces pervasive toxic blooms in upwelling regions along the U.S. west coast, led to notable shellfish closures for the first time in NBay's history in 2016 and 201714,15,16. Communicating these changes to diverse audiences is important for increasing science literacy and for promoting continued support of phytoplankton monitoring studies.
The goal of this project was to utilize microscopic images of phytoplankton from NBay, as well as data synthesized from NBPTS, to visualize the literal shifts in phytoplankton taxa and biomass that are occurring in NBay to communicate and enhance the importance of phytoplankton to general audiences. NBPTS provides 60+ years of publicly available weekly phytoplankton counts and biomass to leverage data from (https://web.uri.edu/gso/research/plankton/). The final product was a large mural of plankton patterns representative of the time series data (e.g., phytoplankton biomass and taxa, temperature) within the art piece itself. This approach represents a visualization method that can be utilized for many other plankton time series throughout the world and can be adapted for monitoring programs with short-term, seasonal data as well. The benefits of implementing this protocol include increasing efforts in data visualization, science communication, education, and engagement with local communities.
Protocol
1. Converting phytoplankton images into vector graphics
- Select phytoplankton microscopic images taken from the Narragansett Bay Long Term Plankton Time Series (NBPTS) as either .JPG, .PNG, or .PDF files (Figure 1A).
NOTE: Taxa include Thalassiosira nordenskioeldii, Thalassionema nitzschioides, Tripos spp., Odontella aurita, Skeletonema species complex, Chaetoceros diadema, Eucampia zodiacus, Dinophysis spp., and Pseudo-nitzschia spp. Images were taken with a light microscope. - Open specific software vector graphics editor or the illustrator used for this study (see Table of Materials). Vector graphic software has been mentioned as illustrator further in the manuscript.
- Place a .JPG or .PNG microscopic image into the illustrator workspace by opening a file from the computer or drag and dropping it into a new workspace.
- Go to View > Show Transparency Grid to reveal the checkerboard background that indicates transparency.
- Click on Window > Image Trace in the dropdown menu to open the Image Trace window.
- Click on the Selection tool (black arrow) in the toolbar and click on the phytoplankton image.
- Click on Object > Expand in the dropdown menu.
- Use the Direct Selection tool (white arrow) in the toolbar to click and select the background parts of the image to get rid of around the phytoplankton. Press delete.
- Repeat for each background region of the image.
- Click on File > Export to save the file as .PNG file. Ensure the Background Transparent box is selected.
- Place .PNG microscopic image with background removed into a new workspace in the illustrator by opening the file from the computer or drag and drop it into a new workspace.
- Click on Window > Image Trace in the dropdown menu to open the Image Trace window.
- Under the Image Trace options, click Preset > Black and White Logo and Mode > Black and White.
- Use the Threshold as well as the Advanced options (i.e., Paths, Corners, and Noise) to refine the image.
- Under Properties, select Expand to vectorize it.
- Select View > Show Transparency Grid.
- Click on the vector image then right click and select Ungroup.
- Choose the Direct Selection tool (white arrow) in the toolbar. Drag and draw a box around the whitespace only. Press delete to remove it.
- Repeat until all the whitespace is removed.
- Click File > Save As and select .EPS to save as a vector graphic.
- Repeat for the phytoplankton taxa from 1.1 (Figure 1B).
2. Creating phytoplankton patterns
- Utilize phytoplankton count data from the NBPTS dataset to determine the average abundance of each taxon from 1970-1979 (1970s), 1990-1999 (1990s), and 2010-2019 (2010s).
- Calculate mean ± standard deviation for each phytoplankton taxa for each decade in a statistical software program by clicking on or typing 'mean() and sd()'.
- Click-on or type 'aov() ' to use an ANOVA to test for significant differences among decades in a statistical software program.
NOTE: Some species (e.g., Tripos spp., Chaetoceros diadema) do not have large enough sample sizes in the 1990s. In this case, click-on or type 't.test()' in a statistical software program to compare mean abundances in the 1970s to the 2010s. - Use the 'Artboard tool ' (square) in the toolbar to click and create a new Artboard in a new workspace of the specific illustrator used in this study.
- Make three identical Artboards of the same size. Adjust the size within Properties > Transform.
NOTE: For this project, Artboards for the phytoplankton images were 1224 px by 545 px. - Drag and drop the .EPS files of the different phytoplankton taxa onto the three Artboards.
- Color the phytoplankton with different colors representative of the decade by using the Direct Selection tool (white arrow) to draw a box around an individual phytoplankton.
- Under Properties, select Fill and then click on the desired color from the color palette. Press enter to fill the vector.
- Use the Selection Tool (black arrow) to highlight a particular phytoplankton then select Edit > Copy and Edit > Paste.
- Paste each phytoplankton vector qualitatively based on the relative proportions of each taxa in the dataset as determined in 2.2 for each of the three decades (Figure 1C).
NOTE: The abundance of phytoplankton on each panel is a qualitative representation of Table 1. For example, if a higher abundance of Pseudo-nitzschia spp. is observed in the 2010s than in the 1990s then copy more Pseudo-nitzschia graphics onto the 2010 artboard than on the 1990 artboard. - Select Object > Pattern > Make to create a color swatch phytoplankton pattern for each of the three decades.
3. Incorporating phytoplankton biomass and temperature data
- Click-on or type 'mean()' to calculate the average chlorophyll a (chl a, proxy for phytoplankton biomass) for each week of every decade in a statistical software program.
- Click-on or type 'plot()' in a statistical software program to graph the mean decadal biomass (dependent variable) by each week (independent variable) and click on Save the graph as a .JPG or .PNG.
- Place a .JPG or .PNG of the chl a decadal biomass figure into the illustrator workspace by opening the file from the computer or drag and drop it into a new workspace.
- Repeat steps 1.3 to 1.8 to vectorize each of the three chl a seasonal cycles.
- Go to View > Show Transparency Grid to reveal the checkerboard background that indicates transparency.
- Click on Window > Image Trace in the dropdown menu to open the Image Trace window.
- Click on the Selection tool (black arrow) in the toolbar and click on the image.
- Click on Object > Expand in the dropdown menu.
- Use the Direct Selection tool (white arrow) in the toolbar to click and select the background parts of the image to get rid of around the line indicating the seasonal cycle. Press delete. Repeat for each background region of the figure.
- Click on File > Export to save the file as .PNG file. Ensure the Background Transparent box is selected.
- Place .PNG figure with background removed into a new workspace of the specific illustrator used by opening the file from the computer or drag and dropping it into a new workspace.
- Click on Window > Image Trace in the dropdown menu to open the Image Trace window.
- Under Properties, select Expand to vectorize it.
- Select View > Show Transparency Grid.
- Click on the vector image then right click and select Ungroup.
- Choose the Direct Selection tool (white arrow) in the toolbar. Drag and draw a box around the whitespace only. Press delete to remove it.
- Repeat until all the whitespace is removed for each of the lines from the 1970s, 1990s, and 2010s.
- Click File > Save As and select .EPS to save each line as a separate vector graphic.
- Use the 'Artboard tool' (square) in the toolbar to click drag, and create a new Artboard in a new illustrator workspace.
- Make three identical Artboards of the same size. Adjust the size within Properties > Transform.
NOTE: For this project, dimensions were 1224 px by 3456 px. - Drag and drop one of the chl a .EPS files onto one of the three Artboards, respectively.
- Create a new Layer by clicking on the 'sticky note icon'.
- Create a rectangle within the new layer with the Rectangle tool from the toolbar.
- Fill the rectangle with a light blue gradient using the Gradient tool from the toolbar.
- Copy the vectorized trend line and add it to the layer with the rectangle in it.
- Use the 'Line Segment' tool from the toolbar to create a box attached to the trend line. Hold shift to make the lines straight and aligned.
- Press the control key and select all the components, including the lines, rectangle, and trend line within the layer.
- Select Object > Clipping Mask > Make. This will remove the top fill of the shape.
- Fill the shape with the phytoplankton pattern saved as a color swatch from 2.11.
- Repeat this process for each of the three decades.
- Repeat steps 3.9 & 3.10 to create a rectangle colored with a red to blue color gradient to represent warming water temperature across the three decadal panels.
- Right click on the object and move it back behind the phytoplankton patterns.
4. Adding detail to phytoplankton panels
- To add images of photographed phytoplankton onto the phytoplankton patterns, select Open and click on the image file to open it in the illustrator used here.
- Create a circle with the Ellipse tool from the toolbar and overlay it on top of the phytoplankton image.
- Hold the shift key to select both the shape and the image, then in the menu click on Object > Clipping Mask > Make to fill the shape with the image.
- Repeat for select phytoplankton images and distribute across the three decades to look like a magnifying glass zooming in on the illustrative phytoplankton (Figure 1D).
NOTE: Steps 1.3 to 1.8 can be repeated to add artistic elements of boats and birds to the panels to make the chl a seasonal cycles look like ocean waves. - Use the 'Rectangle tool' from the toolbar to create a textbox on each of the decade artboards.
- Use the 'Type tool' (T) to click and type informational text about each decade. Add text at the top of each decade with the name of the decade and add the names of the corresponding seasons at the bottom of each of the three panels.
- Save the workspace in the illustrator.
5. Mural production
- Import the saved illustrator file and select to only import the three completed decades. Select all and export as a .PDF file.
- Open the plankton pattern .PDF file with a Large-format Plotter to scale the three decadal artboards into 96 inch by 34 inch panels.
- Print panels on Heavyweight Matter Paper and install with hanging hardware.
Representative Results
Results document a decline in phytoplankton biomass from the 1970s to 1990s to 2010s (Figure 1). All decades exhibited a bimodal peak in chlorophyll a (chl a) concentration with the first peak occurring in winter and the second occurring in summer. The 1970s exhibited higher average chl a in winter than in summer. Conversely, the 1990s showed lower chl a in winter than in summer. The 2010s returned to a higher mean chl a concentration in winter than in summer. These results are reflected in the final product through different chl a peaks in the panels as well as with the text boxes added to emphasize different components of the chl a dataset (Figure 2).
Analysis of ecologically relevant phytoplankton taxa from Narragansett Bay revealed a wide range in abundance over time. This variation often masked any statistically significant differences in the taxa among the three decades although the HAB taxa, Dinophysis spp., and Tripos spp. (previously referred to as Ceratium) decreased (Table 1). In contrast, Thalassiosira nordenskioeldii and Skeletonema spp. increased (Table 1). Other taxa oscillated in abundance such as Eucampia zodiacus (Table 1). These results were illustrated in the final product by the increased presence of more E. zodiacus images in the 2010s compared to the 1970s and 1990s, as well as an overlayed microscopic image of E. zodiacus to bring the actual species to 'real-life' for the audience (Figure 2 & 3).
| Taxa Name | Type | 1970-79 Mean ± SD (Cells L-1) | 1990-99 Mean ± SD (Cells L-1) | 2010-19 Mean ± SD (Cells L-1) | p-value |
| Pseudo-nitzschia spp. | Diatom | 3701 ± 18235 | 5123 ± 24396 | 12919 ± 58632 | > 0.05 |
| Thalassionema nitzschioides | Diatom | 81797 ± 245710 | 22909 ± 59246 | 62656 ± 292940 | > 0.05 |
| Tripos spp. | Dinoflagellate | 1933 ± 703 | 500 ± 706 | 841 ± 353 | < 0.001 |
| Eucampia zodiacus | Diatom | 27266 ± 27675 | 7500 ± 2121 | 90764 ± 181415 | > 0.05 |
| Thalassiosira nordenskioeldii | Diatom | 76800 ± 150545 | 27000 ± 28284 | 362411 ± 376064 | 0.008 |
| Odontella aurita | Diatom | 5571 ± 8541 | 5000 ± 2645 | 17750 ± 23485 | > 0.05 |
| Chaetoceros diadema | Diatom | 103027 ± 239802 | 18000 ± 0 | 40402 ± 46128 | > 0.05 |
| Skeletonema spp. | Diatom | 2457847 ± 7814228 | 1884674 ± 4888589 | 1349184 ± 3732765 | 0.003 |
| Dinophysis spp. | Dinoflagellate | 5166 ± 8983 | 1978 ± 1840 | 2331 ± 2504 | < 0.001 |
Table 1: Phytoplankton counts. Mean (cells L-1) and standard deviation of phytoplankton concentrations for each taxa for each decade. Type designates whether the phytoplankton is classified as a diatom or dinoflagellate. ANOVA or t-test was performed to test for statistically significant differences in mean abundance among the three decades (ANOVA) or two (t-test) when low sample size was present in the 1990s (i.e., Tripos spp., Eucampia zodiacus, Thalassiosira nordenskioeldii, Odontella aurita, and Chaetoceros diadema). Significant p-values determined at α = 0.05 and indicated in bold.
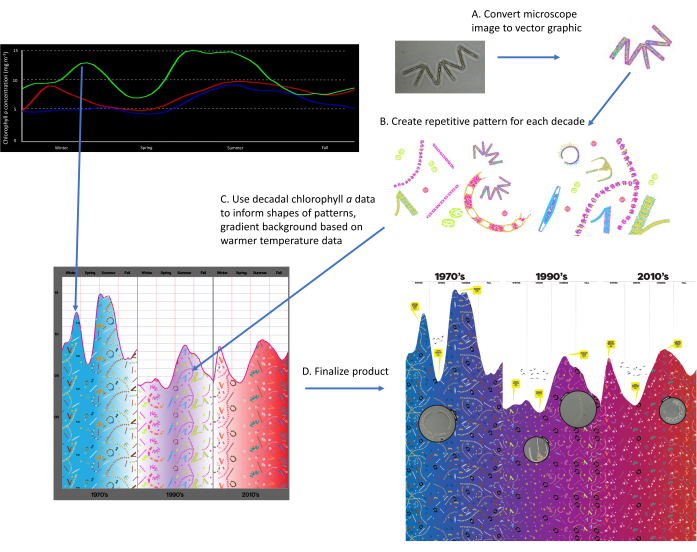
Figure 1: Schematic of methodology. A) Convert microscopic image into a vector illustrative graphic, B) Create repetitive pattern for each decade (1970s, 1990s, 2010s), C) Use decadal chlorophyll a data to inform shapes of patterns. Fill background with blue to red color scheme to represent increasing water temperature, and D) Finalize product by adding text to inform distinct features in patterns and microscopic images of phytoplankton used to create illustrative graphics. Please click here to view a larger version of this figure.
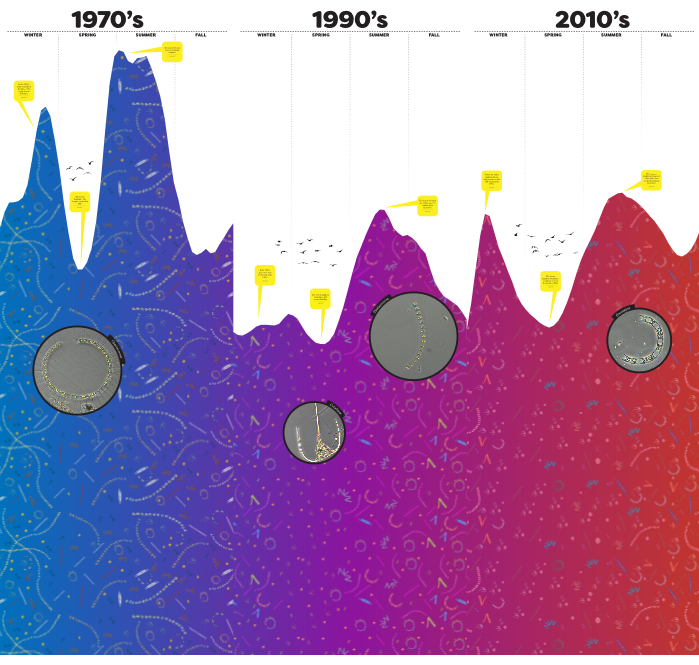
Figure 2: Completed visualization. Finalized phytoplankton visualization made in the illustrator. Taxa include Thalassiosira nordenskioeldii, Thalassionema nitzschioides, Tripos spp., Odontella aurita, Skeletonema species complex, Chaetoceros diadema, Eucampia zodiacus, Dinophysis spp., and Pseudo-nitzschia spp. Please click here to view a larger version of this figure.
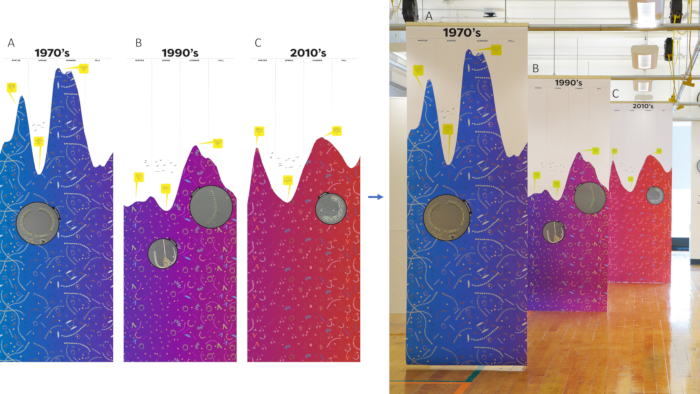
Figure 3: Completed art piece. Finalized phytoplankton visualization made in the illustrator alongside printed version for A) the 1970s, B) the 1990s, and C) the 2010s. Please click here to view a larger version of this figure.
Discussion
Critical steps of the protocol include obtaining microscopic images of phytoplankton and converting them into vector graphics. Making the images of phytoplankton, which are not noticeable to the naked eye, large enough to be seen without a magnifying glass on the mural, helps to bring them to life for the viewer. To accomplish this mural as not only a work of art but also a data visualization method, it is important to incorporate observed data into the project. In the case of the phytoplankton mural, the chlorophyll a (chl a) annual cycles that were averaged by decade represent the actual data and show how chl a declined by decade on the different panels. For phytoplankton abundance, the average concentrations of certain taxa varied among the decades, therefore, a higher abundance of a taxa observed in a particular decade would have more graphics of that taxa copied onto the decadal panel compared to another panel with lower mean abundance. Using observed data to inform the artistic elements, such as a gradient of color from blue to red to represent temperature increases, also aid in visualizing these scientific data.
Modifications of the method could include obtaining microscopic images of phytoplankton from open-access image repositories as well as utilizing other phytoplankton imaging systems for photographic images besides a microscope (e.g., Imaging Flow-Cytobot). Further, microscopic images and scientific data could include daily phytoplankton counts and images, rather than decades, for shorter time series datasets, as well as zooplankton images to reveal food web interactions. Finally, average temperatures recorded for each decade could be included on the panels to quantify changes in temperature, or a trend line drawn near the bottom of the panels, in addition to the illustrative changes shown through the gradient background. Limitations include troubleshooting these scientific data to scale within the confines of the physical art piece as well as obtaining instrumentation for printing on large panels. It is also important to ensure that the background color is transparent enough to reveal the changes in phytoplankton abundance distinctly over time, which can be difficult to distinguish until printed. Finally, Adobe Illustrator is a proprietary software, which may limit accessibility for certain users, but free software illustration programs are available (e.g., Inkscape, GIMP, Vectr, Vectornator). Adapting the protocol to produce the phytoplankton murals on these free programs represents useful future work to increase accessibility.
Given that phytoplankton represent the base of the food web in almost all marine systems, communicating their importance is critical; However, most studies in phytoplankton ecology only present results as data graphs reducing their accessibility to general audiences. The protocol presented here of developing the phytoplankton mural shows the impact of visualizing scientific data through an artistic lens17. Through analysis of this mural, the viewer can see that phytoplankton biomass has decreased in Narragansett Bay (NBay) since the 1970s. This decline occurs over a period when there have been long-term increases in seawater temperature in NBay13. Similar shifts in plankton communities (i.e., zooplankton) with warming seawater temperatures were also observed in San Francisco Bay Estuary, which, like NBay, supports a large human population18,19. This approach represents a visualization method that can be utilized for many other plankton time series, like San Francisco Bay Estuary, throughout the world.
At a far glance, the shape and color of the panels change over time. Viewing the panels more closely, the patterns of phytoplankton are reflective of shifts in the abundance and biomass of different taxa. This is where the worlds of art and science collide in that the scientific patterns are the literal patterns shown on the mural. It is evident that there is much more to NBay than what appears on the water's surface by visualizing phytoplankton data through art.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was supported by the National Science Foundation (OIA-1655221, OCE-1655686) and Rhode Island Sea Grant (NA22-OAR4170123, RISG22-R/2223-95-5-U). We thank the multiple captains for providing field assistance and the many students and researchers who collected data since 1970. We thank Stewart Copeland and Georgia Rhodes for developing the Vis-A-Thon project that produced the plankton mural as well as Rafael Attias from the Rhode Island School of Design for his artistic guidance during project development.
Materials
| Adobe Illustrator | Adobe | version 23.0.6 | Free alternatives include: Inkscape, GIMP, Vectr, Vectornator |
| Eclipse E800 | Nikon | ECLIPSE Ni/Ci Upright Microscope | Now succeeded by Eclipse Ni-U |
| Epson Large Format Printer | Epson | SCT5475SR | |
| Heavy Matte Paper | Epson | S041596 | |
| RStudio | Rstudio, PBC | version 2022.07.1 | Any statistical software tool will suffice |
References
- Cloern, J. E., Jassby, A. D. Complex seasonal patterns of primary producers at the land-sea interface. Ecology Letters. 11 (12), 1294-1303 (2008).
- Cloern, J. E., Jassby, A. D. Patterns and Scales of Phytoplankton Variability in Estuarine-Coastal Ecosystems. Estuaries and Coasts. 33 (2), 230-241 (2010).
- Hays, G. C., Richardson, A. J., Robinson, C. Climate change and marine plankton. Trends in Ecology & Evolution. 20 (6), 337-344 (2005).
- Harvey, C. J., et al. The importance of long-term ecological time series for integrated ecosystem assessment and ecosystem-based management. Progress in Oceanography. 188, 102418 (2020).
- Leeuwe, M. A., et al. Annual patterns in phytoplankton phenology in Antarctic coastal waters explained by environmental drivers. Limnology and Oceanography. 65 (7), 1651-1668 (2020).
- Hunter-Cevera, K. R., et al. Physiological and ecological drivers of early spring blooms of a coastal phytoplankter. Science. 354 (6310), 326-329 (2016).
- Church, M. J., Lomas, M. W., Muller-Karger, F. Sea change: Charting the course for biogeochemical ocean time-series research in a new millennium. Deep Sea Research Part II: Topical Studies in Oceanography. 93, 2-15 (2013).
- Bates, N. R., Johnson, R. J. Acceleration of ocean warming, salinification, deoxygenation and acidification in the surface subtropical North Atlantic Ocean. Communications Earth & Environment. 1 (1), 33 (2020).
- Wolanski, E., Spagnol, S., Gentien, P., Spaulding, M., Prandle, D. Visualization in Marine Science. Estuarine, Coastal and Shelf Science. 50 (1), 7-9 (2000).
- United Nations. Factsheet: People and Oceans (2017). , (2017).
- Oviatt, C. A. The changing ecology of temperate coastal waters during a warming trend. Estuaries. 27 (6), 895-904 (2004).
- Oviatt, C., et al. Managed nutrient reduction impacts on nutrient concentrations, water clarity, primary production, and hypoxia in a north temperate estuary. Estuarine, Coastal and Shelf Science. 199, 25-34 (2017).
- Fulweiler, R. W., Oczkowski, A. J., Miller, K. M., Oviatt, C. A., Pilson, M. E. Q. Whole truths vs. half truths – And a search for clarity in long-term water temperature records. Estuarine, Coastal and Shelf Science. 157, A1-A6 (2015).
- Trainer, V. L., et al. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae. 14, 271-300 (2012).
- Sterling, A. R., et al. Emerging harmful algal blooms caused by distinct seasonal assemblages of a toxic diatom. Limnology and Oceanography. 67 (11), 2341-2359 (2022).
- Roche, K. M., Sterling, A. R., Rynearson, T. A., Bertin, M. J., Jenkins, B. D. A Decade of Time Series Sampling Reveals Thermal Variation and Shifts in Pseudo-nitzschia Species Composition That Contribute to Harmful Algal Blooms in an Eastern US Estuary. Frontiers in Marine Science. 9, 889840 (2022).
- Li, . Qi Data visualization as creative art practice. Visual Communication. 17 (3), 299-2222312 (2018).
- Cloern, J. E., et al. Projected Evolution of California’s San Francisco Bay-Delta-River System in a Century of Climate Change. PLoS ONE. 6 (9), e24465 (2011).
- Bashevkin, S. M., et al. Five decades (1972-2020) of zooplankton monitoring in the upper San Francisco Estuary. PLOS ONE. 17 (3), e0265402 (2022).

