Automated Impactor for Contusive Spinal Cord Injury Model in Mice
Summary
Presented here is a novel automated spinal cord injury contusion device for mice, which can accurately produce spinal cord injury contusion models with varying degrees.
Abstract
Spinal cord injury (SCI) due to traumatic injuries such as car accidents and falls is associated with permanent spinal cord dysfunction. Creation of contusion models of spinal cord injury by impacting the spinal cord results in similar pathologies to most spinal cord injuries in clinical practice. Accurate, reproducible, and convenient animal models of spinal cord injury are essential for studying spinal cord injury. We present a novel automated spinal cord injury contusion device for mice, the Guangzhou Jinan University smart spinal cord injury system, that can produce spinal cord injury contusion models with accuracy, reproducibility, and convenience. The system accurately produces models of varying degrees of spinal cord injury via laser distance sensors combined with an automated mobile platform and advanced software. We used this system to create three levels of spinal cord injury mice models, determined their Basso mouse scale (BMS) scores, and performed behavioral as well as staining assays to demonstrate its accuracy and reproducibility. We show each step of the development of the injury models using this device, forming a standardized procedure. This method produces reproducible spinal cord injury contusion mice models and reduces human manipulation factors via convenient handling procedures. The developed animal model is reliable for studying spinal cord injury mechanisms and associated treatment approaches.
Introduction
Spinal cord injury usually results in permanent spinal cord dysfunction below the injured segment. It is mostly caused by objects striking the spine and hyperextension of the spine, such as traffic accidents and falls1. Due to the limited availability of effective treatment options for spinal cord injury, elucidation of the pathogenesis of spinal cord injuries using animal models will be informative for the development of appropriate treatment approaches. The contusion model of spinal cord injury caused by impact on the spinal cord results in the development of animal models with similar pathologies to most clinical spinal cord injury cases2,3. Therefore, it is important to produce accurate, reproducible, and convenient animal models for spinal cord injury contusion.
Since Allen's invention of the first animal model of spinal cord injury in 1911, there have been major advances in the development of instruments for establishing spinal cord injury animal models4,5. Based on injury mechanisms, spinal cord injury models are classified as contusion, compression, distraction, dislocation, transection, or chemical6. Among them, the contusion models, which use external forces to displace and injure the spinal cord, are closest to the clinical etiology of most spinal cord injury patients. Therefore, the contusion model has been used by many investigators in spinal cord injury studies3,7. Various instruments are used to develop spinal cord injury contusion models. The New York University (NYU)-multicenter animal spinal cord injury studies (MASCIS) impactor produces spinal cord injury contusions by weight-drop device8. After several updated versions, the MASCIS impactor is widely used to develop spinal cord injury contusion animal models9. However, when the impact rod of MASCIS falls and hits the spinal cord, multiple injuries may occur, which affects the degree of injury in spinal cord injury models. Moreover, achieving mechanical precision to ensure the accuracy of the instrument and the repeatability of the manufacturing model is also challenging. The infinite horizon impactors cause contusions by controlling the force applied to the spinal cord rather than heavy drops10. It uses a computer connected to a sensor to directly measure the impact force between the impactor and the spinal cord. When the threshold is reached, the impactor is immediately retracted, thereby avoiding weight rebound and improving accuracy10,11. However, the use of this fine motor modality to inflict damage can result in inconsistent damage and functional deficits6. The Ohio State University (OSU) device compresses the dorsal surface of the spinal cord at a transient rate by an electromagnetic driver12,13. This device is similar to the infinite horizon impactors, as it uses short-distance compressions to cause spinal cord injuries. However, it has various limitations in that initial determination of the zero point will cause errors due to the presence of the cerebrospinal fluid6,14. In summary, there are many instruments that can be used to develop spinal cord injury contusion animal models, but they all have some limitations that lead to insufficient accuracy and reproducibility of animal models. Therefore, in order to more accurately, conveniently and reproducibly create mouse contusion models of spinal cord injury, an automated and intelligent spinal cord injury impactor is needed.
We present a novel spinal cord injury impactor, Guangzhou Jinan University smart spinal cord injury system (G smart SCI system; Figure 1), for producing spinal cord injury contusion models. The device uses a laser rangefinder as a positioning device, combined with an automated mobile platform to automate strikes according to set strike parameters, including strike speed, strike depth and dwell time. Automated operation reduces human factors and improves the accuracy as well as reproducibility of animal models.
Protocol
The studies involving animals were reviewed and approved by the Ethics Committee of Jinan University.
1. Anesthetization of animals and T10 spinal laminectomy
- Use 8 weeks-old female young adult C57/6J mice for this study. Anesthetize the mice by intraperitoneal injection of ketamine (100 mg/kg) and diazepam (5 mg/kg). Check for successful anesthetization indicated by loss of pain reflex. Apply vet ointment on eyes to prevent dryness under anesthesia.
- Shave the hair on the back of the mice using a shaver to reveal the skin. Disinfect the skin with three alternating rounds of iodophor and alcohol.
- Make 2.5 cm medial longitudinal incision in the dorsal skin using a scalpel and expose the spine at the T9-T11 level using tweezers.
- Bilaterally fix T10 facets using a spinal fixator. Ensure the spine is stably fixed. Ensure that the paravertebral muscles are stripped and remove the spinous process as well as laminae using micro-grinding drill to expose the spinal cord of the T10 segment.
2. Contusion of the T10 spinal cord using the G smart SCI system
- Turn on the switch and wait for the device to automatically return to its original state. Place the spinal fixator into the G smart SCI system and secure it using screws.
- Using the operation touch screen (Figure 2A), set damage parameters, including impact speed (1 m/s), impact depth (0.5 mm, 0.8 mm, and 1.1 mm for three different sets of mice) and dwell time (500 ms)15.
- Align the laser rangefinder at the center of the exposed spinal cord by moving the platform. (Figure 2B)
- Click the Ready button on the touch screen (Figure 2C). The impact head will automatically adjust to a specific height based on setting parameters. The carrier table automatically moves the spinal cord impact site below the impact head.
- Manually press the impact head to further determine the impact site. Click the Start button, the impact head will hit the spinal cord based on set parameters.
- Remove the mice from the device and observe under a Stereomicroscope (20x) to determine spinal cord injury (Figure 3). To determine the success of model development, observe local congestion, collapse, and spinal membrane rupture.
- Suture the muscle, fascia and skin layer by layer using 3-0 sutures. Place the mice in a warm box and wait for their recovery.
3. Post-operative care
- Inject meloxicam (5 mg/kg) subcutaneously daily for 7 days after surgery. Manually empty the bladder every 8 h until bladder functions are restored.
- At 14 days after operation, remove the suture threads.
4. Testing effects of spinal injury
- Calculate the BMS scores for mice from the first postoperative day16,17.
- On the 30th postoperative day, perform animal behavioral experiments, including catwalk, foot fault and rotarod16,17. Catwalk: Record distance of 45 cm; Maximum run duration 8 s; Camera gain 28.02; Intensity threshold 0.01. Foot fault: Record 60 steps for each mouse. Rotarod: Speed 20 rpm. Record the time for the mouse to fall and record it as 120 s for more than 120 s.
- On the 31st postoperative day, anesthetize the mice by intraperitoneal injection of ketamine (100 mg/kg) and diazepam (5 mg/kg) and then euthanize the mice by perfusion using 4% PFA. Remove the spinal cord carefully, and intercept 5 mm above and below the injury site for paraffin embedding. Make a 5 µm section of the center of the mouse spinal cord injury and perform Hematoxylin and eosin staining17.
- For statistical analysis use commercial software. Express data as mean ± standard error of the mean (SEM) and compare using one-way ANOVA; p < 0.05 was considered significant.
Representative Results
Laminectomy was performed on 24 female mice (8 weeks old) as described above. Mice in the sham group (n=6) were not subjected to spinal cord injury, while the rest of the mice, including 0.5 mm group (n=6), 0.8 mm group (n=6), and 1.1 mm group (n=6) were subjected to different depths of spinal cord impingement. The BMS scores were regularly recorded until 1 month postoperatively (Figure 4). There were significant differences in postoperative BMS scores of mice in different groups. After 1 month, mice in the 0.5 mm group had 4 to 6 postoperative scores and recovered to a similar level as the sham group. Mice in 0.8 mm and 1.1 mm groups had 1 to 2 postoperative scores. After 1 month, the 0.8 mm group recovered to 4 to 6 scores, while mice in the 1.1 mm group barely recovered.
After 1 month, animal behavioral assays, including foot fault, rotarod and catwalk were performed. In the foot fault assay (Figure 5A), there were no significant differences in hindlimb foot fault between the 0.5 mm and sham groups. However, hindlimb foot fault for the 0.8 mm group was significantly different from all other groups. The foot fault rate of mice in the 1.1 mm group was 100% because the hind limbs could not support the animal on the ground and was significantly different from the other groups. In the rotarod test (Figure 5B), we recorded the time of latency to fall in different groups of mice. Both 0.8 mm and 1.1 mm groups were significantly different from the other groups, but the sham group had similar results to 0.5 mm group. In the catwalk test (Figure 6), we recorded and analyzed the regularity index and hind max contact area in different groups of mice. There were significant differences in regularity index and hind max contact areas between mice in different groups, implying significant differences in walking functions between mice with different depths of spinal cord injury. Therefore, mice models with different depths of spinal cord injury and with significant differences in their hindlimb functions can be created using the developed device.
Finally, we resected the spinal cords of mice (Figure 7A) and made sections for hematoxylin and eosin (HE) staining (Figure 7C). There were various degrees of damage in both spinal cord images and HE-stained sections. In summary, we accurately developed different grades of spinal cord injury mice models using the proposed instrument.
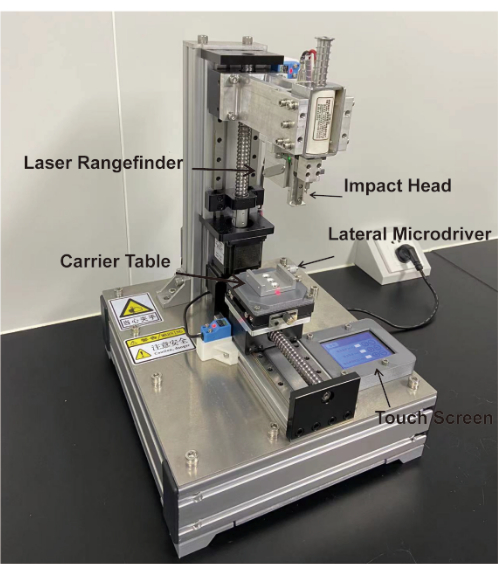
Figure 1: Guangzhou Jinan university Smart spinal cord injury system (G smart SCI system). Fix the spinal immobilizer for immobilizing the mouse on the carrier table. Set impact parameters via touch screen. Use lateral microdriver to adjust the lateral positions of the carrier and use the touch screen to adjust the frontal positions. The laser rangefinder confirms the impact position and measures the impact height so that the impact head can accurately strike according to the set strike position and depth. Please click here to view a larger version of this figure.
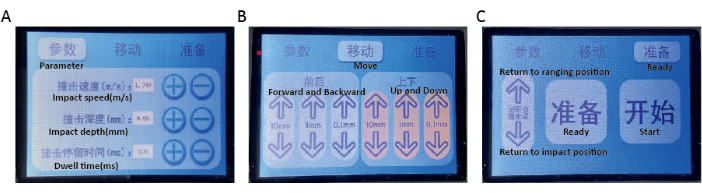
Figure 2: The operation touch screen. (A) In the parameters page, the damage parameters, including impact speed (0.5 -2.0 m/s), impact depth (0 -3 mm) and dwell time (500 -2 ms) can be set. (B) In the moving page, the height of the impact head and spinal fixator position can be set. (C) In the preparation page, clicking the Ready button will result in automatic adjustment of the impact head and carrier table to a specific position based on the set parameters. By clicking the Start button, the impact head will hit the spinal cord based on the set parameters. Please click here to view a larger version of this figure.
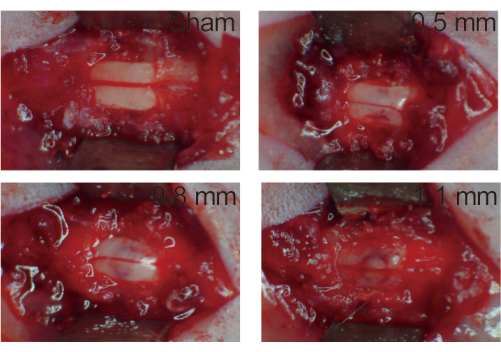
Figure 3: Spinal cord after injury. (A) Sham group. The spinal immobilizer immobilized the T10 vertebrae, and the spinal cord is exposed after laminectomy without damage. (B) 0.5 mm group. The spinal cord was slightly injured after being hit at a depth of 0.5 mm and there was a small amount of congestion. (C) 0.8 mm group. The spinal cord was moderately injured and had obvious congestion after being hit at a depth of 0.8 mm. (D) 1.1 mm group. The spinal cord was severely injured after being hit at a depth of 1.1 mm and there was a lot of congestion. Please click here to view a larger version of this figure.
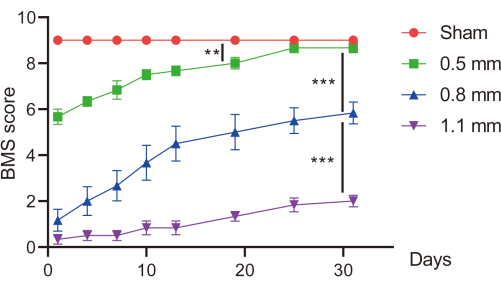
Figure 4: The BMS scores. The BMS scores of mice were recorded from the first postoperative day to one month postoperatively (n =6 /group). *p< 0.05, ** p < 0.01, *** p < 0.001 compared using one-way ANOVA. Date is expressed as mean ± standard error of the mean (SEM). Please click here to view a larger version of this figure.
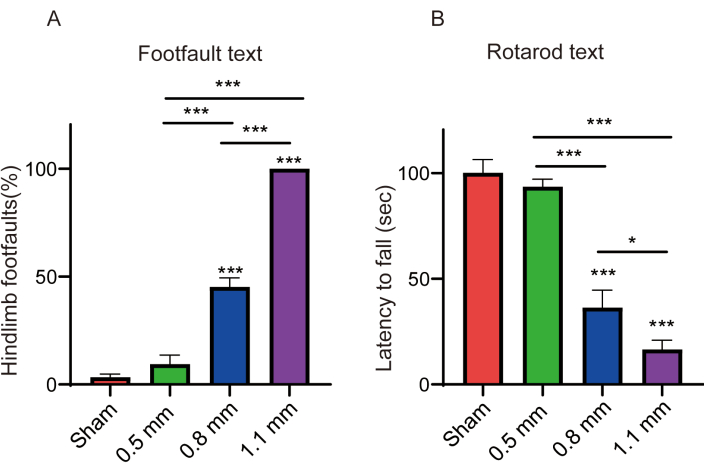
Figure 5: Footfall test and rotarod test. (A) Hindlimb foot faults in mice with different degrees of spinal cord injuries (n =6 /group). *** p <0.001 compared using one-way ANOVA. (B) The latency to falling during the accelerating rotarod was compared among mice with different degrees of spinal cord injuries (n =6 /group). * p < 0.05, *** p < 0.001 compared using one-way ANOVA. Date is expressed as mean ± standard error of the mean (SEM). Please click here to view a larger version of this figure.
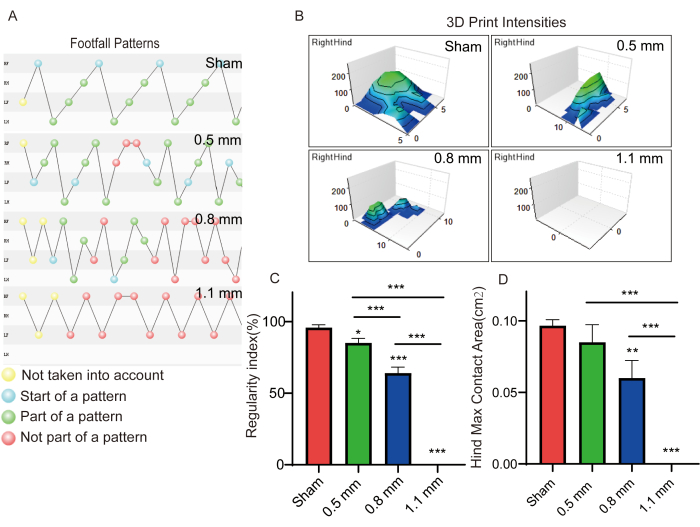
Figure 6: Catwalk test. (A, C) Regularity indices in mice with different degrees of spinal cord injuries (n =6 /group). * p < 0.05, *** p < 0.001 compared using one-way ANOVA. (B, D)Hind max contact areas were automatically analyzed using the software (n =6 /group). ** p < 0.01, *** p < 0.001 compared using one-way ANOVA. Date is expressed as mean ± standard error of the mean (SEM). Please click here to view a larger version of this figure.
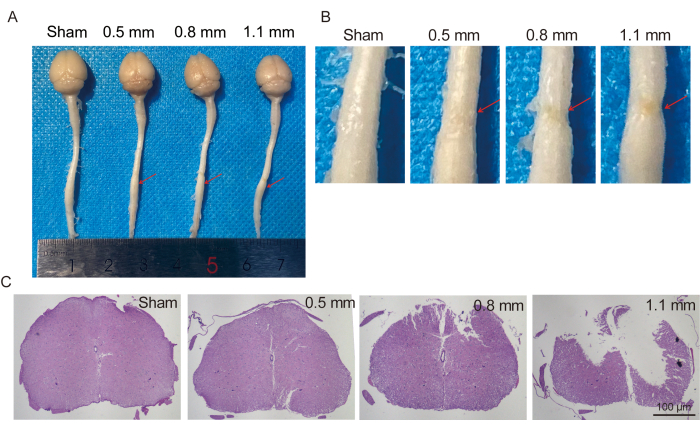
Figure 7: Spinal cord and H&E staining. (A) Spinal cord of mice with different degrees of injury 1 month after surgery. (B) Enlarged figures. (C) H&E staining of spinal cord injury sites in mice with different degrees of injury. Please click here to view a larger version of this figure.
Discussion
Spinal cord injury can lead to sensory and motor deficits, which can result in severe physical and mental impairments. In China, incidences of spinal cord injuries in different provinces vary from 14.6 to 60.6 per million18. The increase in the prevalence of SCI will put more pressure on the healthcare system. Currently, there are limited effective treatment option for spinal cord injury, injuries because its pathomechanisms and repair processes are yet to be fully understood19. There is a need to create accurate and reproducible spinal cord injury animal models to investigate the pathomechanisms and repair processes of spinal cord injuries. To this end, we developed an accurate, repeatable, and simple spinal cord injury instrument.
Many devices, including the NYU-MASCIS impactor, infinite horizon impactor and OSU impactor are used to create contusion models of spinal cord injuries8,9,12,13,20. These devices are complex to operate and may produce large errors due to different proficiencies of personnel. Moreover, there are various flaws in the designs of these devices that make them less accurate and reproducible6. The specific location of the spinal cord injury determines its severity, so during the establishment of spinal cord injury models, the positioning method determines the accuracy of modeling21. The G smart SCI system uses a laser rangefinder to locate the spinal cord injury site and adjusts the height of the impact head based on the rangefinder data to set the strike depth. Another percussion device that uses laser positioning is the Louisville injury system apparatus (LISA) impactor from Indiana University School of Medicine15. Although LISA uses laser positioning, it requires manual determination and adjustment of the height of the impact head to achieve controlled strike depth, which increases human intervention. Meanwhile, LISA uses pneumatic strikes and laptop control, which require more experimental space and increases the operating costs10. G smart SCI system reduces human errors by semi-automating the operation process and is easy to move because of its light design.
Based on set parameters, the device can accurately impact the spinal cords of mice, thereby creating models with different degrees of spinal cord injuries. In this study, mice with different degrees of spinal cord injuries exhibited significant differences in hindlimb functions. Importantly, the assays are reproducible and can consistently generate SCI models.
In the protocol, the most critical steps include accurately performing laminectomy, stabilizing the spines of mice, and using the laser for accurate positioning to ensure the accuracy and repeatability of the experiment. During the design process, we made some improvements to the device. We found that after the strike, the stage could not easily return to the ranging point, therefore, we added a button to return to the ranging point. Moreover, the desired parameters could not be quickly adjusted, thus, we added a numeric keyboard for input. The slow charging electromagnetic drive in the first version was also improved. This device is currently limited to the establishment of thoracic spinal cord injury contusion mice models. Studies should be performed to support the use of this instrument in the establishment of rat spinal cord injury models or cervical spinal cord injury models.
Furthermore, Dr Bilgen from University of Kansas Medical School described a computer-controlled impactor device, which can induce central nervous system (CNS) injuries, including traumatic brain injury (TBI) and spinal cord injury (SCI)22. Similar to our device, this device also uses a variety of commercialized equipment and systems, so it was successfully commercialized and used23. The equipment we describe has the characteristics of automation, accuracy and convenience, and is expected to be commercialized in the future and benefit more spinal cord injury researchers.
In summary, we designed an automated mouse spinal cord impactor to create SCI contusion models. The device improves accuracy with a laser rangefinder and reduces human errors via an automated operating process. Moreover, the G smart SCI system is easier to operate and carry than other devices, bringing convenience to spinal cord injury research. Importantly, the device can accurately and reproducibly create different classes of SCI mice models depending on the needs of the experiment.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China, Nos. 82102314 (to ZSJ), and 32170977 (to HSL) and Natural Science Foundation of Guangdong Province, Nos. 2022A1515010438 (to ZSJ) and 2022A1515012306 (to HSL). This study was supported by the Clinical Frontier Technology Program of the First Affiliated Hospital of Jinan University, China, Nos. JNU1AF- CFTP- 2022- a01206 (to HSL). This study was supported by Guangzhou Science and Technology Plan Project, Nos. 202201020018 (to HSL), 2023A04J1284 (to ZSJ) and 2023A03J1024 (to HSL).
Materials
| 0.01M PBS (powder, pH7.2-7.4) | Solarbio Life Sciences | P1010 | |
| 2,2,2-Tribromoethanol | Macklin | 75-80-9 | |
| 4% paraformaldehyde tissue fixative | Biosharp life science | BL539A | |
| Biomicroscope | Leica | LCC50 HD | |
| CatWalk | Noldus Information Technology | CatWalk XT 9.1 | |
| Cover glass | CITOTEST Scientific | 10212432C | |
| Embedding machine | Changzhou Zhongwei Electronic Instrument | BMJ-A | |
| Ethanol absolute | DAMAO | 64-17-5 | |
| FootFaultScan | Clever Sys Inc. | – | |
| Glass slide | CITOTEST Scientific | 80302-2104 | |
| Hematoxylin and Eosin Staining Kit | Beyotime Biotechnology | C0105S | |
| micro-grinding drill | FEIYUBIO | 19-7010 | |
| Mouse spinal fixator | RWD Life Science | 68094 | |
| Paraffin microtome | Thermo | shandon finesse 325 | |
| RotaRod for Mice | Ugo Basile | 47600 | |
| Stereomicroscope | KUY NICE | SZM-7045 | |
| Tert-Amyl alcohol | Macklin | 75-85-4 | |
| Xylene | China National Pharmaceutical | #10023418 |
References
- Venkatesh, K., Ghosh, S. K., Mullick, M., Manivasagam, G., Sen, D. Spinal cord injury: pathophysiology, treatment strategies, associated challenges, and future implications. Cell and Tissue Research. 377 (2), 125-151 (2019).
- Chiu, C. W., Cheng, H., Hsieh, S. L. Contusion Spinal Cord Injury Rat Model. Bio Protocol. 7 (12), e2337 (2017).
- Thygesen, M. M., Guldbæk-Svensson, F., Rasmussen, M. M., Lauridsen, H. Contusion Spinal Cord Injury via a Microsurgical Laminectomy in the Regenerative Axolotl. Journal of Visualized Experiments. (152), 60337 (2019).
- Anderson, T. E. A controlled pneumatic technique for experimental spinal cord contusion. Journal of Neuroscience Methods. 6 (4), 327-333 (1982).
- Allen, A. R. SURGERY OF EXPERIMENTAL LESION OF SPINAL CORD EQUIVALENT TO CRUSH INJURY OF FRACTURE DISLOCATION OF SPINAL COLUMN: A PRELIMINARY REPORT. Journal of the American Medical Association. LVII (11), 878-880 (1911).
- Cheriyan, T., et al. Spinal cord injury models: a review. Spinal Cord. 52 (8), 588-595 (2014).
- Yan, R., et al. A modified impactor for establishing a graded contusion spinal cord injury model in rats. Annals of Translational Medicine. 10 (8), 436 (2022).
- Gruner, J. A. A monitored contusion model of spinal cord injury in the rat. Journal of Neurotrauma. 9 (2), 123-126 (1992).
- Ghnenis, A. B., et al. Evaluation of the Cardiometabolic Disorders after Spinal Cord Injury in Mice. Biology (Basel). 11 (4), 495 (2022).
- Scheff, S. W., Rabchevsky, A. G., Fugaccia, I., Main, J. A., Lumpp, J. E. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. Journal of Neurotrauma. 20 (2), 179-193 (2003).
- Hong, Y. R., et al. Ultrasound stimulation improves inflammatory resolution, neuroprotection, and functional recovery after spinal cord injury. Scientific Reports. 12 (1), 3636 (2022).
- Noyes, D. H. Electromechanical impactor for producing experimental spinal cord injury in animals. Medical & Biological Engineering & Computing. 25 (3), 335-340 (1987).
- Stokes, B. T., Noyes, D. H., Behrmann, D. L. An electromechanical spinal injury technique with dynamic sensitivity. Journal of Neurotrauma. 9 (3), 187-195 (1992).
- Pearse, D. D., et al. Histopathological and behavioral characterization of a novel cervical spinal cord displacement contusion injury in the rat. Journal of Neurotrauma. 22 (6), 680-702 (2005).
- Wu, X., et al. A Tissue Displacement-based Contusive Spinal Cord Injury Model in Mice. Journal of Visualized Experiments. (124), 54988 (2017).
- Forgione, N., Chamankhah, M., Fehlings, M. G. A Mouse Model of Bilateral Cervical Contusion-Compression Spinal Cord Injury. Journal of Neurotrauma. 34 (6), 1227-1239 (2017).
- Ji, Z. S., et al. Highly bioactive iridium metal-complex alleviates spinal cord injury via ROS scavenging and inflammation reduction. Biomaterials. 284, 121481 (2022).
- Chen, C., Qiao, X., Liu, W., Fekete, C., Reinhardt, J. D. Epidemiology of spinal cord injury in China: A systematic review of the chinese and english literature. Spinal Cord. 60 (12), 1050-1061 (2022).
- Flack, J. A., Sharma, K. D., Xie, J. Y. Delving into the recent advancements of spinal cord injury treatment: a review of recent progress. Neural Regeneration Research. 17 (2), 283-291 (2022).
- Khuyagbaatar, B., Kim, K., Kim, Y. H. Conversion Equation between the Drop Height in the New York University Impactor and the Impact Force in the Infinite Horizon Impactor in the Contusion Spinal Cord Injury Model. Journal of Neurotrauma. 32 (24), 1987-1993 (2015).
- Alizadeh, A., Dyck, S. M., Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Frontiers in Neurology. 10, 282 (2019).
- Bilgen, M. A new device for experimental modeling of central nervous system injuries. Neurorehabilitation and Neural Repair. 19 (3), 219-226 (2005).
- Khan, M., et al. GSNOR and ALDH2 alleviate traumatic spinal cord injury. Brain Research. 1758, 147335 (2021).

