Fabrication of Monolayer Graphene-Coated Grids for Cryoelectron Microscopy
Summary
Here, we describe a protocol for applying a single monolayer of graphene to electron microscopy grids and how to prepare them for use in cryoEM structure determination.
Abstract
Cryogenic electron microscopy (cryoEM) has emerged as a powerful technique for probing the atomic structure of macromolecular complexes. Sample preparation for cryoEM requires preserving specimens in a thin layer of vitreous ice, typically suspended within the holes of a fenestrated support film. However, all commonly used sample preparation approaches for cryoEM studies expose the specimen to the air-water interface, introducing a strong hydrophobic effect on the specimen that often results in denaturation, aggregation, and complex dissociation. Further, preferred hydrophobic interactions between regions of the specimen and the air-water interface impact the orientations adopted by the macromolecules, resulting in 3D reconstructions with anisotropic directional resolution.
Adsorption of cryoEM specimens to a monolayer of graphene has been shown to help mitigate interactions with the air-water interface while minimizing the introduction of background noise. Graphene supports also offer the benefit of substantially lowering the required concentration of proteins required for cryoEM imaging. Despite the advantages of these supports, graphene-coated grids are not widely used by the cryoEM community due to the prohibitive expense of commercial options and the challenges associated with large-scale in-house production. This paper describes an efficient method for preparing batches of cryoEM grids that have nearly full coverage of monolayer graphene.
Introduction
Single-particle cryogenic electron microscopy (cryoEM) is an increasingly applicable technology used to investigate the 3D structures of biomacromolecules. Technological advances in electron microscope optics, direct electron detection1, and computer algorithms2,3,4 over the past decade have enabled cryoEM users to determine the structures of biochemically stable macromolecular complexes to near-atomic resolution5,6,7,8. Despite these advances, there remain notable barriers to preserving samples for cryoEM imaging, which prevent the majority of biological specimens from being resolved to such high resolutions.
Sample preparation for high-resolution cryoEM analysis involves trapping macromolecules that are evenly distributed in a wide range of orientations within a thin layer of vitrified ice. The "blot and plunge" methods of freezing are the most widely used methods employed to generate thin films of biological samples on grids for cryoEM studies9,10. These methods involve applying a few microliters of sample solution to an EM grid containing a fenestrated film that has been made hydrophilic and subsequently blotting away the majority of the sample with filter paper before rapidly plunging the grid into a cryogen of liquid ethane or ethane-propane mixture9.
While this method has successfully been used to determine structures of a wide range of biological specimens, all commonly used cryoEM specimen preparation methods expose specimens to the hydrophobic air-water interface (AWI), which often introduces issues that limit high-resolution structure determination. It is established that biological specimens have a high propensity to denature when exposed to the AWI, which can lead to complex aggregation and disassembly11,12,13,14. Furthermore, hydrophobic patches on the surfaces of biological specimens cause particles to adopt preferred orientations in the ice12. In many scenarios, a single hydrophobic region of the sample forces all particles to adopt a singular orientation in the ice, thereby abolishing the ability to generate a reliable reconstruction. In addition to issues with the AWI, specimens may demonstrate an affinity for the surface of the fenestrated layer of film, limiting the number of particles suspended in the ice within the holes15.
Several methodological and technological solutions have been developed to diminish these issues arising from interactions with the AWI or the films16,17. One popular approach is to coat the fenestrated film of the EM grids with a thin (tens of nanometers) layer of amorphous carbon. This coating provides a continuous surface across the holes to which particles can adsorb, with the benefit of partially shielding the sample from interactions with the AWI15,18,19,20. However, the additional carbon layer elevates the amount of background signal in the imaged regions, introducing noise that can compromise attainable resolution, particularly for small (<150 kDa) specimens. In recent years, the use of graphene oxide (GO) flakes to produce supporting films on cryoEM grids has been shown to have advantages over traditional amorphous carbon. GO flakes are produced through the oxidation of graphite layers, resulting in pseudo-crystalline sheets of monolayer graphite that are hydrophilic due to their substantial oxygen content in the form of carboxyl, hydroxyl, and epoxy groups on the surfaces and edges. Commercial GO flakes in aqueous suspensions are inexpensive, and there are numerous published methods for applying GO flakes to EM grids18,21. However, these methods often result in grids that are only partially covered with GO flakes, as well as regions that contain multiple layers of GO flakes. Further, GO flakes contribute a noticeable background signal to cryoEM images that is close to that observed with thin amorphous carbon22,23.
Pristine monolayer graphene, which consists of a single 2D crystalline array of carbon atoms, is distinct from GO in that it does not produce phase contrast in the electron microscope. Monolayer graphene thus can be used to generate an invisible support layer for imaging biological samples. Monolayer graphene is also stronger than GO and can be applied as a single monolayer on an EM grid, and recent advancements in the fabrication of graphene-coated EM grids have made it possible to prepare high-coverage monolayer graphene grids in-house24,25,26,27,28,29,30. However, despite the benefits of using graphene-coated grids for cryoEM structure determination, they are not widely used due to the prohibitive expense of commercial options and the complexity of in-house production. Here, we describe a step-by-step guide to effectively producing EM grids covered with a monolayer of graphene for cryoEM structure determination of biological specimens (Figure 1). By following this detailed protocol, cryoEM researchers can reproducibly prepare dozens of high-quality graphene support grids in a single day. The quality of the graphene-coated grids can be readily examined using a low-end transmission electron microscope (TEM) equipped with a LaB6 filament.
Protocol
1. Preparation of materials and accessories necessary for the fabrication of graphene grids
NOTE: Graphene readily contaminates, which reduces the efficiency of graphene coating and the quality of the graphene grids; therefore, it is important to thoroughly clean all materials that come into contact with the graphene. Preparation of materials and all steps should be carred out in a fume hood.
- Gather the necessary materials that will be used for coating the grids with graphene (Figure 2A).
- Rinse the glassware several times with deionized (DI) water to remove any dust, lint, and oily residues.
- Use disposable wipes to clean glass coverslips with 75% ethanol and use an air-duster to remove any contaminants.
NOTE: The clamping TEM grid holder block used in this protocol can hold up to 45 grids. For a large batch production of graphene grids, 45 grids or less can be prepared at once. However, it is recommended to start with a small batch production (four to six grids) until the method is established in the lab.
2. Preparation of 0.2 M ammonium persulfate (APS) in water
NOTE: This APS solution is used as an etchant to remove the copper (Cu) support from the graphene/Cu sheet in a later step. Always prepare fresh APS solution. Reused or old solutions will not etch copper effectively and may leave copper residue on the graphene in the later steps.
- Rinse a 500 mL flask with deionized water (DI) water, then add 200 mL of DI water and microwave using maximum settings for approximately 1 min to degas the water.
- Add 9 g of ammonium persulfate to 200 mL of DI water to produce a solution of 0.2 M APS.
CAUTION: APS is toxic, it is advised to wear personal protective equipment (PPE) when handling APS. Dispose of APS waste in an approved waste disposal plant. - Stir the solution with a stir bar on a magnetic stirrer while connecting the flask to a vacuum source under a fume hood.
NOTE: Degassing APS solution will help prevent the formation of bubbles, which can reduce the efficiency of Cu etching in step 6.
3. Transfer graphene/copper to a clean coverslip with a piece of blotting paper
NOTE: We use a 15 x 15 cm chemical vapor deposition (CVD) graphene film on Cu from a graphene supplier. Commercially purchased monolayer graphene/Cu sheets should be stored under a vacuum. As graphene is grown on both sides of Cu by the CVD method, graphene suppliers generally conduct quality checks and recommend the better side for use. We refer to this recommended side of the graphene as the "top" side while the other side is the "back" side in this protocol.
- Cut a piece of blotting paper into a rectangular shape approximately 20 mm x 40 mm. This blotting paper is used as padding for the graphene/Cu sheet and will absorb excess methyl methacrylate (MMA (8.5) MMA EL6) used to coat the graphene/Cu sheet; therefore, be sure to cut it into a size that is bigger than the graphene/Cu sheet to be used.
- Use polyimide tape to tape the four corners of the blotting paper to the top of a clean coverslip that will fit into a homemade spin coater.
NOTE: Polyimide tape is used because it is thin and can be removed easily, which makes handling easy. - Remove one piece of the graphene/Cu sheet from the vacuum storage.
- Use clean and dust-free scissors to carefully cut a small square of the Cu-graphene sheet that will be sufficient to completely cover the number of grids to be prepared. For 25 grids arranged in a 5 x 5 array, for example, cut a piece that is 18 mm x 18 mm. Place the graphene/Cu sheet on top of the blotting paper with clean, dry tweezers. Make sure that the back side of the graphene/Cu sheet is facing down and be careful not to accidentally flip the graphene/Cu sheet since it is difficult to discern the top side from the back side.
- Tape the four corners of the graphene/Cu sheet to the blotting paper/coverslip, minimizing the amount of contact between the tape and the graphene/Cu sheet, since any regions covered with tape will not be covered with MMA in the next step.
NOTE: Static discharge can damage graphene films, and thus, it is advisable to remain electrically grounded and minimize the accumulation of static charge when handling graphene or graphene grids. This can be accomplished by wearing a wrist grounding strap or touching a grounded metal object immediately prior to handling graphene or graphene grids.
4. Coat the single-layer graphene/Cu sheet with a thin layer of MMA(8.5)MMA EL6 (MMA)
NOTE: After the Cu is etched away, this layer of MMA will support the graphene monolayer to enable the handling of the graphene sheet in future steps. MMA coating also enables the visualization of the graphene film since a monolayer of graphene alone would be transparent.
- Place the coverslip with the taped graphene/Cu sheet on a homemade spin coater (this can be assembled using a computer fan) inside a fume hood (Figure 2B), as previously described by Han et al.25.
- Wearing safety goggles, add two drops of MMA using a glass pipette on the graphene sheet and immediately begin spinning at maximum speed.
- While spinning, add two more drops in the center. Spin for 1 min.
NOTE: Be sure that sufficient MMA has been applied to fully coat the graphene. If unsure, add a few more drops. If graphene is not fully coated, "holes" appear in the film after Cu is etched away. - Let air-dry for 10 min inside a fume hood.
5. Remove graphene on the back side of the graphene/Cu sheet
NOTE: Graphene grown on the back side of the copper (the side not coated with MMA) must be removed before proceeding to the subsequent steps because this excess graphene will reduce the effectiveness of Cu etching. We remove this graphene by exposing the graphene to plasma, which can be accomplished using any glow discharge device typically used to prepare EM grids for biological sample preparation.
- Carefully remove the tape and tlift the MMA-coated graphene/Cu sheet from the coverslip with clean, dry tweezers.
- Tape the piece of the MMA/graphene/Cu sheet with the MMA side down to a clean and dust-free glass coverslip.
- Place the coverslip with the MMA/graphene/Cu sheet into the glow discharge device and apply settings that would typically be used when preparing grids for negative stain or cryoEM sample preparation.
NOTE: Prolonged glow discharge should be avoided to prevent oxidation of Cu on the back side, which can result in contamination of copper oxide (CuO) (nano)particles on graphene film.
6. Etch away the Cu from the MMA/graphene/Cu sheet in APS solution
- In a fume hood, pour 200 mL of the freshly made APS solution into a clean and dust-free crystallizing dish (150 x 75 mm).
- Remove the MMA/graphene/Cu sheet from the glass slide. Keep track of which side contains the MMA.
- To begin the etching, place the MMA/graphene/Cu sheet with the plasma-treated Cu side down on the surface of the APS solution. Cover the wide glass beaker with a piece of aluminum foil or a lid to prevent dust from entering.
- Incubate for 3 h. If most of the Cu is not etched after 1 h, repeat the steps in sections 2-6 and then continue to the next step.
NOTE: If most of the Cu is not etched after 1 h, it is likely that the MMA/graphene side of the film has been placed down on the surface of the APS solution instead of the Cu side. Cu should be etched completely after 3 h, and the MMA/graphene film is colorless if Cu is etched completely. Graphene readily contaminates so be sure to cover any containers to avoid the accumulation of lint or any greasy residues on surfaces, as this will negatively impact the quality of the graphene.
7. Rinse the MMA/graphene film in DI water
- In a fume hood, fill a clean and dust-free crystallizing dish with DI water.
- Gently scoop up the graphene-MMA film with a clean, dust-free glass coverslip positioned at ~45° angle relative to the APS solution surface.
- Position the glass slide at a nearly 90° angle to the water, and gently lower the glass coverslip into the water so that the MMA/graphene slowly slides off the slide and floats on the water's surface.
- Leave the MMA/graphene film on the water surface for 1 h to wash off any APS.
8. Clean the grids to be coated with a monolayer of graphene
NOTE: The grids to which the graphene will be transferred must be as clean as possible to maximize the attachment of the graphene to the grid foil surface. Commercially purchased grids often contain residual contaminants that must be removed prior to graphene transfer.
- Clean and dry three crystallizing dishes so that they are free of dust.
- In a fume hood, pour 200 mL of chloroform, acetone, and isopropyl alcohol (IPA) into each of the three crystallizing dishes. Cover the crystallizing dishes with aluminum foil to minimize the evaporation of the organic solvents.
CAUTION: Chloroform and acetone cause skin irritation and can be toxic if inhaled. Limit exposure to these organic solvents and wear PPE. - Place the base of the clamping TEM grid holder block at the bottom of the first crystallizing dish containing 200 mL of chloroform.
- Transfer each grid individually from the grid box to a well in the grid holder block, ensuring that the fenestrated film side is facing up. Cover the crystallizing dish with aluminum foil, place it on an orbital shaker, and gently shake for 30 min.
- Place the metal lid on the grid holder block, which will secure the grids during the transfer to the next crystallizing dish. Carefully lift the grid holder block out of the crystallizing dish with a bent fork and long tweezers and place it at the bottom of the second crystallizing dish containing 200 mL of acetone. Remove the lid from the grid holder block and gently shake on an orbital shaker for 30 min.
- Place the lid on the grid holder block and transfer to a crystallizing dish containing 200 mL of IPA solution to clean off acetone residue. Remove the lid from the grid holder block and gently shake for 20 min.
- Individually transfer the grids with the fenestrated film side facing up from the grid holder block to a glass Petri dish covered with blotting paper.
- Dry the grids for at least 30 min under the fume hood, ensuring that the grids are covered to prevent dust from landing on it.
9. Transfer the clean grids to blotting paper placed on a stainless-steel wire mesh or perforated tray under DI water
NOTE: Grids must be submerged under DI water on a flat surface so that the graphene can be floated onto the water and lowered onto the grids. This can be performed using a commercial grid coating trough or with a Petri dish and a peristaltic pump, as used for generating graphene-oxide grids, as described by Palovcak et al.18.
- Place the stainless-steel mesh or tray in a grid coating trough and rinse with DI water.
- Cut the blotting paper that is slightly smaller than the stainless-steel mesh/tray and place it on top of the platform, submerged in the DI water.
NOTE: The blotting paper is slightly smaller than the platform to enable movement and handling. - Gently transfer the cleaned grids with the fenestrated film side facing up on top of the blotting paper. The grids will likely be hydrophobic, so submerge the grids into the water vertically or they may bend due to water tension. Position the grids in a square array so that they are as close to one another as possible, but not overlapping (Figure 2C).
- The grids are now ready to be coated with a graphene monolayer. Fill the grid coating trough with more DI water so that the surface of the water is at least 5 mm above the grids.
10. Transfer graphene to the grids
- Carefully scoop out the MMA/graphene film from the crystallizing dish with a clean coverslip by slowly lowering the coverslip into the trough at an angle, some distance away from the graphene film. Position the coverslip underneath the graphene-MMA sheet so that the edges of the sheet and the coverslip are parallel, and then lift the coverslip vertically out of the water, bringing the MMA/graphene film with it.
- Transfer the MMA/graphene film to the trough by lowering the coverslip into the water at a ~45° angle, so that the MMA/graphene film detaches from the coverslip and floats on the surface of the water.
- Position the MMA/graphene film directly above the grids before the water level is lowered. Use a glass Pasteur pipette whose tip has been melted to seal the opening to carefully manipulate the position of the MMA/graphene film.
- Slowly lower the water level with the syringe, at approximately 1.25 mL/min such that the MMA/graphene film fully covers the grid surfaces as it lands on the filter paper
NOTE: Further manipulation of the graphene-MMA sheet may be necessary to maintain its position above the grids as the water level drops. - Use a pair of clean, dry tweezers to lift the blotting paper holding the grids to a clean, dry, dust-free Petri dish, or transfer the entire stainless-steel platform.
- Air-dry the MMA/graphene grids for at least 30 min under the fume hood. Keep the grids covered with aluminum foil or a lid to prevent any contamination from dust particles.
- Transfer the grids to an incubator and bake the grids in a 65 °C incubator for 30 min.
- Remove the grids from the incubator and leave them covered for 5 min at room temperature to cool the grids to the room temperature.
11. Removing MMA and cleaning the grids
NOTE: MMA must be thoroughly washed off in acetone to prevent any residual MMA on the graphene-coated grids.
- In a fume hood, prepare two crystallizing dishes containing 200 mL of acetone and one crystallizing dish containing 200 mL of isopropanol (IPA) at room temperature. Cover the crystallizing dishes with aluminum foil to minimize the evaporation of the organic solvents.
CAUTION: Wear PPE when handling acetone as it can cause skin irritation and can be harmful if inhaled. - Place the base of the clamping TEM grid holder block at the bottom of the first crystallizing dish containing 200 mL of fresh acetone.
- Transfer each grid individually from the blotting paper to a well in the grid holder block, ensuring that the MMA side is facing up. Cover the crystallizing dish with foil, place it on an orbital shaker, and gently shake for 30 min.
- Place the metal lid on the grid holder block, which will secure the grids during the transfer to the next crystallizing dish. Carefully lift the grid holder block out of the crystallizing dish with a bent fork and long tweezers and place it at the bottom of the second crystallizing dish containing 200 mL of fresh acetone. Remove the lid from the grid holder block and gently shake on an orbital shaker for 30 min.
- Place the lid on the grid holder block and transfer to the crystallizing dish containing 200 mL of IPA solution to clean off acetone residue. Remove the lid from the grid holder block and gently shake for 20 min.
- Transfer the grids to a small glass Petri dish covered with blotting paper and air-dry the grids for at least 10 min. Keep the grids covered with a lid to prevent any contamination from dust particles.
- Grids are ready to be used or transferred to a grid box wrapped with aluminum foil inside a vacuum desiccator.
NOTE: Storing graphene grids inside a vacuum desiccator can prevent contamination of hydrophobic particles from ambient conditions. These grids can be stored for up to several months before use27.
12. Render graphene grids hydrophilic with UV/Ozone treatment
NOTE: Graphene is extremely hydrophobic, which is not compatible with cryoEM sample preparation, since blot-plunge approaches require a hydrophilic surface upon which a drop of sample can spread evenly. While traditional glow-discharge devices can be configured to gently pulse plasma to make the graphene surface hydrophilic, these devices tend to destroy the thin graphene monolayer. It was previously shown that a UV/ozone cleaner can be used to partially oxygenate the surface of the graphene25, rendering it hydrophilic for cryoEM sample preparation without damaging the monolayer.
- If using a UV/Ozone system that requires priming, turn on the system and prime the lamp for 10 min (at this step, ensure that no grids are exposed). While the UV/ozone cleaner is priming, remove grids from the vacuum desiccator and transfer them to a clean coverslip.
- When the UV/Ozone system is ready, place the coverslip containing the grids with the graphene side facing up into the UV/ozone cleaner, and expose the grids to the ozone gas for 4 min.
- After exposure to ozone, use the grids immediately for cryoEM sample preparation.
NOTE: If using a UV/Ozone system that requires priming, grids must be placed in the cleaner immediately after the lamp has been primed for 10 min, or it will be too cool to produce sufficient ozone gas to oxygenate the graphene for sample preparation. Do not expose the grids to the ozone gas for more than 6 min, as it will destroy the graphene layer.
Representative Results
Successful execution of the graphene grid fabrication protocol described here will result in EM grids that are fully coated with a single monolayer of graphene. Graphene coverage of the grids can be checked using any TEM. Since a monolayer of clean graphene is nearly invisible in the TEM, one must examine it using the diffraction mode of the microscope and observe Bragg spots corresponding to the hexagonal organization of the carbon atoms that comprise the graphene (Figure 3A). It is normal to occasionally observe some wrinkles of monolayer graphene, which are introduced during MMA-coating (Figure 3B). One can also check the level of contamination present on the graphene by acquiring a high magnification image in the center of one of the graphene-covered holes (Figure 3C). If acquired with a high-resolution detector, a Fourier transform of this image should contain Bragg spots corresponding to carbon-carbon spacing at 2.14 Å (Figure 4C). A monolayer of carbon atoms does not produce sufficient electron scattering to generate phase contrast, and thus an image of clean graphene will not present Thon rings associated with the contrast transfer function in a Fourier Transform of the image. However, it is very difficult to prevent contamination of graphene grids after they are produced, and insufficient washing of the EM grids or removal of MMA after graphene coating will result in notable contaminants on the grids that are visible in the real-space images (Figure 3C). As shown in Figure 4, graphene grids have a concentrating effect on a sample, as observed when comparing 0.5 mg/mL of apoferritin is applied to holey gold grids with (Figure 4A) and without the graphene support (Figure 4B). Similar graphene fabrication protocols have been previously described to solve cryoEM structures of proteins such as apoferritin at high resolution25,27.
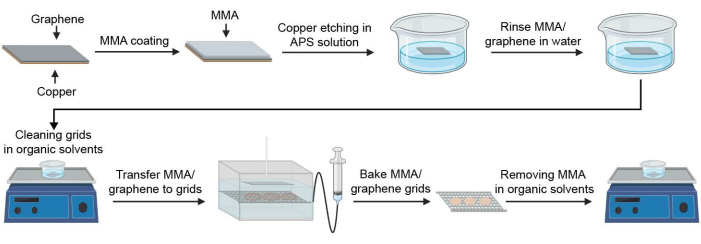
Figure 1: Schematic for preparing graphene-coated cryoEM grids. Key steps in the process of graphene grid fabrication are illustrated. Abbreviations: cryoEM = cryogenic electron microscopy; MMA = methyl methacrylate; APS = ammonium persulfate. Please click here to view a larger version of this figure.
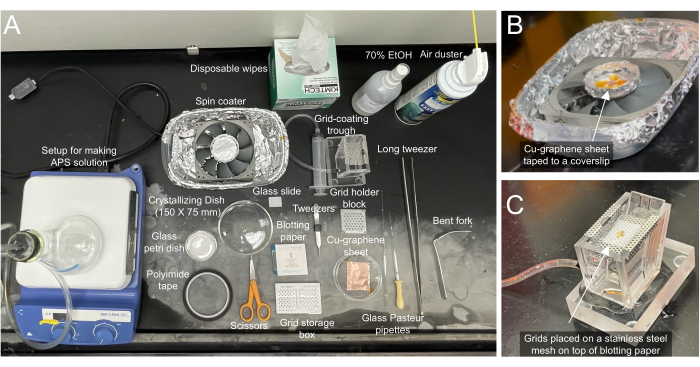
Figure 2: Required materials for making graphene grids. (A) Necessary materials for coating cryo-EM grids are labeled accordingly. (B) Closeup view of the coater with graphene/Cu sheet taped onto a blotting paper on a glass slide. The spin coater can be assembled by purchasing parts from a local computer/hardware store. (C) Closeup view of the grid coating trough attached to a syringe that can be used to control the water level. Grids are placed on top of a blotting paper on a stainless-steel mesh. The blotting paper aids in maneuvering the location of the grids so the graphene sheet can be matched to it. Please click here to view a larger version of this figure.
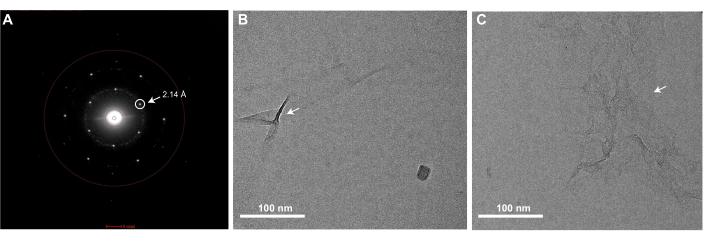
Figure 3: A representative diffraction pattern image and bright field images of a graphene grid showing wrinkles or MMA contamination. (A) EM grids covered with a monolayer of graphene will show Bragg peaks corresponding to the hexagonal lattice of the graphene when imaged in a TEM in diffraction mode. The Bragg peak corresponding to the 2.14 Å carbon-carbon spacing is circled and denoted with an arrow. (B) A bright-field image of a monolayer graphene grid with some wrinkles (denoted with an arrow) in the graphene monolayer. (C) A bright-field image of monolayer graphene with MMA contamination (denoted with an arrow). Scale bars = 100 nm (B,C). Abbreviations: EM = electron microscopy; MMA = methyl methacrylate. Please click here to view a larger version of this figure.
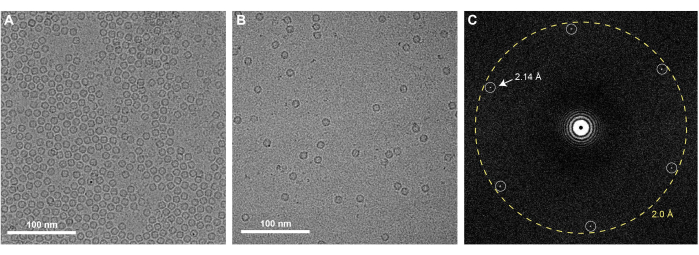
Figure 4: Apoferritin on graphene-covered gold grids: (A) CryoEM micrograph of 0.5 mg/mL apoferritin on graphene-covered gold grids. (B) Apoferritin imaged at the same concentration is visible at substantially lower concentration when prepared using holey gold grids without graphene. (C) FFT of the cryoEM micrograph of 0.5 mg/mL apoferritin on graphene-covered gold grids, with the Bragg peaks corresponding to the hexagonal graphene lattice denoted. Scale bars = 100 nm (A,B). Abbreviations: cryoEM = cryogenic electron microscopy; FFT = fast Fourier transform. Please click here to view a larger version of this figure.
Discussion
The preservation of biological samples in a thin layer of vitreous ice is a critically important step for high-resolution cryoEM structure determination. However, researchers often encounter problems arising from interactions with the AWI, which introduces preferred orientation, complex disassembly, denaturation, and aggregation. Furthermore, samples cannot always be concentrated sufficiently to populate the thin ice suspended across the holes of a fenestrated film. Several research groups have developed methods to coat EM grids with a monolayer of graphene to help overcome some of these limitations24,25,26,27,28,29,30, and graphene grids have been used with great success. Here, we provide step-by-step instructions for effectively preparing batches of graphene grids in-house and examining the quality of the graphene grids by TEM. We emphasize that particular care should be exercised during some of the critical steps, which we outline below.
Graphene has a strong tendency to attract airborne contaminants. Therefore, during the graphene grid fabrication process, it is important to make sure all the tools that make contact with the graphene/Cu sheet or the grids are clean and dust-free. Glass coverslips used to transfer graphene can be cleaned by rinsing with ethanol and DI water or using an air-duster. It is also advised to work under a fume hood and keep graphene sheets and grids covered with foil or a clean glass plate at all times. Dust or contaminants on the grids may prevent graphene from thoroughly adhering to the EM grids. When handling graphene or graphene-coated grids, it is important to be electrically grounded to prevent damage to the graphene film from static discharge. Static discharge can be avoided by using a wrist grounding strap, touching a grounded metal object each time graphene or graphene grids are handled, and/or not wearing a glove on the hand holding the tweezers24.
Since a monolayer of graphene is very thin (the width of a carbon atom), it is important to support graphene with an organic layer such as MMA or poly-MMA (PMMA) during the transfer of graphene to grids. PMMA is the most widely used material for graphene transfer. However, PMMA has a strong affinity with graphene and can often result in polymer contamination on the graphene film. MMA is used in this protocol, as it leaves less residual contamination25. However, both PMMA and MMA have the disadvantage of forming wrinkles and cracks that can be observed in some areas of the graphene film (Figure 3B). It can be challenging to avoid these wrinkles as they commonly occur during graphene growth by the CVD method31. A method has been recently developed for growing ultra-flat graphene without wrinkles, whereby the copper foil is replaced by a Cu(111)/sapphire wafer as a growth substrate32.
Based on our experience, it is better to purchase graphene/Cu sheets and support the graphene with MMA in-house than purchasing polymer-covered Cu-graphene sheets from manufacturers, which become brittle after copper etching and are difficult to handle in subsequent steps. The spin coater we used for MMA coating can be cheaply built using parts from a local computer/hardware store, as previously described25.
During the step of MMA coating, it is important to cover the entirety of the graphene surface on the Cu-graphene sheet with MMA. After the Cu has been etched away, MMA-graphene will become semi-transparent, and areas lacking MMA coverage will look like empty holes. To prevent MMA coating on the copper side, it is important to place a small piece of blotting paper underneath it during coating such that it soaks up any excess MMA that spins out from the CVD film.
After etching and rinsing, the MMA/graphene sheet is ready to be transferred to EM grids by using a commercial or homemade trough system with a syringe or peristaltic pump to control the water level. Prior to the transfer step, it is important to thoroughly prerinse the grids in successive baths of chloroform, acetone, and IPA. Baking graphene-coated grids at 65 °C helps to preserve graphene integrity and promotes the adsorption of graphene to the grids. Lastly, to prevent MMA contamination on the grids, it is important to thoroughly remove MMA in an acetone bath and clean the grids in IPA. Any unwashed MMA residue will be observed on EM grids and diminish the signal-to-noise ratio of the images (Figure 3C). The acetone-IPA washing process can be repeated to further clean the graphene surfaces.
To render graphene grids hydrophilic, we exposed the grids to UV/Ozone. Different models of UV/ozone cleaners may require optimization to sufficiently oxygenate the graphene layer for cryoEM sample preparation without damaging the graphene. Regardless of the system, it is critical to use these grids for cryoEM sample application immediately after UV/Ozone treatment. Alternative methods to render graphene grids hydrophilic are described in other studies33,34.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Xiao Fan for helpful discussions while establishing these methods at Scripps Research. B.B. was supported by a postdoctoral research fellowship from the Hewitt Foundation for Medical Research. W.C. is supported by a National Science Foundation predoctoral fellowship. D.E.P is supported by the National Institutes of Health (NIH) grant NS095892 to G.C.L. This project was also supported by NIH grants GM142196, GM143805, and S10OD032467 to G.C.L.
Materials
| 70% EtOH | Pharmco (190 pf EtOH) | 241000190CSGL | |
| Acetone | Sigma Aldrich | 650501-4L | |
| Ammonium persulfate (APS) | Sigma Aldrich | 215589-500g | Hazardous; use extreme caution |
| Chloroform | Sigma Aldrich | C2432-1L | |
| Clamping TEM Grid Holder Block for 45 Grids | PELCO | 16830-45 | |
| Computer fan | Amazon (Noctua) | B07CG2PGY6 | |
| Cover slip | Bellco Glass | 1203J71 | Standard cover slips |
| Crystallizing dish | Pyrex | 3140-100 | |
| Electronics duster | Falcon Safety Products | 75-37-6 | |
| Falcon Dust-off Air Duster | Staples | N/A | |
| Filter papers | Whatman | 1001-055 | |
| Fine tip tweezer | Dumont | 0508-L4-PO | |
| Flask | Pyrex | 4980-500 | |
| Fork | Supermarket | N/A | |
| Glass pasteur pipette | VWR | 14672-608 | |
| Graphene/Cu | Graphenea | N/A | CVD monolayer graphene cu |
| Grid Coating Trough | Ladd Research Industries | 10840 | Fragile |
| Isopropanol | Fisher Scientific | 67-63-0 | |
| Kapton Tape | Amazon (MYJOR) | MY-RZY001 | Polyimide tape |
| Kimwipes | Fisher Scientific | 06-666 | |
| Long twzeer | Cole Parmer Essentials | UX-07387-15 | |
| Metal grid holder | Ted Pella | 16820-81 | |
| MMA(8.5)MMA EL 6 | KAYAKU Advanced Materials | M31006 0500L 1GL | Flammable |
| Model 10 Lab Oven | Quincy Lab, Inc. | FO19013 | |
| Petri dish | Pyrex | 3610-102 | |
| Plasma cleaner (Solarus 950) | Gatan, Inc. | N/A | |
| Scissors | Fiskars | 194813-1010 | |
| Standard Analog Orbital Shaker | VWR | 89032-088 | |
| UltrAuFoil R1.2/1.3 – Au300 | Quantifoil | N/A | Holey gold grids |
| Ultraviolet Ozone Cleaning Systems | UVOCS | model T10X10/OES |
References
- Li, X., Zheng, S. Q., Egami, K., Agard, D. A., Cheng, Y. Influence of electron dose rate on electron counting images recorded with the K2 camera. Journal of Structural Biology. 184 (2), 251-260 (2013).
- Tang, G., et al. EMAN2: An extensible image processing suite for electron microscopy. Journal of Structural Biology. 157 (1), 38-46 (2007).
- Zivanov, J., et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife. 7, 1-22 (2018).
- Punjani, A., Rubinstein, J. L., Fleet, D. J., Brubaker, M. A. CryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nature Methods. 14 (3), 290-296 (2017).
- Grigorieff, N., Harrison, S. C. Near-atomic resolution reconstructions of icosahedral viruses from electron cryo-microscopy. Current Opinion in Structural Biology. 21 (2), 265-273 (2011).
- Nakane, T., et al. Single-particle cryo-EM at atomic resolution. Nature. 587 (7832), 152-156 (2020).
- Zhang, K., Pintilie, G. D., Li, S., Schmid, M. F., Chiu, W. Resolving individual atoms of protein complex by cryo-electron microscopy. Cell Research. 30 (12), 1136-1139 (2020).
- Yip, K. M., Fischer, N., Paknia, E., Chari, A., Stark, H. Atomic-resolution protein structure determination by cryo-EM. Nature. 587 (7832), 157-161 (2020).
- Schultz, P. Cryo-electron microscopy of vitrified specimens. Quarterly Reviews of Biophysics. 21 (2), 129-228 (1988).
- Nguyen, H. P. M., McGuire, K. L., Cook, B. D., Herzik, M. A. Manual blot-and-plunge freezing of biological specimens for single-particle cryogenic electron microscopy. Journal of Visualized Experiments. 2022 (180), 1-16 (2022).
- Glaeser, R. M. Proteins, interfaces, and cryo-em grids. Current Opinion in Colloid & Interface Science. 25 (3), 289-313 (2016).
- Glaeser, R. M., Han, B. -. G. Opinion: hazards faced by macromolecules when confined to thin aqueous films. Biophysics Reports. 3 (1-3), 1-7 (2017).
- Han, B. G., Watson, Z., Cate, J. H. D., Glaeser, R. M. Monolayer-crystal streptavidin support films provide an internal standard of cryo-EM image quality. Journal of Structural Biology. 200 (3), 307-313 (2017).
- Noble, A. J., et al. Routine single particle CryoEM sample and grid characterization by tomography. eLife. 7, 1-42 (2018).
- Drulyte, I., et al. Approaches to altering particle distributions in cryo-electron microscopy sample preparation. Acta Crystallographica Section D: Structural Biology. 74 (6), 560-571 (2018).
- Liu, N., Wang, H. W. Better cryo-EM specimen preparation: how to deal with the air-water interface. Journal of Molecular Biology. 435 (9), 167926 (2022).
- Noble, A. J., et al. Reducing effects of particle adsorption to the air-water interface in cryo-EM. Nature Methods. 15 (10), 793-795 (2018).
- Palovcak, E., et al. A simple and robust procedure for preparing graphene-oxide cryo-EM grids. Journal of Structural Biology. 204 (1), 80-84 (2018).
- Patel, A., Toso, D., Litvak, A., Nogales, E. Efficient graphene oxide coating improves cryo-EM sample preparation and data collection from tilted grids. bioRxiv. , (2021).
- Marr, C. R., Benlekbir, S., Rubinstein, J. L. Fabrication of carbon films with ~500nm holes for cryo-EM with a direct detector device. Journal of Structural Biology. 185 (1), 42-47 (2014).
- Pantelic, R. S., Meyer, J. C., Kaiser, U., Baumeister, W., Plitzko, J. M. Graphene oxide: A substrate for optimizing preparations of frozen-hydrated samples. Journal of Structural Biology. 170 (1), 152-156 (2010).
- Pantelic, R. S., et al. Graphene: substrate preparation and introduction. Journal of Structural Biology. 174 (1), 234-238 (2011).
- Russo, C. J., Passmore, L. A. Progress towards an optimal specimen support for electron cryomicroscopy. Current Opinion in Structural Biology. 37, 81-89 (2016).
- Passmore, L. A., Russo, C. J. Specimen preparation for high-resolution cryo-EM. Methods in Enzymology. 579, 51-86 (2016).
- Han, Y., et al. High-yield monolayer graphene grids for near-atomic resolution cryoelectron microscopy. Proceedings of the National Academy of Sciences of the United States of America. 117 (2), 1009-1014 (2020).
- Zheng, L., et al. Robust ultraclean atomically thin membranes for atomic-resolution electron microscopy. Nature Communications. 11 (1), 541 (2020).
- Ahn, E., Kim, B., Cho, U. -. S. Batch production of high-quality graphene grids for cryo-EM: cryo-EM structure of Methylococcus capsulatus soluble methane monooxygenase hydroxylase. bioRxiv. (Cvd), (2021).
- Naydenova, K., Peet, M. J., Russo, C. J. Multifunctional graphene supports for electron cryomicroscopy. Proceedings of the National Academy of Sciences of the United States of America. 116 (24), 11718-11724 (2019).
- Fan, H., Sun, F. Developing graphene grids for cryoelectron microscopy. Frontiers in Molecular Biosciences. 9, 937253 (2022).
- D’Imprima, E., et al. Protein denaturation at the air-water interface and how to prevent it. eLife. 8, e42747 (2019).
- Zhang, X., et al. Evolution of copper step beams during graphene growth by CVD method. Applied Surface Science. 610, 155518 (2023).
- Zheng, L., et al. Uniform thin ice on ultraflat graphene for high-resolution cryo-EM. Nature Methods. 20 (1), 123-130 (2023).
- Fujita, J., et al. Epoxidized graphene grid for highly efficient high-resolution cryoEM structural analysis. Scientific Reports. 13, 2279 (2023).
- Lu, Y., et al. Functionalized graphene grids with various charges for single-particle cryo-EM. Nature Communications. 13, 6718 (2022).

