Characterization of Vascular Morphology of Neovascular Age-Related Macular Degeneration by Indocyanine Green Angiography
Summary
Currently, fluorescein angiography (FA) is the preferred method for identifying leakage patterns in animal models of choroidal neovascularization (CNV). However, FA does not provide information about vascular morphology. This protocol outlines the use of indocyanine green angiography (ICGA) to characterize different lesion types of laser-induced CNV in mouse models.
Abstract
Age-related macular degeneration (AMD) is a leading cause of blindness among older individuals, and its prevalence is rapidly increasing due to the aging population. Choroidal neovascularization (CNV) or wet AMD, which accounts for 10%-20% of all AMD cases, is responsible for an alarming 80%-90% of AMD-related blindness. Current anti-VEGF therapies show suboptimal responses in approximately 50% of patients. Resistance to anti-VEGF treatment in CNV patients is often associated with arteriolar CNV, while responders tend to have capillary CNV. While fluorescein angiography (FA) is commonly used to assess leakage patterns in wet AMD patients and animal models, it does not provide information about CNV vascular morphology (arteriolar CNV vs. capillary CNV). This protocol introduces the use of indocyanine green angiography (ICGA) to characterize lesion types in laser-induced CNV mouse models. This method is crucial for investigating the mechanisms and treatment strategies for anti-VEGF resistance in wet AMD. It is recommended to incorporate ICGA alongside FA for comprehensive assessment of both leakage and vascular features of CNV in mechanistic and therapeutic studies.
Introduction
Age-related macular degeneration (AMD) is a prevalent condition that leads to severe vision loss in older individuals1. In the United States alone, the number of AMD patients is projected to double, reaching nearly 22 million by 2050, compared to the current 11 million. Globally, the estimated number of AMD cases is expected to reach a staggering 288 million by 20402.
Choroidal neovascularization (CNV), also known as "wet" or neovascular AMD, can have devastating effects on vision due to the formation of abnormal blood vessels beneath the central retina. This leads to hemorrhaging, retinal exudation, and significant vision loss. The introduction of anti-vascular endothelial growth factor (VEGF) therapies, which target extracellular VEGF, has revolutionized CNV treatment. However, despite these advancements, up to 50% of patients exhibit suboptimal responses to these therapies, with ongoing disease activity such as fluid accumulation and unresolved or new hemorrhages3,4,5,6,7,8,9,10,11,12,13,14.
Clinical studies have indicated that anti-VEGF resistance in CNV patients often corresponds to the presence of arteriolar CNV, characterized by large-caliber branching arterioles, vascular loops, and anastomotic connections9. Repeated anti-VEGF treatment can contribute to vessel abnormalization, the development of arteriolar CNV, and ultimately, resistance to anti-VEGF therapies14,15. In cases of arteriolar CNV, persistent fluid leakage is likely due to heightened exudation caused by inadequately formed tight junctions at arteriovenous anastomotic loops, particularly under conditions of high blood flow9. Conversely, individuals who respond well to anti-VEGF treatment tend to exhibit capillary CNV.
In our studies using animal models, we have demonstrated that laser-induced CNV in older mice develops arteriolar CNV and shows resistance to anti-VEGF treatment16,17. Conversely, laser-induced CNV in younger mice leads to the development of capillary CNV and high responsiveness to anti-VEGF treatment. Thus, it is crucial to differentiate between CNV vascular types for both mechanistic and therapeutic investigations.
In clinical settings, CNV is commonly classified based on fluorescein angiography (FA) leakage patterns (e.g., Type 1, Type 2), which use fluorescein dye to track exudation and identify areas of pathological leakage. In AMD research, CNV is predominantly studied using FA in animal models. However, FA fails to reveal the vascular morphology of CNV. Moreover, FA only captures images in the visible light spectrum and cannot visualize the choroidal vasculature beneath the retinal pigment epithelium (RPE). In contrast, indocyanine green (ICG), which exhibits strong affinity for plasma proteins, facilitates predominant intravascular retention and enables visualization of vascular structure and blood flow9. By utilizing the near-infrared fluorescence property of ICG, it becomes feasible to image the retinal and choroidal pigment using ICG angiography (ICGA). In this context, a protocol is presented that combines FA and ICGA to investigate the leakage and vascular morphology of laser-induced choroidal neovascularization (CNV) in young and old mice, where capillary and arteriolar CNV are observed.
Protocol
The animal experiments conducted in this study received approval from the Institutional Animal Care and Use Committees (IACUC) at Baylor College of Medicine. All procedures were carried out in compliance with the guidelines outlined in the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Young (7-9 weeks) and old (12-16 months) C57BL/6J male and female mice were used for the present study. The animals were obtained from a commercial source (see Table of Materials).
1. Preparation of the imaging system
- Position a heating pad on the imaging platform (see Table of Materials) to ensure the mouse's body temperature is maintained during imaging. Activate the heating pad and adjust the temperature to 35 °C.
- Remove the dust cover and turn on the laser scanning ophthalmoscope. Place a 55° lens on the machine.
- Set up the imaging software (see Table of Materials) and input essential information, including genotype, gender, age, etc. When the imaging session commences, select the IR (Infrared channel) for capturing ICGA images.
2. Animal preparation prior to ICGA and FA
- Weigh the mouse to determine the amount of anesthesia (Ketamine/Xylazine 70-100/2.5-10 mg/kg, see Table of Materials) needed.
NOTE: Optimizing the dosage is crucial as the metabolic profile varies among different mouse strains. It is essential to determine the appropriate dosage to avoid the mouse waking up before completing the imaging or the risk of an overdose and potential animal mortality. - Use a 1 mL sterile syringe with a 30-32 G needle to deliver anesthesia by intraperitoneal injection. Gently pinch one of the mouse's paws to check whether the mouse is adequately anesthetized. If the animal exhibits any response or movement, it is advisable to wait for additional time before proceeding to the next step. This allows the animal to settle and ensures optimal conditions for the subsequent procedures.
- Administer 1% tropicamide ophthalmic solution drops to dilate both eyes of the mouse. Wait at least 30 s before using 0.5% proparacaine hydrochloride drops (see Table of Materials) on both eyes to reduce eye movement and blinking. Follow this up with lubricant eye gel drops.
NOTE: Throughout the entire duration of anesthesia, it is important to address the issue of extreme dryness in the mouse's eyes, which can lead to corneal opacity. This opacity makes subsequent imaging challenging due to hindered visibility. To prevent this, it is crucial to apply lubricant eye gel drops continuously to keep the mouse's eyes moisturized. - Place the mouse on a heating water pad.
NOTE: Mice possess a high surface area to volume ratio, resulting in increased heat loss to the environment. When combined with the temperature drop induced by anesthesia, this can pose a significant risk to the mouse, potentially leading to death due to hypothermia. It is crucial to take necessary precautions to prevent hypothermia and ensure the mouse's well-being during the procedure. - Prepare a 1:1 volume mixture of 2 mg/mL ICG and 20 mg/mL Fluorescein dye (see Table of Materials). Administer 250 µL of the mixture through intraperitoneal injection using a 1 mL syringe and a 32 G needle.
- Insert the needle in the bottom left quadrant of the mouse abdomen near the hind legs at an angle parallel to the mouse's skin to avoid perforating any organs.
- Carefully withdraw the plunger and verify that no blood has entered the syringe cap. Proceed to inject the dye slowly and steadily, maintaining a consistent pace.
NOTE: The ICG dye must be filtered with a 0.22 µm syringe filter before use.
3. ICGA and FA
- Place the mouse on the heating pad of the imaging platform to start imaging.
- Position the mouse's body at a 45-degree angle to the camera and rotate its head slightly downwards. This allows the optic nerve to be in the center of the camera's focus.
- Using a cotton swab, delicately wipe the eye to remove the layer of lubricant eye drops or gels on the eye being imaged first. Ensure to apply the lubricant gel drops immediately after completing the imaging procedure.
NOTE: Leaving the eye without lubrication for more than 1 min while under anesthesia is not recommended. - Move the camera toward the mouse's eye. Select the FA channel from the acquisition module. The luminescence emitted from the FA channel can be used to position in the center of the mouse cornea for faster placement.
- Position the mouse's head in a way that the optic nerve is centered on the screen, avoiding the need to tilt the laser scanning ophthalmoscope at an angle. Make minor adjustments to the mouse's head position to achieve the desired alignment.
- On the acquisition module, select the ICGA channel. Ensure that the laser intensity is set to 100% and select the 55° option to match the appropriate lens. This ensures optimal settings for the laser scanning ophthalmoscope.
NOTE: To prevent oversaturation, it may be necessary to use a lower laser intensity, typically around 25%-50%, when imaging the early stage. Adjusting the laser intensity in this range can help capture clear and accurate images without causing oversaturation. - When the eye occupies the entire screen on the imaging software, make adjustments to the sensitivity and focus settings to obtain the clearest image of the CNV membrane.
- Rotate the round black button on the acquisition module to adjust the sensitivity of the image.
- Rotate the knob on the ophthalmoscope to adjust the focus. The optimal focus for visualizing the retinal vasculature using FA is typically within the range of 35-45 D (diopters). On the other hand, the ideal focus for visualizing the choroid using ICGA is generally between 10-15 D.
NOTE: Due to the different sizes of CNV membrane, it may be necessary to adjust the focus in 10-30 D to obtain optimal imaging of the vascular morphology.
- Once the focus and the sensitivity have been adjusted to achieve the best possible image, press the round black button on the acquisition module to normalize the image. Once normalization is complete (all frames captured), click on the acquire button on the touchscreen panel to save the image. Moving the camera around to image CNV from different angles may be necessary. The mouse can also be oriented in different positions to provide the best image of the CNV lesion.
NOTE: One may have to readjust the focus or positioning of the laser scanning ophthalmoscope when viewing vasculature farther from the optic nerve. - Switch back to the FA channel using the acquisition module. Complete the same steps listed in steps 3.6-3.7 to adjust the sensitivity and focus of each image to capture the leakage of the CNV lesion.
NOTE: Ensure the correct sensitivity is selected to avoid oversaturation of the CNV leakage and artificially increase the leakage area. - Image the "early phase" of ICGA and FA 3-4 min post-injection.
NOTE: The "early phase" is the time when the choroidal vasculature can be clearly and distinctly seen. During the middle phase, which typically occurs between 4-8 min, the retinal and choroidal vessels are much more faded and diffused. As the late phase (>8-10 min) sets in, both the choroidal and retinal vessels become indiscernible. Hyperfluorescent CNV lesions, however, exhibit maximal contrast against the diminished background. These mentioned timings are variable and depend on the concentration and amount of ICG dye injected. A greater amount of ICG dye tends to increase the timeline of each phase and provide more distinct vessels. A phase should be defined based on the key features listed above rather than an absolute time. - Once all images have been acquired, apply a gel lubricant or ointment to the mouse's eye and carefully monitor the mouse on the heating pad for recovery. It usually takes 1.5 h for the mouse to recover fully.
- Place the mice back into their cages and the designated holding area once they have fully recovered from the anesthesia and are awake.
- Before shutting down the imaging system and laser, export the images as either TIFF or JPEG files for subsequent analysis.
4. RPE/choroid flat-mount and staining
- Fix the eyes in 4% paraformaldehyde overnight. Wash the eyes with PBS three times. Remove the lens and cornea.
- Incubate the eyes in blocking solution (10% bovine serum albumin, 0.6% Triton X-100 in PBS) for 1 h at room temperature. Repeat PBS wash three times.
- Incubate the eyes with isolectin GS-IB4 Alexa-flour 568 conjugate and anti-α-smooth muscle actin antibody (see Table of Materials) overnight in the blocking solution.
- Wash three times with PBS. Incubate the samples with an Alexa Fluor 488 goat anti-rabbit secondary antibody (see Table of Materials) for 2 h at room temperature.
- Perform 4 radial cuts from the edge to the equator. Carefully remove the retina17.
NOTE: Care must be taken to ensure the neovascular membrane is not accidentally detached. - Mount the flat-mounted choroid on a glass slide. Visualize the CNV using confocal microscopy.
Representative Results
Following the protocol, ICGA and FA were performed on laser-induced CNV in young (7-9 weeks) and old (12-16 months) C57BL/6J mice. FA provides information about the location and leakage of the CNV lesions (Figure 1, left panels), while ICGA reveals the vascular morphology of the CNV lesions (Figure 1, right panels). In young mice, capillary CNV dominates the CNV lesions. In contrast, old mice exhibit arteriolar CNV characterized by large caliber vessels, vascular loops, and anastomotic connections. Both young and old mice show clear visibility of the retinal vasculature in FA (Figure 1, left panels). In the ICGA images of young mice, the retinal vasculature is not visible, and the choroidal vessels appear faded, indicating the middle phase of ICGA with the focus on choroidal vasculature. In the ICGA images of old mice, partial retinal vasculature can be observed while the choroidal vessels appear faded, suggesting the middle phase with the focus between the retina and choroid due to the larger size of arteriolar CNV in old mice. Arteriolar CNV in old mice exhibits larger CNV size (Figure 2) and significantly more leakage compared to capillary CNV in young mice. Immunostaining with an anti-smooth muscle actin antibody extensively labels the CNV vasculature in old mice, confirming the arteriolar morphology (Figure 3). In contrast, minimal staining with α-smooth muscle actin is observed in the lesion site vasculature of young mice, consistent with capillary morphology.
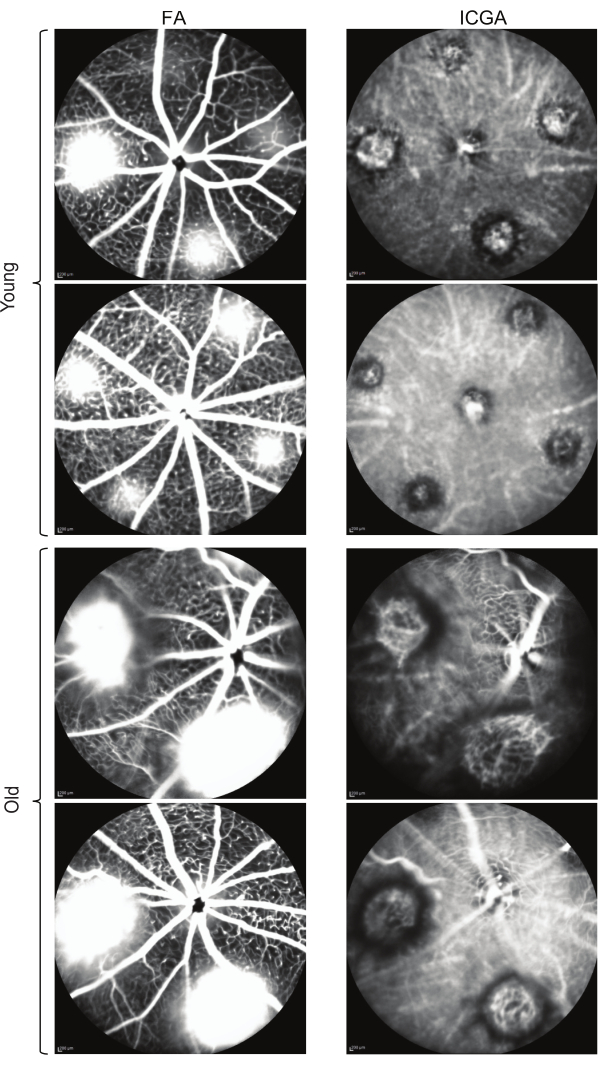
Figure 1: Comparison of FA and ICGA images depicting laser-induced CNV in young and old mice. The FA images display the leakage of CNV lesions, while ICGA provides visualization of the vascular morphology. Scale bars: 200 µm. Please click here to view a larger version of this figure.
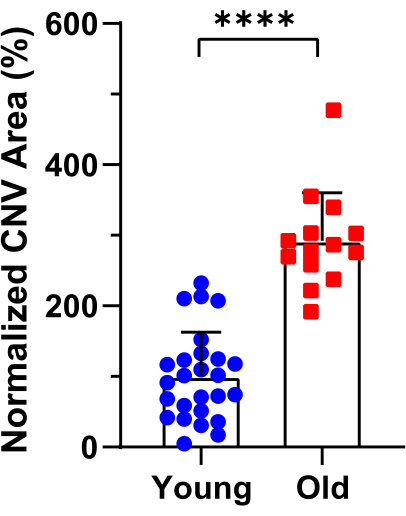
Figure 2: Quantification of CNV lesion size in young and old mice based on ICGA images. CNV areas were measured, with a total of 26 and 14 laser spots analyzed in young and old mice, respectively. Error bars represent mean ± SD. Statistical analysis was conducted using an unpaired t-test. ****P < 0.0001. Please click here to view a larger version of this figure.
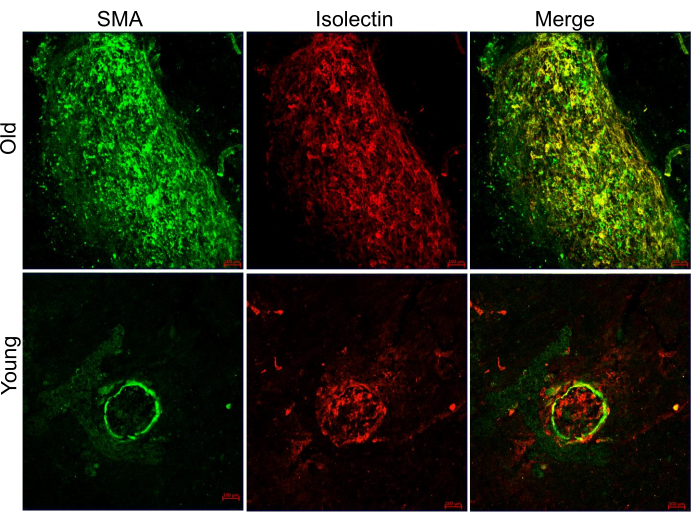
Figure 3: Representative images of CNV lesions in young and old mice, co-labeled with Alexa 568 isolectin and anti-α-smooth muscle actin antibody on RPE/choroid flat-mounts. The red color represents Alexa 568 isolectin, while the green color represents α-smooth muscle actin (SMA). Scale bars: 100 µm. Please click here to view a larger version of this figure.
Discussion
This study demonstrated the use of indocyanine green angiography (ICGA) to identify the vascular morphology of arteriolar and capillary choroidal neovascularization (CNV) in mouse models with laser-induced CNV. The hemoglobin-bound and infrared light properties of indocyanine green (ICG) dye enabled the detection of CNV morphology, which is challenging to achieve using fluorescein angiography (FA), the current method employed by the research community.
The first critical step in the protocol is to ensure that the dye is injected into the intraperitoneal cavity without penetrating organs. Proper injection placement in the lower left quadrant, with a small angle between the skin and bevel, while avoiding insertion of the entire needle, allows for improved uptake of indocyanine dye. Injecting the dye into an organ can result in slower uptake and potential complications such as laceration of abdominal organs, internal bleeding, or infection. Another key aspect of the procedure is to center the optic nerve before acquiring images to view the entire diameter of the eye. This requires overlapping the luminescence emitted by the FA channel and the mouse eye while paying attention to the image on the computer screen. To fix the longitudinal angle, it is best to tilt the mouse head directly in place rather than adjusting the machine up or down, ensuring the full field of view is captured.
Previous research has shown that the use of ketamine/xylazine anesthetics can cause corneal opacity18,19. This can be minimized by reducing the amount of xylazine20. Additionally, it is important to maintain consistent corneal moisture to avoid cataract formation. This can be achieved using lubricating eye drops or gel. These factors become particularly important with increased imaging frequency and the aging of the animal model, as sustained corneal damage affects the clarity of ICGA images. For prolonged imaging periods, the procedure can be modified by employing a polymethyl methacrylate contact lens above a gel-based buffer solution to prevent cataract formation21.
The method of injection is another crucial component. While this study focuses on intraperitoneal (IP) injection, the procedure can be performed with slight modifications using intravenous (IV) injection, specifically tail-vein injection. Intraperitoneal injection was chosen due to its ease of accomplishment, especially with pigmented mice, and its reliability during the procedure. This is an important consideration as quantitative experiments on CNV require efficient processing of large numbers of mice. Regardless of the injection method, the angiographic features of CNV can still be acquired due to its large size and location between the choroid and retina when characterizing different types of choroidal lesions in an animal model. However, this differs for polypoidal choroidal vasculopathy (PCV), another subtype of wet AMD, which is primarily located inside the choroid and requires IV-ICGA time course imaging for accurate diagnosis22.
One limitation of combined FA/ICGA is the increased variability when capturing various phases of CNV exudation. The optimal timings for early and late stages do not always align for ideal ICGA and FA images, requiring additional time to adjust the focus between the two modes for each eye. This aspect is magnified by the IP injection procedure, which introduces more variability in the timing of the three phases and necessitates longer imaging time compared to tail-vein injection22. However, these factors have minimal impact on detecting CNV vascular morphology, and the benefits of combined FA/ICGA outweigh these limitations.
Recent studies indicate that different types of CNV lesions, such as capillary or arteriolar CNV, respond differently to current anti-VEGF therapies9,16,17. Therefore, determining the vascular morphology of CNV lesions is crucial. However, the current method of choice, FA, does not provide this essential information. It is recommended to use ICGA in the research community for imaging neovascular AMD models. This study demonstrated that ICGA and FA can be conveniently performed together to assess both leakage and vascular features of CNV for mechanistic and therapeutic studies.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from BrightFocus Foundation, Retina Research Foundation, Mullen Foundation, and the Sarah Campbell Blaffer Endowment in Ophthalmology to YF, NIH core grant 2P30EY002520 to Baylor College of Medicine, and an unrestricted grant to the Department of Ophthalmology at Baylor College of Medicine from Research to Prevent Blindness.
Materials
| 32-G Insulin Syringe | MHC Medical Products | NDC 08496-3015-01 | |
| Alexa Fluor 488 goat anti-rabbit secondary antibody | Invitrogen | A11008 | |
| Anti-α smooth muscle Actin antibody | Abcam | ab5694 | |
| Bovine Serum Albumin | Santa Cruz Biotechnology, Inc. | sc-2323 | |
| C57BL/6J mice (7-9 weeks) | The Jackson Laboratory | Strain #:000664 | |
| Fluorescein Sodium Salt | Sigma-Aldrich | MFCD00167039 | |
| Gaymar T Pump Heat Therapy System | Gaymar | TP-500 | Water circulation heat pump for mouse recovery after imaging |
| GenTeal Gel | Genteal | NDC 58768-791-15 | Clear lubricant eye gel |
| GS-IB4 Alexa-Flour 568 conjugate | Invitrogen | I21412 | |
| Heidelberg Eye Explorerer | Heidelberg Engineering, Germany | HEYEX2 | |
| Indocyanine Green | Pfaultz & Bauer | I01250 | |
| Ketamine | Vedco Inc. | NDC 50989-996-06 | |
| Paraformaldehyde | Acros Organics | 416785000 | |
| Proparacaine Hydrochloride Ophthalmic Solution (0.5%) | Sandoz | NDC 61314-016-01 | |
| Spectralis Multi-Modality Imaging System Heidelberg Engineering, Germany SPECTRALIS HRA+OCT Tropicamide ophthalmic solution (1%) Bausch & Lomb NDC 24208-585-64 for dilation of pupils GenTeal Gel Genteal NDC 58768-791-15 clear lubricant eye gel Ketamine Vedco Inc NDC 50989-996-06 Xylazine Lloyd Laboratories NADA 139-236 Acepromazine Vedco Inc NDC 50989-160-11 32-G Needle Steriject PRE-32013 1-ml syringe BD 309659 Indocyanine Green Pfaltz & Bauer I01250 | Heidelberg Engineering, Germany | SPECTRALIS HRA+OCT | |
| Triton X-100 | Sigma-Aldrich | X100-1L | |
| Tropicamide ophthalmic solution (1%) | Bausch & Lomb | NDC 24208-585-64 | For dilation of pupils |
| Xylazine | Lloyd Laboratories | NADA 139-236 |
References
- Fleckenstein, M., et al. Age-related macular degeneration. Nature Reviews Disease Primers. 7 (1), 1-25 (2021).
- Wong, W. L., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health. 2 (2), e106-e116 (2014).
- Maguire, M. G., et al. Five-Year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 123 (8), 1751-1761 (2016).
- Yang, S., Zhao, J., Sun, X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review. Drug Design, Development and Therapy. 10, 1857-1867 (2016).
- Ehlken, C., Jungmann, S., Böhringer, D., Agostini, H. T., Junker, B., Pielen, A. Switch of anti-VEGF agents is an option for nonresponders in the treatment of AMD. Eye. 28 (5), 538-545 (2014).
- Heier, J. S., et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 119 (12), 2537-2548 (2012).
- Rofagha, S., Bhisitkul, R. B., Boyer, D. S., Sadda, S. R., Zhang, K. SEVEN-UP Study Group Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 120 (11), 2292-2299 (2013).
- Krebs, I., Glittenberg, C., Ansari-Shahrezaei, S., Hagen, S., Steiner, I., Binder, S. Non-responders to treatment with antagonists of vascular endothelial growth factor in age-related macular degeneration. British Journal of Ophthalmology. 97 (11), 1443-1446 (2013).
- Mettu, P. S., Allingham, M. J., Cousins, S. W. Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Progress in Retinal and Eye Research. 82, 100906 (2021).
- Otsuji, T., et al. Initial non-responders to ranibizumab in the treatment of age-related macular degeneration (AMD). Clinical Ophthalmology (Auckland, N.Z). 7, 1487-1490 (2013).
- Cobos, E., et al. Association between CFH, CFB, ARMS2, SERPINF1, VEGFR1 and VEGF polymorphisms and anatomical and functional response to ranibizumab treatment in neovascular age-related macular degeneration. Acta Ophthalmologica. 96 (2), e201-e212 (2018).
- Kitchens, J. W., et al. A pharmacogenetics study to predict outcome in patients receiving anti-VEGF therapy in age related macular degeneration. Clinical Ophthalmology (Auckland, N.Z.). 7, 1987-1993 (2013).
- Rosenfeld, P. J., Shapiro, H., Tuomi, L., Webster, M., Elledge, J., Blodi, B. Characteristics of patients losing vision after 2 Years of monthly dosing in the phase III Ranibizumab clinical trials. Ophthalmology. 118 (3), 523-530 (2011).
- Spaide, R. F. Optical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularization. American Journal of Ophthalmology. 160 (1), 6-16 (2015).
- Lumbroso, B., Rispoli, M., Savastano, M. C., Jia, Y., Tan, O., Huang, D. Optical coherence tomography angiography study of choroidal neovascularization early response after treatment. Developments in Ophthalmology. 56, 77-85 (2016).
- Zhu, L., et al. Combination of apolipoprotein-A-I/apolipoprotein-A-I binding protein and anti-VEGF treatment overcomes anti-VEGF resistance in choroidal neovascularization in mice. Communications Biology. 3 (1), 386 (2020).
- Zhang, Z., Shen, M. M., Fu, Y. Combination of AIBP, apoA-I, and Aflibercept overcomes anti-VEGF resistance in neovascular AMD by inhibiting arteriolar choroidal neovascularization. Investigative Ophthalmology & Visual Science. 63 (12), 2 (2022).
- Koehn, D., Meyer, K. J., Syed, N. A., Anderson, M. G. Ketamine/Xylazine-induced corneal damage in mice. PloS One. 10 (7), e0132804 (2015).
- Li, X. -. T., Qin, Y., Zhao, J. -. Y., Zhang, J. -. S. Acute lens opacity induced by different kinds of anesthetic drugs in mice. International Journal of Ophthalmology. 12 (6), 904-908 (2019).
- Zhou, T. E., et al. Preventing corneal calcification associated with xylazine for longitudinal optical coherence tomography in young rodents. Investigative Ophthalmology & Visual Science. 58 (1), 461-469 (2017).
- Ikeda, W., Nakatani, T., Uemura, A. Cataract-preventing contact lens for in vivo imaging of mouse retina. BioTechniques. 65 (2), 101-104 (2018).
- Kumar, S., Berriochoa, Z., Jones, A. D., Fu, Y. Detecting abnormalities in choroidal vasculature in a mouse model of age-related macular degeneration by time-course indocyanine green angiography. Journal of Visualized Experiments. 84, e51061 (2014).

