Exploring Life History Choices: Using Temperature and Substrate Type as Interacting Factors for Blowfly Larval and Female Preferences
Summary
Herein, two protocols for assessing food source and oviposition preferences in larvae and females of blowflies are detailed. These comprise four choices with two interacting factors: substrate type and temperature. The assays enable the determination of the food source preference of the larvae and the oviposition site preference for the females.
Abstract
Blowflies (Diptera: Calliphoridae) present a wide range of larval lifestyles, typically classified as obligate parasitism, facultative parasitism, and complete sapro-necrophagy. Several parasitic species, both obligate and facultative, are considered to be of sanitary and economic importance, as their larvae can cause myiasis (maggot infestation in live tissue). However, it is noteworthy that the adult female plays a decisive role as she chooses the oviposition site, and, therefore, largely determines the feeding habit and developmental conditions of the larvae. In this study, two protocols are proposed to test larval feeding preference and female oviposition site preference considering two interacting factors: meat substrate type and temperature. The setups presented here allowed to test Lucilia cuprina larvae and gravid females in a four-choice assay with two temperatures (33 ± 2 °C and 25 ± 2 °C) and two types of meat substrates (fresh meat supplemented with blood and 5-day-old rotten meat). Larvae or gravid females can choose to burrow or lay their eggs, respectively, in either of the following: rotten meat at 25 °C (simulating a necrophagous species condition), fresh meat supplemented with blood at 33 °C (simulating a parasitic species condition), and two controls, rotten meat at 33 °C, or fresh meat supplemented with blood at 25 °C. The preference is assessed by counting the number of larvae or eggs laid in each option for each replicate. Comparing the observed results to a random distribution allowed for the estimation of the statistical significance of the preference. The results indicated that L. cuprina larvae have a strong preference for the rotten substrate at 25 °C. Conversely, oviposition-site preference by females was more varied for the meat type. This methodology can be adapted to test the preference of other insect species of similar size. Other questions can also be explored by using alternative conditions.
Introduction
Flies, particularly calyptrate muscoids (including blowflies, house flies, bot flies and flesh flies among others), exhibit a wide range of lifestyles, encompassing parasitic and necro-saprophagous behaviors1. Parasitic species typically cause myiasis, an infestation of live tissues by maggots (larvae)2. In the Calliphoridae family, both obligate and facultative parasitic species are major livestock pests responsible for economic losses and poor animal welfare due to maggot infestations2,3,4,5,6,7. Obligate parasites, such as the New World and Old World screwworms (Cochliomyia hominivorax and Chrysomyia bezziana, respectively), are especially problematic4,7,8,9,10 along with facultative parasites, such as the sheep blowflies (Lucilia cuprina and Lucilia sericata)2,5,6,7. Non-parasitic species, including sapro-necrophagous ones, develop in decaying and necrotic organic matter and are commonly found in unsanitary environments. Their strictly non-parasitic lifestyle can be successfully used for maggot therapy, which uses fly larvae to clean wounds of necrotic tissues11,12,13. Blowflies are also used in forensic science, as they are among the first organisms to locate and colonize recently deceased bodies, with the developing larvae serving as a means of estimating the time of death14.
Blowfly lifestyles have been the subject of various research studies (e.g.,15,16,17,18,19,20,21) due to their significance in relation to human interests. Understanding the biological mechanisms governing a species' lifestyle can provide valuable insights into improving methods aimed at controlling pest species. Moreover, the diversity and evolution of blowfly lifestyles offer an ideal context to study the origins and mechanisms of complex traits (e.g., parasitism). Parasitism due to maggots feeding on live tissue has evolved independently several times within the Calliphoridae family22,23. However, the evolutionary history of the feeding habits of blowflies is still largely unknown, with studies restricted to mapping the habits along phylogenies (e.g.,16,19,22) without the aid of functional assays. For instance, it is uncertain whether obligate parasites evolved from generalists (i.e., facultative parasites) or directly from necrophagous species. The molecular, physiological, and behavioral processes accompanying the evolutionary shifts in lifestyle are also largely unknown.
In this context, facultative parasites, such as the sheep blowfly Lucilia cuprina, that can develop as parasites on a host or as necrophages on cadavers offer the possibility to explore the factors and mechanisms controlling lifestyle choices. Lucilia cuprina is a cosmopolitan species known for causing sheep flystrike, especially in Australia where it is considered a pest3,16. Myiasis due to L. cuprina can also occur in other livestock animals, pets, and humans3,24,25,26,27,28,29,30. However, its larvae can also develop in necrotic tissues and decaying matter and this species has been successfully used in forensic entomology as it is very fast to locate and colonize corpses31,32,33,34. Although the parasitic versus non-parasitic lifestyle of blowflies is defined by the larval stage, it is the adult female that selects the oviposition site. Consequently, the adult female heavily influences the larvae's lifestyle, as the latter have limited mobility. However, the female's choice does not necessarily imply that the larvae would prefer the same substrate when presented with a choice35. One hypothesis is that behavioral changes leading to females laying their eggs on live tissue could have been part of an early switch towards a parasitic lifestyle. Pre-adaptations or physiological capabilities of the resulting larvae would have been essential for their successful development on the live tissue, leading to the emergence of the parasitic lifestyle. As such, the processes affected and selected may not necessarily align between both life stages.
In this context, two methods were developed to test behavioral preference in blowflies, in particular, for, L. cuprina, regarding larval feeding substrate (larval preference assay) and oviposition site (female preference assay). These methods take into account two interacting factors: temperature and meat freshness. Temperature was chosen as a crucial factor since most cases of myiasis occur in homeothermic animals2. Hence, a temperature of 33 °C was selected as a proxy for the "parasitic lifestyle factor", whereas a temperature of 25 °C (room temperature) represents the "non-parasitic factor". A temperature of 25 °C was chosen as it is representative of the average yearly temperature recorded in Brazil (National Institute of Meteorology, INMET). Additionally, two types of meat substrates were considered, both from bovine sources: (i) fresh meat supplemented with blood, mimicking the substrate for the parasitic lifestyle, which is used to rear the parasitic blowfly Co. hominivorax in laboratory conditions36, and (ii) 5-day-old rotten meat, emulating the substrate for the necrophagous lifestyle. The bovine substrate is commonly used to rear L. cuprina in laboratory conditions27,37,38,39 as it offers several advantages in terms of availability, cost-effectiveness, and practicality while being an ecologically justifiable substrate. Other studies40,41 comparing the effect of rotten versus fresh substrates in blowflies have used 7-day-old rotten substrate (in anaerobic conditions) and showed an adverse effect of the rotten substrate on developmental rates, survival, and growth. As L. cuprina is known to colonize fresh cadavers which are usually exposed to air, we resolved to use 5-day-old rotten meat (ground beef) in non-hermetic pots (aerobic and anaerobic decomposition) to mimic a necrophagous substrate.
The experimental designs presented here offer the advantage of discerning preferences for individual factors as well as their combined effects. Moreover, the phenotypes scored, namely the choice of the larval feeding substrate and the number of eggs laid, are directly relevant to the biological and ecological aspects of blowfly species. The suitability of these protocols is highlighted by demonstrating their effectiveness in L. cuprina. Additionally, a script for statistical analysis is provided, which can be used to compare the observed results obtained in L. cuprina to simulated random data, ensuring robust statistical analysis and interpretations.
Protocol
Fly samples were obtained using traps and not on infested animals. A SISBIO license (67867-1) was issued to collect and keep flies of the Calliphoridae family in captivity in laboratory conditions. Insect samples are exempt from ethical evaluation in research in Brazil. Bovine meat and blood were obtained commercially, and no ethical clearance was required.
1. Larval feeding preference
- Preparation of the Petri dishes containing 2% agar
- Prepare four Petri dishes with 2% agar. To do so, add 6 g of bacteriological agar to 300 mL of water and melt this mix in a microwave. Then, divide the volume evenly into four glass Petri dishes (150 x 20 mm), using around 70 mL in each dish.
NOTE: Prepare Petri dishes equal to the number of experimental replicates desired. In this study, 36 replicates were used. - Once the agar has solidified, use a 50 mL conical tube (3 cm diameter) to punch four holes in the agar, two on each side of the Petri dish, following the cutting pattern provided (Figure 1).
NOTE: This setup is similar to previously described protocols by Fouche et al. (2021)40 and Boulay et al. (2016)42.
- Prepare four Petri dishes with 2% agar. To do so, add 6 g of bacteriological agar to 300 mL of water and melt this mix in a microwave. Then, divide the volume evenly into four glass Petri dishes (150 x 20 mm), using around 70 mL in each dish.
- Preparation of substrates
- To prepare the fresh meat with blood, add 12 mL of diluted bovine blood to 200 g of fresh bovine ground meat. Mix thoroughly. Be sure to use different graduated cylinders and spoons for each type of meat to avoid cross-contamination between substrates.
NOTE: The diluted blood is made up of 50% pure blood mixed with an anticoagulant (3.8% sodium citrate) and 50% filtered water. - To prepare the rotten substrate, add 12 mL of filtered water to 200 g of 5-day-old rotten bovine ground meat and mix it well.
NOTE: Rotten meat was obtained by incubating fresh ground meat for five days at 25 °C in non-hermetic plastic pots (mix of aerobic and non-aerobic decomposition). Each pot contained 200 g of fresh ground meat. It was then frozen until use. - Fill two holes in each Petri dish with the fresh meat and blood mixture and the remaining two holes with the rotten meat and water mixture.
NOTE: To avoid position biases, vary the placement of the different meat types in the Petri dishes. For example, some Petri dishes should have the same type of meat facing each other, while in other dishes, the meat type should be crossed, as shown in Figure 2.
- To prepare the fresh meat with blood, add 12 mL of diluted bovine blood to 200 g of fresh bovine ground meat. Mix thoroughly. Be sure to use different graduated cylinders and spoons for each type of meat to avoid cross-contamination between substrates.
- Experimental setup
- At a room temperature (RT) of 25 °C, position the heating pad directly below a light source to evenly illuminate the experimental area and avoid any behavior bias towards or against the light. Place paperboard pads around the heating pad to ensure that the experimental setup remains leveled.
NOTE: The light source used was white low heating emitting light, such as a neon tube. The heating pad was positioned on a table right below the ceiling light bulbs (Figure 3). - Cover the heating pad and leveling paperboard pads with black cardboard and switch the heating pad on.
NOTE: The black cardboard cover should be used to avoid visual cues that may bias the behavior assay. - Place six Petri dishes with agar and meat substrate over the black cardboard with two substrates, one of each type, on the heating pad and the other two off the surface of the heating pad (Figure 2). Let the substrates heat for approximately 10 min.
NOTE: Condensation may form on the lid of the Petri dishes.
- At a room temperature (RT) of 25 °C, position the heating pad directly below a light source to evenly illuminate the experimental area and avoid any behavior bias towards or against the light. Place paperboard pads around the heating pad to ensure that the experimental setup remains leveled.
- Larval test
- Check the temperature of the substrates (cold side: 25 ± 2 °C; hot side: 33 ± 2 °C) with an infrared thermometer.
NOTE: The heating pad stays on during the whole length of the experiment. Temperature measurements were made at the beginning and at the end of the experiment. Although the temperature did fluctuate by ± 2 °C, there was still at least an 8 °C temperature difference between the hot and cold conditions. - After reaching the desired temperature, place five third-instar larvae in the center of each Petri dish using tweezers (Figure 2) and cover the Petri dishes with the lids. Let the choice experiment run for 10 min.
NOTE: Some larvae may crawl around the edges and on the lid of the Petri dishes. If any larva escapes, use a tweezer to place it back to the center of the Petri dish. - After 10 min, get all the Petri dishes off of the heating pad and place them on a different surface to avoid continuing heating the substrates. Then, count the number of larvae in each substrate, as well as those that did not choose any substrate.
NOTE: Lucilia cuprina larvae stay in their chosen substrate, as observed in this experiment.
- Check the temperature of the substrates (cold side: 25 ± 2 °C; hot side: 33 ± 2 °C) with an infrared thermometer.
2. Female oviposition site preference
- Experimental setup
- Use a regular shelf previously covered up with black cardboard and evenly illuminated with white LED light strips.
NOTE: The black cardboard covers should be used to avoid visual cues that may bias the behavior assay. The white LED strips are secured lengthwise in the middle of the shelf right above the experiment. The shelves used in the setup were placed 45 cm apart. - At RT (25 °C), place a heating pad in the center of the shelf. Use paperboard pads around the heating pad as a support to ensure that the experimental setup is leveled.
- Cover the heating pad and leveling paperboard pads with a black cardboard to keep the same visual pattern below all the substrates.
- Place two cross-shaped-glass containers on one shelf, each should have two arms over the black cardboard and heating pad. Switch on the white LED light strips and the heating pads prior to the start of the experiment.
- Use 70% ethanol to clean the crosses (inside the cross and the lid) to avoid odor contamination.
- Use a regular shelf previously covered up with black cardboard and evenly illuminated with white LED light strips.
- Preparation of substrates
- Prepare four Petri dishes (60 mm x 15 mm) per cross with 5 g of 5-day-old rotten or fresh meat (two of each type of substrate).
NOTE: Prepare Petri dishes equal to the number of experimental replicates desired times four. In this study 30 replicates were used, totaling 120 prepared Petri dishes. - Add 1 mL of diluted bovine blood (50% pure blood with anticoagulant and 50% filtered water) onto the fresh meat and 1 mL of filtered water onto the rotten meat. Thoroughly mix the meat (fresh or rotten) with the liquid (blood or water) using a different spoon for each type of meat.
NOTE: The preparation of the meat for the female assay is very similar to the larval assay, although the quantities are different because the female test will be running for longer than the larval test. Remember to use different pipette tips and spoons for each type of meat to avoid any odor cross-contamination between substrates. - Check if the alcohol has completely evaporated from the crosses. Then, place four Petri dishes (one of each type of meat on the heating pad and the other two off the heating pad's surface) at the extremity of each arm of the cross (Figure 4). Close the crosses with their lids and allow the substrates to heat for approximately 10 min.
NOTE: In addition, to avoid position biases, vary the placement of the different meat types in the crosses. For example, some crosses should have the same type of meat on adjacent arms, while in other crosses, the same meat type should be across from one another, as shown in Figure 4.
- Prepare four Petri dishes (60 mm x 15 mm) per cross with 5 g of 5-day-old rotten or fresh meat (two of each type of substrate).
- Female test
- Collect gravid females in the fly cage and isolate them into individual tubes.
NOTE: Gravid females are characterized by having an enlarged and whitish-yellow abdomen in contrast to non-gravid females (Figure 5). Gravid females were collected between 10 and 16 days after emergence for the experiments. - Check the temperature of the substrates in the crosses (cold side: 25 ± 2 °C; hot side: 33 ± 2 °C) using an infrared thermometer.
NOTE: Just like in the larval test, the heating pad stays on during the whole length of the experiment. Temperature measurements were made at the beginning and end of the experiment. Although the temperature did fluctuate by ± 2 °C, there was still at least an 8 °C temperature difference between the hot and cold conditions. - Place the tube upside down containing one gravid female in the opening in the center of each cross. After the female enters the cross, remove the tube and close the opening with its small lid. After closing all the crosses, place a black cardboard in the front of the shelf to enclose the experimental setup. Let the experiment run for 4 h.
- After that, remove the female, by carefully catching it with a tube, and check if there were any eggs on the substrates.
- Identify the lid of each Petri dish with substrate type from each cross. Use 70% ethanol to clean the crosses (inside the cross and the lid) from any odor from the test.
NOTE: In case the eggs cannot be counted right after the experiment, the Petri dishes with substrates can be stored at -20 °C.
- Collect gravid females in the fly cage and isolate them into individual tubes.
- Egg count
NOTE: If the substrates in the Petri dishes were frozen, thaw them prior to counting.- Use a stereomicroscope to count the number of eggs laid in each substrate. Use a brush and water to help separate the eggs to count them.
3. Data analysis and statistics
- Preference indexes calculation
- For each replicate of larval (n = 36) and female (n = 30) tests, calculate the Preference Index for meat (designated as PImeat) by determining the ratio of larvae or eggs present on Fresh substrates (Fresh hot and Fresh cold) to the total count of larvae or eggs on all substrates (fresh hot + fresh cold + rotten hot + rotten cold).
PImeat = (# larvae or eggs on Fresh substrates) / # Total larvae or eggs
NOTE: The terms "Hot" and "Cold" are indicative of temperature conditions of 33 ± 2 °C and 25 ± 2 °C, respectively. - Likewise, calculate the Preference Index for temperature (PItemp) for each replicate of larval and female tests as the number of larvae or eggs present on Hot substrates (fresh Hot and rotten Hot) divided by the total number of larvae or eggs on all substrates (fresh hot + fresh cold + rotten hot + rotten cold).
PItemp = (# larvae or eggs on Hot substrates) / # Total larvae or eggs
NOTE: Values close to 1 reflect a preference for Fresh or Hot substrates and values close to zero indicate a preference for Rotten or Cold substrates. The PIs can be calculated manually or using the code provided (Supplemental Files S1 and Supplemental Files S2).
- For each replicate of larval (n = 36) and female (n = 30) tests, calculate the Preference Index for meat (designated as PImeat) by determining the ratio of larvae or eggs present on Fresh substrates (Fresh hot and Fresh cold) to the total count of larvae or eggs on all substrates (fresh hot + fresh cold + rotten hot + rotten cold).
- Comparison of the observed preference to simulated random data
- Run the provided code (Supplementary File S1 and Supplementary File S1) to generate the simulated data and to compare it with the observed data.
NOTE: This code generates 1000 simulated random dataset for larvae and females and calculates the Preference Indexes (PIs) for each replicate of the simulated, and the observed data of L. cuprina. The simulations assume that both larvae and females exhibit no substrate preference and make random choices. The simulations incorporate key behavioral aspects of the animals, encompassing various scenarios such as: the probability of larvae not selecting any substrate, and adult females concentrating their egg-laying on a single substrate or distributing their eggs either uniformly or not among different substrates. Generalized Linear Models (GLM, family: quasibinomial; link: logit) were used to compare the observed data from the behavior assays with the simulated random data. The employed GLM was well-suited for this analysis due to the bounded nature of the Preference Index (PI), ranging between 0 and 1. GLMs are adept at handling non-normally distributed response variables and allow for robust statistical comparisons. This choice facilitated meaningful insights by enabling to effectively compare observed data from behavior assays with complex patterns generated by simulated random data. Minor adjustments to the code may be needed for other structured datasets.
- Run the provided code (Supplementary File S1 and Supplementary File S1) to generate the simulated data and to compare it with the observed data.
Representative Results
To demonstrate the effectiveness of the presented methods, the experiments were conducted using a laboratory population of Lucilia cuprina (family: Calliphoridae), a facultative parasitic blowfly2. The entire raw dataset obtained for this species can be found in Supplementary File S3 with the results for the larval and female substrate preference tests. To assess if the larvae and females display a preference for any substrate, the observed data were compared to 1000 simulated datasets, each representing a random choice (see code in Supplementary File S1). The percentage of statistically significant comparisons (p < 0.05) was used as a measure to assess preference. From this analysis, it was evident that larvae exhibited a marked preference for the rotten substrate at 25 °C (Figure 6A, Table 1) as all 1000 comparisons between the observed data and each of the simulated random choice datasets were found significantly different for the meat and temperature conditions. Likewise, females also displayed a noticeable preference for 25 °C: 69.7% of the comparisons between the observed data and the random choice were found significantly different (Figure 6B, Table 1). However, their preference for rotten meat was more nuanced (Figure 6B, Table 1) as only 27.1% of the observed versus random choice comparisons were significant. Another observation from this study was that larvae of L. cuprina usually made a swift choice and burrowed in the meat substrate within the first 2 min of the experiment. They rarely changed to another condition during the 10 min experiment.
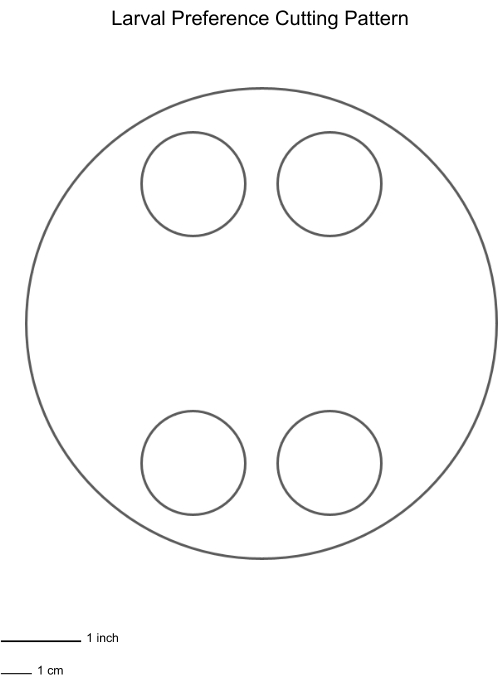
Figure 1: Larval feeding preference cutting pattern for Petri dishes with agar. Please click here to view a larger version of this figure.
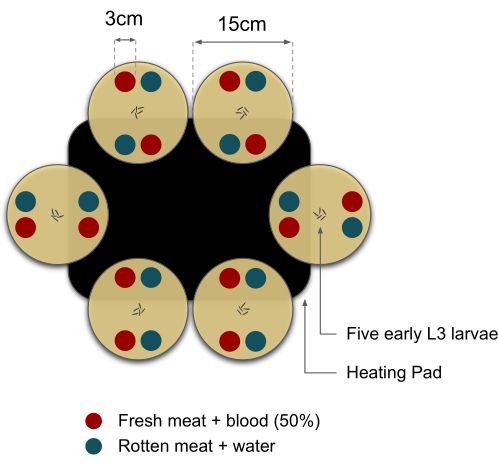
Figure 2: Top-view diagram of the layout of the larval feeding preference assay. The choices were randomly positioned, and the tests were performed at RT (25 ± 2 °C). The black rectangle represents the heating pad, which holds temperatures at 33 ± 2 °C. Red and blue circles represent fresh meat supplemented with diluted blood (50%) and rotten meat supplemented with water, respectively. Please click here to view a larger version of this figure.
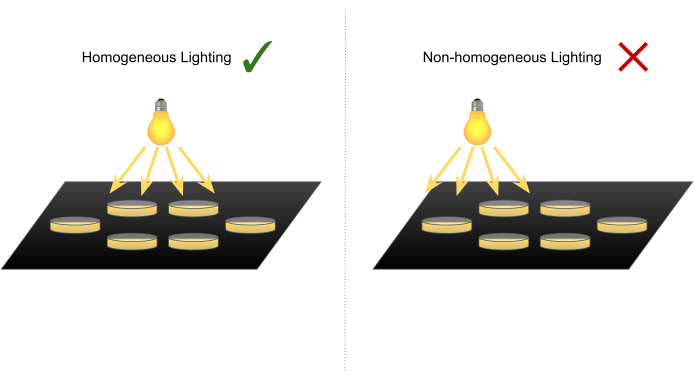
Figure 3: Diagram illustrating how to position the larval experimental setup below the light source to avoid biases towards or against light. The light source used was white low heating emitting light (neon tube). The heating pad was positioned on a table right below the ceiling light. Please click here to view a larger version of this figure.
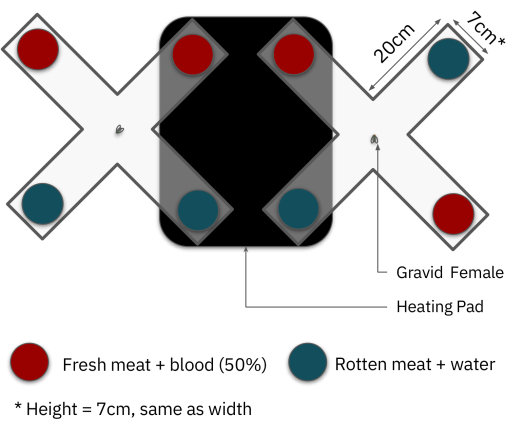
Figure 4: Top-view diagram of the layout of the Female oviposition site preference assay. The choices were randomly positioned, and the tests were performed at RT (25 ± 2 °C). The black rectangle represents the heating pad which holds the temperature at 33 ± 2 °C. Red circles represent fresh meat with diluted blood (50%) and blue circles, rotten meat with water. Please click here to view a larger version of this figure.

Figure 5: Photograph of a gravid (right) versus a non-gravid (left) female. Please click here to view a larger version of this figure.
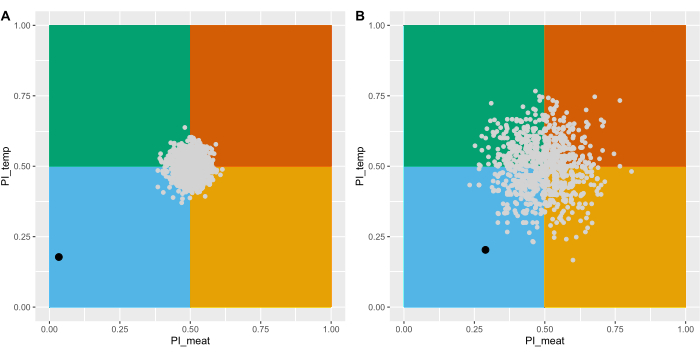
Figure 6: Mean Preference Indexes (PIs) for meat type and temperature for larvae (A) and females (B) displayed on a cartesian plane. The black circles represent the mean PIs taking into account all the experimental replicates (n = 36 for larvae and n = 30 for female experiments) obtained for L. cuprina. Each of the gray circles denotes the mean PIs for meat and temperature of a simulated dataset with similar characteristics to the observed dataset (e.g. same number of replicates) but representing a random choice. The colored panes serve as a visual aid to picture the PI areas of preference for each of the four choices with blue indicating rotten meat at 25 ± 2 °C, green as rotten meat at 33 ± 2 °C, yellow as fresh meat at 25 ± 2 °C and orange as fresh meat at 33 ± 2 °C. Please click here to view a larger version of this figure.
| Stage | Comparison | Significant comparisons for PImeat | Significant comparisons for PItemp |
| Larvae | Simulated vs. null hypothesis (p < 0.05) | 3.8% | 2.1% |
| Observed vs. simulated (p < 0.05) | 100.0% | 100.0% | |
| Females | Simulated vs. null hypothesis (p < 0.05) | 3.3% | 4.6% |
| Observed vs. simulated (p < 0.05) | 27.1% | 69.7% |
Table 1. Percentage of significant PImeat and PItemp (p-values < 0.05) of the comparisons between (i) the simulated random data (no preference) and the statistical null hypothesis, and (ii) the observed data and the simulated random data. The results are presented separately for larvae and females.
Supplementary File S1: Code used for data analysis and statistics in R markdown. Please click here to download this File.
Supplementary File S2: Report of the statistical analysis. Please click here to download this File.
Supplementary File S3: Raw counts of Lucilia cuprina for larval and female preference on each of the four substrate choices. Please click here to download this File.
Discussion
Understanding the evolution of feeding habits, particularly in the context of parasitism in blowflies, requires the examination of substrate preferences throughout different life stages for feeding or oviposition. Therefore, in this study, robust and straightforward methods were proposed for investigating substrate preferences in larvae and females of blowflies. These methods were tested in Lucilia cuprina, a facultative parasitic blowfly2. Interestingly, the experiments unveiled a distinct inclination for rotten meat at 25 °C among L. cuprina larvae, aligning with conditions typically used by necrophagous species. This differed from a study of Fouche et al40, which showed a preference for fresh liver substrate in Lucilia sericata and Calliphora vicina and showed that the rotten substrate negatively impacted survival and growth. However, it is difficult to compare the results of both studies as the degree of meat decay (seven days versus five in our case) and the decomposition process (purely anaerobic versus aerobic and anaerobic in our case) were different. The species used were also different. Additionally, observations from the experiments presented here indicated that females preferred laying their eggs at 25 °C, while exhibiting only a slight preference for rotten meat. These results show that larvae and female choices are not the same and that the females display a more varied choice for their oviposition site selection than the larvae for burrowing and food choices. This suggests that the parasitic habit in L. cuprina is led by changes in the female oviposition choice and not by larval feeding preference. Notably, these findings serve as a proof-of-concept demonstration of the effectiveness and utility of the methods in elucidating the lifestyle of blowflies at different developmental stages.
Distinct meat and temperature conditions were used to mimic parasitic and necrophagous lifestyle factors. This approach facilitated the evaluation of larval feeding and female oviposition site preferences in a four-choice assay, using two interacting factors. The protocols adopted represent an approach that deviates from the traditional two-choice technique typically used in previous studies43,44,45,46,47,48,49,50. To minimize variations resulting from environmental factors that could influence behavior, such as light, visual, or odor cues, rigorous control measures were implemented. Uniform and consistent illumination from above was sustained in the assays to avoid any biases towards or against light, complemented by the use of a black background to prevent potential visual cues' impact on larval and female preferences. Furthermore, the risk of cross-contamination between rotten and fresh meat substrates was avoided by employing glass or disposable plastic material, gloves, and separate utensils. The application of these measures proved critical in establishing a controlled and trustworthy experimental framework, thereby assuring the robustness and reliability of the results obtained.
The larval experiment design was similar to previously described two-choice assays40,42, with adaptations made to incorporate the temperature factor. The larval protocol described here proved to be rapid, robust and straightforward, given that the larvae exhibited a strong tendency to remain burrowed within their chosen substrate, thus eliminating the possibility of ambiguous scoring issues arising from the switching of substrates at the end of the experiment. This particular feature enables the experimenter to conduct six or more replicates simultaneously without the risk of unclear or uncertain results. Although the presence of multiple larvae within the same replicate can influence individual choices, the protocol allows for the assessment of general substrate preference through independent replicates. In scenarios where possible aggregative behavior needs to be avoided or controlled, individual testing or the incorporation of control experiments to account for potential influences between larvae can be implemented to counter any bias.
On the other hand, the female oviposition site preference protocol offers the notable advantage of assessing the individual choice independently, free from the influence of other females' preferences, thus avoiding aggregative behavior. Indeed, it is known that calliphorid female oviposition choice can be influenced by the presence of conspecific flies46,47. Nevertheless, it is important to acknowledge the inherent limitation of the experimental assay. Eggs may not be laid within the 4-hour experimental window due to unsuitable conditions or, more likely, the immaturity of the females. This uncertainty results in a subset of the replicates with no eggs laid (78% of trials). Furthermore, the wide range in the number of eggs laid in each replicate (26 to 208, mean ± standard deviation = 132.4 ± 46.2) introduces considerable variability, making it challenging to distinguish between variations driven by female's preference and those influenced by factors such as limited egg reserves or late egg-laying during the experiment. Despite these limitations, the proposed protocols are suitable to effectively assess oviposition site preference.
Overall, the protocols developed hold significant potential for a wide range of applications in studying blowfly behavior. Firstly, these tests can be employed to examine the effects of various treatments, such as different husbandry or developmental conditions, on larval or female preferences within the same species. This could potentially unveil the underlying mechanisms driving behavioral preferences and their genetic basis, particularly when coupled with sequencing techniques. Moreover, these tests can be extended to investigate the substrate preferences of different blowfly species, providing valuable insights into the evolution of parasitism within this group. By delving into the diverse preferences exhibited by blowflies, a deeper understanding of their ecological adaptations can be gained, providing valuable knowledge for the future management and control of pest species.
Lastly, the potential of the protocols extends beyond the study of blowflies alone. With minor modifications, these protocols can be readily applied to assess the fly species from other families, such as house flies and flesh flies, or even insects of similar size. The adaptability of the protocols also allows for the selection of different substrates to match the objectives of specific scientific inquiries. For instance, researchers can modify the degree of meat decay or substitute bovine meat with alternative animal sources (e.g., fish, pork) or non-animal substrates (e.g., fruits) to address diverse ecological questions. These adaptations not only enhance the versatility of the protocols, but also enable the exploration of preferences in a wide range of insect species and ecological contexts, thereby enhancing the capacity to elucidate fundamental aspects of insect behavior and ecological adaptation.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We acknowledge Patrícia J. Thyssen, Gabriela S. Zampim and Lucas de Almeida Carvalho for providing the L. cuprina colony and for their assistance in setting up the experiment. We would also like to thank Rafael Barros de Oliveira for filming and editing the video. This research was supported by the Developing Nation Research Grant from Animal Behavior Society to V.A.S.C. and by a FAPESP Dimensions US-Biota-São Paulo grant to T.T.T. (20/05636-4). S.T. and D.L.F. were supported by a FAPESP (19/07285-7 postdoctoral grant and 21/10022-8 PhD scholarship, respectively). V.A.S.C. and A.V.R. were supported by CNPq PhD scholarships (141391/2019-7, 140056/2019-0, respectively). T.T.T. was supported by CNPq (310906/2022-9).
Materials
| Agar | Sigma-Aldrich | 05038-500G | For microbiology |
| Black cardboards | – | – | 70×50 cm |
| Bovine blood with anticoagulat | – | – | 50% pure bovine blood with anticoagulant (3.8% sodium citrate) + 50% of filtered water |
| Bovine ground Meat | – | – | Around 7-8% of fat |
| Brush | – | – | Made with plastic |
| Conical tube | Falcon or Generic | – | 50 mL |
| Cross-shaped glass containers | Handmade | NA | 48×48 cm, 8 cm of height and 8 cm of width |
| Erlenmeyer | Vidrolabor | NA | 500 mL |
| 70% Ethanol | Synth | A1084.01.BL | 70% ethyl ethanol absolute + 30% filtered water |
| Graduated cylinder | Nalgon or Generic | – | 500 mL and 50 mL |
| Heating pad | Thermolux | – | 30×40 cm dimensions, 40 W, 127 V |
| Infrared thermometer | HeTaiDa | HTD8808 | Non-contact body thermometer (Sample Rate: 0.5 S, Accuracy: ±0.2 °C, Measuring: 5-15 cm) |
| Petri dish (Glass) | Precision | NA | 150×20 mm dimensions |
| (Note: the petri dishes can be plastic if used only once) | |||
| Petri dish PS | Cralplast | 18130 | 60×15 mm dimensions |
| Plastic Pasteur pipette | – | – | 3 mL (total volume) |
| Sodium citrate | Synth | C11033.01.AG | 3.8% Sodium citrate (38 g diluted in 1L of filtered water) |
| Spoons | – | – | More than one spoon is necessary. Use one for each type of meat substrate. Preferably stainless steel. |
| Stainless steel spatula | Generic | – | Flat end and spoon end |
| Stereomicroscope | Bioptika | – | WF10X/22 lenses |
| Tweezer | – | – | Metal made and fine point |
| White led light strips | NA | NA | 4.8 W, 2×0.05 mm², 320 lumens, Color temperature:6500 K (white) |
References
- Kutty, S. N., et al. Phylogenomic analysis of Calyptratae: Resolving the phylogenetic relationships within a major radiation of Diptera. Cladistics. 35 (6), 605-622 (2019).
- Zumpt, F. . Myiasis in Man and Animals in the Old World. A textbook for physicians, veterinarians and zoologists. , (1965).
- Hall, M., Wall, R. Myiasis of humans and domestic animals. Advances in Parasitology. 35, 257-334 (1995).
- Grisi, L., et al. Reassessment of the potential economic impact of cattle parasites in Brazil. Revista Brasileira de Parasitologia Veterinária. 23 (2), 150-156 (2014).
- Sackett, D., Holmes, P., Abbot, K., Jephcott, S., Barber, M. Assessing the economic cost of endemic disease on the profitability of Australian beef cattle and sheep producers. Meat & Livestock Australian Report. AHW.087. Meat & Livestock Australian Report. , (2006).
- Heath, A. C. G., Bishop, D. M. Flystrike in New Zealand: An overview based on a 16-year study, following the introduction and dispersal of the Australian sheep blowfly, Lucilia cuprina Wiedemann (Diptera: Calliphoridae). Veterinary Parasitology. 137 (3-4), 333-344 (2006).
- Mullen, G. R., Durden, L. A. . Medical and veterinary entomology. , (2009).
- Spradbery, J. P. Screw-worm fly: A tale of two species. Agricultural Zoology Reviews. 6 (1), (1994).
- World Organization for Animal Health (OIE). New World screwworm (Cochliomyia hominivorax) and Old World screwworm (Chrysomya bezziana), Manual of diagnostic tests and vaccines for terrestrial animals. World Organization for Animal Health (OIE). , (2013).
- Wardhana, A. H., Abadi, I., Cameron, M. M., Ready, P. D., Hall, M. J. R. Epidemiology of traumatic myiasis due to Chrysomya bezziana in Indonesia. Jurnal Ilmu Ternak dan Veteriner. 23 (1), 45 (2018).
- Linger, R. J., et al. Towards next generation maggot debridement therapy: Transgenic Lucilia sericata larvae that produce and secrete a human growth factor. BMC Biotechnology. 16 (1), 30 (2016).
- Fonseca-Muñoz, A., Sarmiento-Jiménez, H. E., Pérez-Pacheco, R., Thyssen, P. J., Sherman, R. A. Clinical study of Maggot therapy for Fournier’s gangrene. International Wound Journal. 17 (6), 1551 (2020).
- Franciéle, S. M., Demetrius, S. M., Patricia, J. T. Larval Therapy and the application of larvae for healing: review and state of the art in Brazil and worldwide. Revista Thema. 12 (01), 4-14 (2015).
- Greenberg, B. Flies as forensic indicators. Journal of Medical Entomology. 28 (5), 565-577 (1991).
- Stevens, J. R., Wallman, J. F., Otranto, D., Wall, R., Pape, T. The evolution of myiasis in humans and other animals in the Old and New Worlds (Part II): Biological and life-history studies. Trends in Parasitology. 22 (4), 181-188 (2006).
- Stevens, J. R. The evolution of myiasis in blowflies (Calliphoridae). International Journal for Parasitology. 33 (10), 1105-1113 (2003).
- McDonagh, L. M., Stevens, J. R. The molecular systematics of blowflies and screwworm flies (Diptera: Calliphoridae) using 28S rRNA, COX1 and EF-1α Insights into the evolution of dipteran parasitism. Parasitology. 138 (13), 1760-1777 (2011).
- Wallman, J. F., Leys, R., Hogendoorn, K. Molecular systematics of Australian carrion-breeding blowflies (Diptera:Calliphoridae) based on mitochondrial DNA. Invertebrate Systematics. 19 (1), (2005).
- Yan, L., et al. Monophyletic blowflies revealed by phylogenomics. BMC Biology. 19 (1), 230 (2021).
- Cardoso, G. A., Deszo, M. S., Torres, T. T. Evolution of coding sequence and gene expression of blowflies and botflies with contrasting feeding habits. Genomics. 113 (1), 699-706 (2021).
- Cardoso, G. A., Marinho, M. A. T., Monfardini, R. D., Espin, A. M. L. D. A., Torres, T. T. Evolution of genes involved in feeding preference and metabolic processes in Calliphoridae (Diptera: Calyptratae). PeerJ. 4, 2598 (2016).
- Stevens, J. R., Wallman, J. F. The evolution of myiasis in humans and other animals in the Old and New Worlds (part I): Phylogenetic analyses. Trends in Parasitology. 22 (3), 129-136 (2006).
- Wiegmann, B. M., et al. Episodic radiations in the fly tree of life. Proceedings of the National Academy of Sciences. 108 (14), 5690-5695 (2011).
- Azevedo, W. T. D. A., et al. Record of the first cases of human myiasis by Lucilia cuprina (Diptera: Calliphoridae), Rio de Janeiro, Brazil. Journal of Medical Entomology. 52 (6), 1368-1373 (2015).
- Bishop, D., Patel, D., Heath, A. A New Zealand case of nasal myiasis involving Lucilia cuprina (Diptera: Calliphoridae). The New Zealand Medical Journal (Online). 131 (1484), 68-70 (2018).
- Lukin, L. G. Human cutaneous myiasis in Brisbane: a prospective study. Medical Journal of Australia. 150 (5), 237-240 (1989).
- Paulo, D. F., et al. Specific gene disruption in the major livestock pests Cochliomyia hominivorax and Lucilia cuprina Using CRISPR/Cas9. G3 Genes|Genomes|Genetics. 9 (9), 3045-3055 (2019).
- Puttalakshmamma, G. C., Dhanalakshmi, H., D’souza, P. E., Ananda, K. J. Incidence of myiasis in domestic animals in Bangalore. Intas Polivet. 6 (2), 353-356 (2005).
- Rao, M. A. N., Pillay, M. R. Some notes on cutaneous myiasis in animals in the Madras presidency. Indian Journal of Veterinary Science. 6 (3), (1936).
- Soundararajan, C. Traumatic myiasis in an Indian peafowl (Pavo cristatus) due to Lucilia cuprina first report. Journal of Veterinary Parasitology. 34 (1), 49-51 (2020).
- Smith, K. G. V. . A manual of forensic entomology. , (1986).
- Goff, M. L. . A Fly for the Prosecution. , (2001).
- CRC Press. . Forensic entomology: the utility of arthropods in legal investigations. , (2001).
- Greenberg, B., Kunich, J. C. . Entomology and the law: flies as forensic indicators. , (2002).
- Ellis, A. M. Incorporating density dependence into the oviposition preference-offspring performance hypothesis. Journal of Animal Ecology. 77 (2), 247-256 (2008).
- Vargas, M. E. I., Azeredo-Espin, A. M. L. Genetic variability in mitochondrial DNA of the screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae), from Brazil. Biochem Genet. 33, 237-256 (1995).
- Bambaradeniya, Y. T. B., Karunaratne, W. I. P., Tomberlin, J. K., Goonerathne, I., Kotakadeniya, R. B. Temperature and tissue type impact development of Lucilia cuprina (Diptera: Calliphoridae) in Sri Lanka. Journal of Medical Entomology. 55 (2), 285-291 (2018).
- Chaaban, A., et al. Insecticide activity of Curcuma longa (leaves) essential oil and its major compound α-phellandrene against Lucilia cuprina larvae (Diptera: Calliphoridae): Histological and ultrastructural biomarkers assessment. Pesticide Biochemistry and Physiology. 153, 17-27 (2019).
- Selem, G., Geden, C. J., Khater, H., Khater, K. S. Effects of larval diets on some biological parameters and morphometric and biochemical analysis of ovaries of Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Journal of Vector Ecology. 48 (2), (2023).
- Fouche, Q., Hedouin, V., Charabidze, D. Effect of density and species preferences on collective choices: an experimental study on maggot aggregation behaviours. Journal of Experimental Biology. 224 (6), 233791 (2021).
- Richards, C. S., Rowlinson, C. C., Cuttiford, L., Grimsley, R., Hall, M. J. R. Decomposed liver has a significantly adverse affect on the development rate of the blowfly Calliphora vicina. International Journal of Legal Medicine. 127, (2013).
- Boulay, J., Deneubourg, J. -. L., Hédouin, V., Charabidzé, D. Interspecific shared collective decision-making in two forensically important species. Proceedings of the Royal Society B: Biological Sciences. 283 (1824), 20152676 (2016).
- Joseph, R. M., Devineni, A. V., King, I. F. G., Heberlein, U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proceedings of the National Academy of Sciences. 106 (27), 11352-11357 (2009).
- Mierzejewski, M. K., Horn, C. J., Luong, L. T. Ecology of fear: Environment-dependent parasite avoidance among ovipositing Drosophila. Parasitology. 146 (12), 1564-1570 (2019).
- Stensmyr, M. C., et al. A conserved dedicated olfactory circuit for detecting harmful microbes in drosophila. Cell. 151 (6), 1345-1357 (2012).
- Yang, S. -. T., Shiao, S. -. F. Oviposition preferences of two forensically important blow fly species, chrysomya megacephala and C. rufifacies (Diptera: Calliphoridae), and implications for postmortem interval estimation. Journal of Medical Entomology. 49 (2), 424-435 (2012).
- Brodie, B. S., Wong, W. H. L., VanLaerhoven, S., Gries, G. Is aggregated oviposition by the blow flies Lucilia sericata and Phormia regina (Diptera: Calliphoridae) really pheromone-mediated?: Pheromone-mediated Lucilia sericata and Phormia regina flies. Insect Science. 22 (5), 651-660 (2015).
- Horn, C. J., Liang, C., Luong, L. T. Parasite preferences for large host body size can drive overdispersion in a fly-mite association. International Journal for Parasitology. , (2023).
- Liu, W., et al. Enterococci mediate the oviposition preference of Drosophila melanogaster through sucrose catabolism. Scientific Reports. 7 (1), 13420 (2017).
- Parodi, A., et al. Black soldier fly larvae show a stronger preference for manure than for a mass-rearing diet. Journal of Applied Entomology. 144 (7), 560-565 (2020).

